Summary
This study aimed to describe the patterns of care and outcomes of diffuse large B cell lymphoma (DLBCL) after failure of front line anthracycline-based immunochemotherapy (IC). Patients with newly diagnosed lymphoma were prospectively enrolled in Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence. All DLBCL and primary mediastinal B-cell lymphoma (PMBL) patients treated with front-line anthracycline-based IC were followed for relapse. Patients with relapse on follow-up and subsequently retreated were included in this analysis. 1039 patients received anthracycline-based IC between 2002 and 2012, of which 244 relapsed and were subsequently retreated. Across all therapies, overall survival at 4 years (OS4) from relapse was 28% and 103 patients ultimately underwent autologous haematopoietic cell transplant (autoHCT) with OS4 from autoHCT of 51%. Patients relapsing after 12 months from initial diagnosis had OS4 of 47% but those with a transient or no response to initial therapy had OS4 of only 13%. Outcomes of relapsed or refractory DLBCL differ substantially when categorized by response to initial therapy, timing of relapse and opportunity to undergo autoHCT. The design and interpretation of uncontrolled trials should account for this heterogeneity in patients with relapsed DLBCL.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) accounts for 30% to 40% of all non-Hodgkin lymphoma (Al-Hamadani, et al 2015). Front line anthracycline-based immunochemotherapy (IC) with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP, or similar) is expected to cure more than 50% of newly diagnosed DLBCL, but at least one-third of patients will be refractory to first-line chemotherapy, achieve only partial remission, or will relapse after achieving complete remission (Coiffier, et al 2002, Habermann, et al 2006, Pfreundschuh, et al 2006, Sehn, et al 2005, Sud and Friedberg 2008). Management of DLBCL after failure of IC is challenging without a clear optimal salvage strategy (Crump, et al 2014, Gisselbrecht, et al 2010). Although curative intent salvage chemotherapy followed by autologous haematopoietic cell transplant (autoHCT) is considered to have the best potential for durable remission, the significant associated toxicities preclude inclusion of the substantial fraction of patients with comorbidities or advanced age (Danese, et al 2016). Thus, the prognosis of relapsed and refractory DLBCL as a group remains poor with only a small number of patients achieving long-term disease-free survival (Costa, et al 2016, Friedberg 2011, Hitz, et al 2015, Kansara, et al 2014, Nagle, et al 2013, Rovira, et al 2015, Van Den Neste, et al 2016, Van Den Neste, et al 2015).
DLBCL at diagnosis is considered a heterogeneous disease due to host and tumour biology, and the same is true in the relapsed and refractory settings (Sehn and Gascoyne 2015). Biological subsets, such as cell of origin (COO) or chromosomal translocations involving MYC and the BCL gene family, are less likely to change throughout the course of disease. But distinct clinical scenarios, such as primary refractory, early or late relapse, nodal or extranodal relapse, autoHCT eligible or not, and relapse post-autoHCT, may variably apply to a given patient over time, each probably with unique outcomes. Clinical trials with new agents targeting relapsed or refractory DLBCL often include only subsets of this population and the ability to judge the efficacy of these agents is limited by unknown historical outcomes of the enrolled subset (Christian, et al 2015, Coiffier, et al 2016, Gerecitano, et al 2015, Jacobsen, et al 2015, Kochenderfer, et al 2015, Locke, et al 2015, Maddocks, et al 2015, Morschhauser, et al 2014, Moskowitz, et al 2015, Ribrag, et al 2015, Salles, et al 2013, Viardot, et al 2016, Walter, et al 2016, Wang, et al 2015, Witzig, et al 2011a, Witzig, et al 2011b). Population-based studies attempting to define these outcomes to date are limited by lack of inclusivity, treatment and outcome detail, or adequate follow-up (Danese, et al 2016). With a multitude of potential therapeutic strategies available for testing, it is unlikely that a single optimal strategy will benefit all relapsed or refractory DLBCL patients and there is a need to identify subsets that can be defined prospectively along with associated expected outcomes.
We initiated a prospective observational cohort study in 2002, enrolling newly diagnosed lymphoma patients with a protocol-specified methodology for capturing baseline clinical, laboratory and pathology data, initial therapy, and active follow-up of all patients for clinical events including re-treatment, relapse/progression and death. Starting from a cohort of consecutively enrolled patients with de novo DLBCL, the goal of this analysis was to characterize the subsequent patterns of care and outcomes in patients with DLBCL following IC failure and to identify differences among definable subsets
PATIENTS and METHODS
Study Population
Patients were prospectively enrolled in the University of Iowa / Mayo Clinic Specialized Program of Research Excellence Molecular Epidemiology Resource (MER) within 9 months of diagnosis. Clinical management at diagnosis and subsequent therapies were per treating physician.
Patients were followed for relapse, retreatment and death; all events were validated by review of the medical record. This analysis includes patients with DLBCL or primary mediastinal lymphoma (PMBL) who received initial treatment with anthracycline-based IC (R-CHOP or similar) with a focus on patients who subsequently had an event defined by relapse, retreatment, or death. Patients with primary central nervous system (CNS) lymphoma and post-transplant lymphoproliferative disorders (PTLD) were excluded. Response to therapy was retrospectively classified per 2007 Revised Response Criteria for Malignant Lymphoma from available clinical and radiology records (Cheson, et al 2007). Unplanned consolidative radiation (RT) without biopsy proven disease after achieving partial remission (PR) from IC was not classified as a relapse for this analysis. Any relapse consisting of low-grade lymphoma without DLBCL was not considered as a relapse for this analysis.
We defined relapsed/refractory based on response to initial therapy and timing of relapse. Primary refractory disease was defined as no response to frontline anthracycline-based IC. Transient response was defined as either complete remission (CR) or PR noted at assessment in the midst of a planned course of therapy but end of treatment assessment showing progressive disease from the interim response. Relapsed DLBCL was defined as relapse after achieving PR or CR at the end of initial IC. Time from diagnosis to relapse was examined to assess the significance of early vs. late relapses in relapsed DLBCL. We also stratified the treatment into aggressive chemotherapy and less aggressive treatment as a surrogate for patient ability to tolerate salvage chemotherapy. Aggressive salvage treatments at relapse with multi-agent chemotherapy such as R-ICE (rituximab, ifosfamide, carboplatin, etoposide), R-EPOCH (rituximab, etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin), R-GDP (rituximab, gemcitabine, dexamethasone, cisplatin) or R-DHAP (rituximab, dexamethasone, high dose cytarabine, cisplatin) were considered a surrogate for plan to proceed with autoHCT consolidation with curative intent. Patients with CNS involvement at relapse or those treated with radiation only were analysed separately. COO was determined by immunohistochemistry using the Hans algorithm.
Statistical Methodology
Overall survival (OS) was defined as time from the clinical assessment at point of interest (diagnosis, relapse or autoHCT) until death due to any cause. Event-free survival (EFS) was defined as time from clinical assessment until progression/relapse, retreatment, or death due to any cause. OS and EFS were assessed using Kaplan Meier curves and Cox proportional hazards models. Event decomposition was performed by using a competing risk approach (Gray 1988). All p-values reported are 2-sided. All analyses were performed by using SAS v9.4 (SAS Institute, Cary, NC) and R v3.3.1 (R Core Team, 2016).
RESULTS
Patient Characteristics
A total of 1039 patients with de novo DLBCL treated with frontline anthracycline-based IC from 2002–2012 were eligible for this analysis. The median age at diagnosis was 62 years (range 18–92); 56% were male. 647 patients (63%) had stage III/IV disease and International Prognostic Index (IPI) at diagnosis was 0–1 in 350 patients (34%), 2 in 305 patients (29%), 3 in 250 patients (24%) and 4–5 in 134 patients (13%), (Table I). Median follow-up of the entire cohort from diagnosis (reverse Kaplan Meier) was 59 months (range 1–148) (Therneau and Grambsch 2000).
Table I.
Patient characteristics at diagnosis
| Characteristic | All (N=1039) | Relapsed (N=250) | |
|---|---|---|---|
|
| |||
| Median age (range), years | 62 (18–92) | 61 (19–89) | |
|
| |||
| Age at diagnosis | |||
| <60 years | 475 (46%) | 114 (46%) | |
| >60 years | 564 (54%) | 136 (54%) | |
|
| |||
| Sex | |||
| Male | 577 (56%) | 157 (63%) | |
| Female | 462 (44%) | 93 (37%) | |
|
| |||
| Histology at diagnosis | |||
|
| |||
| DLBCL | 983 (95%) | 241 (96%) | |
|
| |||
| PMBL | 56 (5%) | 9 (4%) | |
|
| |||
| IPI at diagnosis | |||
|
| |||
| 0–1 | 349 (34%) | 47 (19%) | |
|
| |||
| 2 | 305 (29%) | 69 (28%) | |
|
| |||
| 3 | 251 (24%) | 93 (37%) | |
|
| |||
| 4 or 5 | 134 (13%) | 41 (16%) | |
|
| |||
| Stage at diagnosis | |||
| I/II | 382 (37%) | 54 (22%) | |
| III/IV | 647 (63%) | 194 (78%) | |
|
| |||
| LDH at diagnosis | |||
| >Upper limit of normal | 393 (42%) | 161 (75%) | |
| <Upper limit of normal | 543 (58%) | 55 (25%) | |
|
| |||
| 2 or more Extranodal sites at diagnosis | |||
| Yes | 215 (21%) | 60 (24%) | |
| No | 824 (79%) | 190 (76%) | |
|
| |||
| ECOG performance status at diagnosis | |||
| 0–1 | 857 (83%) | 186 (75%) | |
| 2–4 | 179 (17%) | 63 (25%) | |
|
| |||
| Any B symptoms at diagnosis | |||
| Yes | 256 (25%) | 84 (34%) | |
| No | 783 (75%) | 166 (66%) | |
|
| |||
| Bulky disease > 10 cm at diagnosis | |||
| Yes | 127 (12%) | 45 (18%) | |
| No | 912 (88%) | 205 (82%) | |
|
| |||
| Bone marrow involvement with DLBCL at diagnosis | |||
| Yes | 102 (10%) | 33 (13%) | |
| No | 937 (90%) | 217 (87%) | |
|
| |||
| Cell of origin (Hans) | |||
| GCB | 375 (64%) | 74 (59%) | |
| Non-GCB | 210 (36%) | 51 (41%) | |
|
| |||
| CNS involvement at diagnosis | |||
| Yes | 18 (2%) | 8 (3%) | |
| No | 1021 (98%) | 242 (97%) | |
|
| |||
| Creatinine at diagnosis | 0.9 (0.4–19.1) | 1.0 (0.4–4.4) | |
CNS, central nervous system; DLBCL, diffuse large B cell lymphoma; ECOG, Eastern Cooperative Oncology Group; GCB, germinal centre B cell-like; IPI, International Prognostic Index; LDH, lactate dehydrogenase; PMBL, primary mediastinal B cell lymphoma.
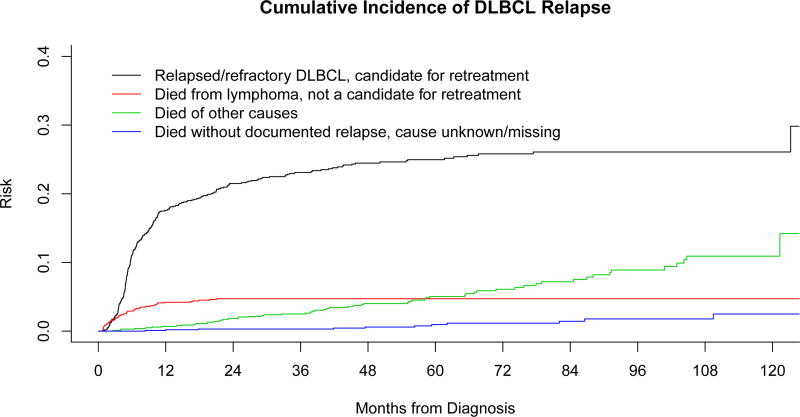
Of the 1039 patients, 250 (24%) had documented relapse and/or retreatment for relapsed or refractory DLBCL. Forty-eight patients died of progressive lymphoma prior to a formal response assessment or died of lymphoma-related causes (unable to complete treatment, clinical deterioration, complications of treatment, etc.) and were not candidates for retreatment. The Kaplan-Meier estimates for EFS and OS of the cohort from the time of diagnosis was 72% (95% confidence interval [CI]: 69%-75%) and 80% (95% CI: 78–83%) at 2 years and 64% (95% CI: 61%-68%) and 71% (95% CI: 68%-74%) at 5 years, respectively. The cumulative incidence of events that include relapse and other competing causes of death for this cohort can be found in Figure 1A.
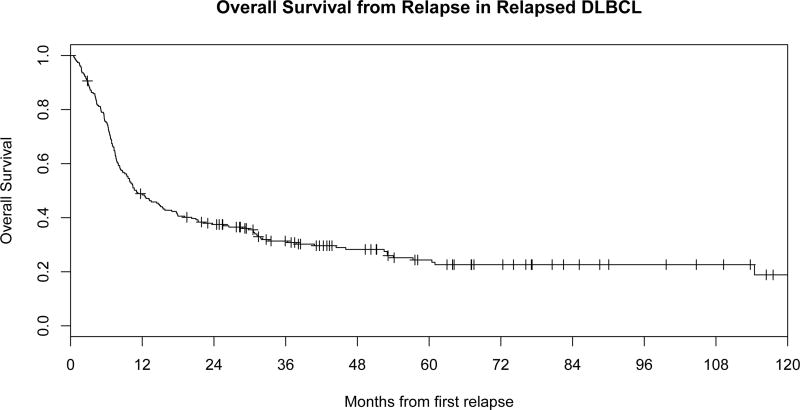
Figure 1.
A: Cumulative incidence of Relapse or Refractory disease in a cohort of patients with de novo diffuse large B cell lymphoma (DLBCL) treated with immunochemotherapy (IC).
B: Overall of survival from the time of relapse or refractoriness in DLBCL.
Initial salvage therapy in patients with relapsed or refractory DLBCL
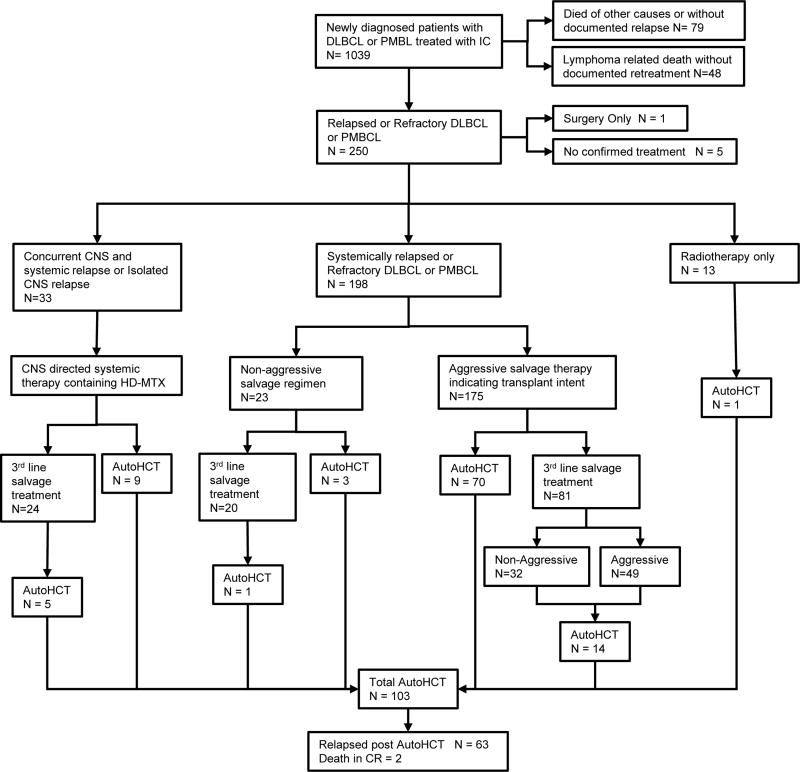
After failure of front-line IC for DLBCL, 244 patients received retreatment for relapsed or refractory disease, one patient underwent surgical resection without systemic therapy, and 5 had no confirmed therapy after documented relapse. Across all therapies, the response rate to first therapy for relapsed disease was 54% with 103 patients (42%) ultimately undergoing autoHCT. At a median follow-up of 58 months from relapse (reverse Kaplan Meier, range 0–125), 175 patients (72%) had died. The median OS of 244 patients from relapse was 11 months (95% CI 8.8–15.5) and overall survival at 4 years (OS4) from relapse was 28% (Figure 1B). The 2-year EFS from first relapse was 23%. Outcomes of the 244 receiving non-surgical therapy are summarized in Figure 2.
Figure 2. Pattern of care after failure of front line immunochemotherapy.
AutoHCT, autologous haematopoietic cell transplant; CNS, central nervous system; CR, complete remission; DLBCL, diffuse large B cell lymphoma; HD-MTX, high dose methotrexate; IC, immunochemotherapy; PMBL, primary mediastinal B cell lymphoma.
Aggressive chemotherapy at first relapse
Patients without CNS involvement at relapse (n=175) were treated with aggressive systemic chemotherapy. The median age of this group of patients at relapse was 61 years (range 20–87). Initial aggressive salvage regimens included R-ICE in 138 patients, R-DHAP in 14, and anthracycline-based therapy in 12 (Table II). One hundred (57%) patients responded to initial aggressive salvage and 84 (48%) subsequently underwent consolidative autoHCT; 70 proceeded to autoHCT after initial salvage therapy and 14 after subsequent salvage therapy(s). In patients undergoing autoHCT, the median survival from autoHCT was 42.7 months (95% CI: 18.9-unreached) with a 2-year EFS rate from autoHCT of 45%. Outcomes were very poor in patients that were treated with aggressive salvage chemotherapy but failed to undergo autoHCT, with a median OS from initial relapse of only 6.9 months (95% CI: 5.7–7.6) and a 2-year OS from initial relapse of 19% (Table III).
Table II.
Outcomes to therapy at first relapse
| Therapy | N | CR/PR to first salvage therapy |
autoHCT after first salvage Therapy |
autoHCT after first or subsequent salvage |
2 year EFS after relapse |
|---|---|---|---|---|---|
| All therapies | 244 | 53% | 88 (36%) | 103 (42%) | 23% |
| All aggressive therapies | 175 | 57% | 70 (40%) | 84 (48%) | 24% |
| R-ICE | 138 | 56% | 62 (45%) | 72 (53%) | 25% |
| R-DHAP | 14 | 36% | 4 (29%) | 7 (50%) | 14% |
| ROAD | 10 | 70% | 5 (50%) | 5 (50%) | 22% |
| Anthracycline-based | 12 | 75% | 2 (17%) | 2 (17%) | 25% |
| R-ESHAP | 1 | 100% | 1 (100%) | 1 (100%) | 100% |
| Non-aggressive therapy | 23 | 52% | 3 (13%) | 4 (17%) | 13% |
| R-GemOX | 4 | 0% | 0 (0%) | 1 (25%) | 0% |
| Zevalin | 1 | 100% | 1 (100%) | 1 (100%) | 100% |
| R-Bendamustine | 8 | 75% | 2 (25%) | 2 (25%) | 13% |
| R monotherapy | 3 | 50% | 0 (0%) | 0 (0%) | 0% |
| Other | 7 | 57% | 0 (0%) | 0 (0%) | 14% |
| CNS directed therapy | 33 | 42% | 9 (27%) | 14 (42%) | 22% |
| RT only | 13 | 38% | 0 (0%) | 1 (8%) | 38% |
autoHCT, autologous haematopoietic cell transplant; CNS, central nervous system; CR, complete remission; EFS, event-free survival; PR, partial remission; R-DHAP, rituximab, dexamethasone, high-dose Ara-C, cisplatin; R-ESHAP, rituximab, etoposide, solumedrol, high-dose Ara-C, cisplatin; R-GemOx, rituximab, gemcitabine, oxaliplatin; R-ICE, rituximab, ifosfamide, cyclophosphamide, etoposide; R, rituximab; ROAD, rituximab, oxaliplatin, Ara-C, dexamethasone ; RT, radiotherapy.
Table III.
Outcomes to therapy for DLBCL after first relapse
| Survival from relapse in all patients | Survival from autoHCT | Survival from relapse if not able undergo autoHCT |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | CR/PR to first salvage therapy |
Median OS from relapse (months) |
4-year OS from relapse |
N | Median OS from autoHCT (months) |
4-year OS from autoHCT |
2-year EFS from autoHCT |
N | Median OS from first relapse (months) |
2-year OS from first relapse |
|
| All patients | 244 | 54% | 11.0 | 28% | 103 (42%) | 48.5 | 51% | 49% | 141 (58%) | 6.8 | 19% |
| Therapy at first relapse | |||||||||||
| Aggressive therapy | 175 | 57% | 11.6 | 29% | 84 (48%) | 42.7 | 49% | 45% | 91 (52%) | 6.9 | 19% |
| Non-aggressive therapy | 23 | 52% | 10.4 | 22% | 4 (17%) | NA | 75% | 75% | 19 (83%) | 9.4 | 19% |
| CNS directed therapy | 33 | 44% | 9.1 | 29% | 14 (42%) | Not reached | 61% | 71% | 19 (58%) | 4.3 | 5% |
| RT only | 13 | 38% | 21.4 | 31% | 1 (8%) | NA | NA | NA | 12 (92%) | 23.8 | 46% |
| Response to initial IC | |||||||||||
| CR at end of IC | 94 | 75% | 25.6 | 37% | 54 (57%) | 35.3 | 48% | 48% | 40 (43%) | 12.3 | 38% |
| PR at end of IC | 57 | 53% | 15.4 | 39% | 30 (53%) | 112.1 | 62% | 53% | 27 (47%) | 7.3 | 12% |
| PR or CR at end of IC | 151 | 67% | 20.3 | 38% | 84 (56%) | 50.8 | 53% | 50% | 67 (44%) | 7.7 | 27% |
| Transient response | 55 | 35% | 7.0 | 13% | 14 (25%) | 35.2 | 42% | 50% | 41 (75%) | 5 | 5% |
| No Response to IC | 30 | 23% | 6.7 | 12% | 4 (13%) | 13.1 | NA | NA | 26 (87%) | 5.8 | 19% |
| SD/PD to IC | 85 | 31% | 6.9 | 13% | 18 (21%) | 25.3 | 37% | 44% | 67 (79%) | 5.6 | 10% |
| Response not assessed/unknown | 8 | 50% | 11.6 | NA | 1 (13%) | NA | NA | NA | 7 (88%) | 7.6 | 29% |
| Timing of Relapse | |||||||||||
| Refractory or progression at end of IC | 93 | 32% | 7.0 | 14% | 19 (20%) | 27.6 | 39% | 47% | 74 (80%) | 5.8 | 12% |
| Post IC relapse within 12 months of diagnosis | 86 | 56% | 12.5 | 30% | 46 (53%) | 22.9 | 45% | 39% | 40 (47%) | 6.5 | 15% |
| Relapse beyond 12 months from diagnosis | 65 | 80% | 37.8 | 47% | 38 (58%) | Not reached | 64% | 63% | 27 (42%) | 21 | 45% |
autoHCT, autologous haematopoietic cell transplant; CNS, central nervous system; CR, complete remission; DLBCL, diffuse large B cell lymphoma; EFS, event-free survival; IC, immunochemotherapy; OS, overall survival; PD, progressive disease; PR, partial remission; RT, radiotherapy;SD, stable disease.
Non-aggressive chemotherapy at first relapse
Twenty-three patients were initially treated with a non-aggressive salvage regimen after failure of front-line therapy, of whom 6 had primary refractory disease and 16 had relapsed after CR or were treated subsequently after PR, with 1 patient having unknown response to front-line therapy. Patients in this group were older [median age 72 years (range 41–92) vs. 61 years (range 20–87) p value < 0.0001] and they were less likely to proceed with autoHCT (17% vs. 48%, p value =0.0040). Median survival from relapse in the 23 patients was 10.4 months (95% CI: 4.5–21.3) with a 2-year EFS rate of 13%, and an OS4 rate of 22%. The most common regimens used in this group included bendamustine-rituximab (n=8), R-GemOx (n=4) and single-agent R (n=3), (Table II). The overall response rate to non-aggressive regimens was 52% but only 4 patients (17%) subsequently underwent autoHCT. This is compared to 48% patients receiving aggressive salvage chemotherapy that proceeded to autoHCT (p value =0.004).
Other therapies at first relapse
Thirty-three patients with CNS relapse were treated with CNS-directed therapy and detailed outcomes will be reported in a separate series. Thirteen of the 244 relapsed/refractory patients were treated with radiotherapy only, with one patient proceeding to autoHCT; EFS of the 13 radiotherapy patients was 38% at 2 years (Table II).
Survival outcomes of specific clinical subsets of relapsed or refractory DLBCL
Outcome based on clinical prognostic factors at diagnosis
Clinical factors at diagnosis that were associated with poor survival at first relapse were IPI at diagnosis (per unit hazard ratio [HR]=1.15, 95% CI: 1.01 – 1.32, p=0.035), age >60 years (HR=1.41, 95% CI: 1.04 – 1.91, p=0.026), and a non-significant trend for sex, with men having improved survival compared to women (HR=0.77, 95% CI: 0.57–1.04, p=0.092). COO was assessed at diagnosis via Hans algorithm and at relapse where available. Concordance between COO at diagnosis and relapse was excellent (88%) and thus COO at diagnosis was used to assess prognosis at relapse.(Qu, et al 2016) There was no difference in survival of patients with non-germinal centre B cell-like (non-GCB) tumours per Hans algorithm (N=51, median survival 11.9 months, 95% CI: 6.7–31.2; HR = 0.79, 95% CI: 0.52–1.22) compared to patients with germinal centre B cell-like (GCB) tumours (N=74, median survival 8.0 months, 95% CI: 6.8–15.1; p=0.29). No other clinical factors at diagnosis were significantly associated with survival post-relapse (Table IV). Finally, in a sensitivity analysis, outcomes after relapse were similar when patients with PMBCL were excluded (results not shown).
Table IV.
Association of patient characteristics at diagnosis with post-relapse survival
| Patient characteristics at diagnosis | HR (95% CI) | p-value |
|---|---|---|
| Age >60 years vs age ≤ 60 years (ref) | 1.41 (1.04–1.91) | 0.026 |
| Sex, male vs. female (ref) | 0.77 (0.57–1.04) | 0.092 |
| PMBL vs DLBCL (ref) | 0.58 (0.24–1.40) | 0.22 |
| IPI at diagnosis (per unit increase) | 1.15 (1.01–1.32) | 0.035 |
| Stage III/IV vs. Stage I/II (ref) | 0.97 (0.67–1.40) | 0.87 |
| Elevated LDH vs. normal range LDH (ref) | 1.13 (0.79–1.63) | 0.50 |
| 2 or more Extranodal sites vs. 0–1 (ref) | 1.24 (0.89–1.74) | 0.22 |
| ECOG PS 2–4 vs. PS 0–1 (ref) | 1.14 (0.81–1.61) | 0.44 |
| B symptoms at diagnosis vs. none (ref) | 0.93 (0.68–1.27) | 0.72 |
| Bulky disease > 10 cm at diagnosis vs. non-bulky disease (ref) | 1.17 (0.81–1.71) | 0.41 |
| Bone marrow involvement with DLBCL at diagnosis vs. no DLBCL bone marrow involvement (ref) | 0.90 (0.57–1.40) | 0.63 |
| Cell of origin (Hans) Non GCB vs GCB (ref) | 0.79 (0.52–1.22) | 0.29 |
CI, confidence interval; DLBCL, diffuse large B cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; GCB, germinal centre B cell type; HR, hazard ratio; IPI, International Prognostic Index; LDH, lactate dehydrogenase; PMBL, primary mediastinal B cell lymphoma; ref, reference.
Outcome based on response to front-line chemotherapy
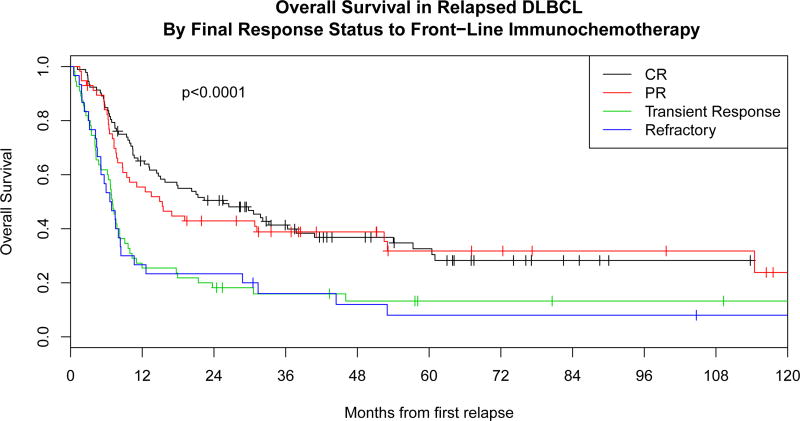
Response to frontline IC was predictive of post-relapse outcome. OS from date of first relapse was superior in the 151 patients with responsive disease (CR or PR) at completion of frontline IC [median OS 20.3 months (95% CI: 13.5–31.8) and 2 year EFS of 31%] compared to the 85 patients who had stable or progressive disease [median OS 6.9 months, 95% CI: 5.1–7.8, p<0.0001; and 2 year EFS of 11%, p=0.0005]. Survival was similar in those progressing after either PR or CR (Figure 3A). Patients achieving a CR or PR to frontline IC who subsequently relapsed were more likely to proceed to autoHCT compared to patients with either stable disease or progressive disease at the end of frontline IC (56% vs. 22% respectively, p<0.0001).
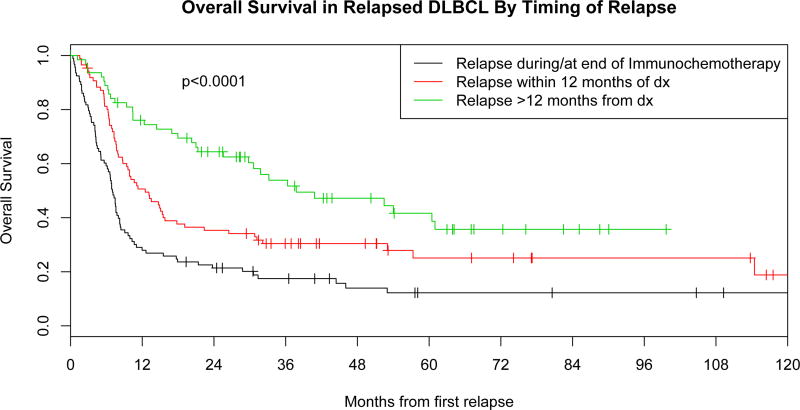
Figure 3. Overall survival.
A: Overall survival in relapsed diffuse large B cell lymphoma (DLBCL) by response to front-line immunochemotherapy.
B: Overall survival in relapsed DLBCL by timing of relapsed after initial diagnosis CR, complete remission; dx, diagnosis; PR, partial remission;
Outcomes in patients with transient response (interim response but progression at or prior to completion of therapy) to front-line IC (n=55) were poor, with a median OS after first relapse of 7.0 months, which was similar to those patients meeting strict definition of refractory with no response to front-line IC (6.7 months, p=0.84) (Figure 3A). Most patients with transient response to front-line IC (67%) received aggressive salvage therapy, which had a response rate of 41%. Ultimately, only 14 of the 55 patients (25%) proceeded to autoHCT, of which only 6 (11%) achieved long-term remission (Table III).
Outcome based on the timing of relapse after achieving complete remission
The timing of relapse from diagnosis was a predictor of outcome. Out of 244 patients with relapse, 93 (38%) patients were primary refractory, 86 (35%) patients achieved remission after completion of IC but relapsed within 12 months of diagnosis, and 65 (27%) patients relapsed beyond 12 months from diagnosis (Table III). Aggressive salvage chemotherapy was administered to 69% of patients with progressive disease at the completion of IC vs. 76% of patients who relapsed between completion of IC and 12 months from diagnosis, and 71% of patients who relapsed > 12 months from diagnosis. Post-relapse outcomes were better in patients who relapsed beyond 12 months from diagnosis (median OS=37.8 months, 80% response rate to first salvage therapy) compared to patients with refractory disease (median OS=7.0 months, 32% response rate) or patients who relapsed within 12 months of diagnosis (median OS=12.5 months, 56% response rate; p<0.0001), (Figure 3B). Patients who relapsed after 12 months from diagnosis were also more likely to proceed to autoHCT (58%) compared to patients with refractory disease to IC (20%, p<0.0001).
DISCUSSION
We report a comprehensive analysis of prospectively collected patterns of care and outcomes of relapsed or refractory DLBCL after receiving front-line anthracycline-based IC. We excluded 48 patients from the analysis who were not candidates for second-line therapy with a goal of making the results relevant to patients, clinicians and clinical research teams contemplating second line therapy. The findings highlight the heterogeneous outcomes of identifiable subgroups of relapsed/refractory DLBCL that will aid in the stratification of patients in phase II or III clinical trials or serve as historical controls for uncontrolled studies. Development of new therapeutic agents targeting relapsed/refractory DLBCL following IC requires an understanding of the patterns of failure and current management strategies with their consequent outcomes.
Most patients with relapsed/refractory DLBCL were treated with aggressive salvage chemotherapy. The response rates to initial salvage therapy reported in this study are somewhat similar for aggressive and non-aggressive regimens, yet the patient cohorts are probably substantially different in regards to age and comorbidities. Similarly, OS is substantially better for the subjects selected for autoHCT. Accordingly, response rates may be an inadequate measure of clinical benefit and clinical trials exploring novel drugs or combinations in this setting might choose endpoints other than simply response rates in trial design (Christian, et al 2015, Coiffier, et al 2016, Gerecitano, et al 2015, Jacobsen, et al 2015, Kochenderfer, et al 2015, Locke, et al 2015, Maddocks, et al 2015, Morschhauser, et al 2014, Moskowitz, et al 2015, Ribrag, et al 2015, Salles, et al 2013, Viardot, et al 2016, Walter, et al 2016, Wang, et al 2015, Witzig, et al 2011a, Witzig, et al 2011b). For patients not deemed appropriate for aggressive therapy, median PFS or even median OS would be a meaningful endpoint without requiring a significant duration of follow up for analysable events. In contrast, clinical trials assessing aggressive combinations that include autoHCT should include 2-year EFS or PFS endpoints, especially if including many non-refractory patients.
In this study, 38% of relapsed/refractory DLBCL patients had primary refractory disease and had particularly poor outcomes. These patients were less likely to respond to salvage chemotherapy and only 20% of these patients underwent autoHCT (Hitz, et al 2015, Rovira, et al 2015, Sarkozy and Coiffier 2015). These poor outcomes in chemotherapy refractory DLBCL are similar to the results reported by the SCHOLAR-1 study (median overall survival of ~6 months and overall response rate of 20–25% to any subsequent treatment) (Crump, et al 2016). Patients with transient response to frontline IC had similarly poor outcomes comparable to those with primary refractory DLBCL suggesting these patients should be included in trials focused on “refractory” patients.
Patients with relapsed DLBCL but chemosensitive disease at the end of front-line treatment (CR or PR) had better survival as compared to patients who were refractory to chemotherapy, even if they had a transient response during the treatment. Similar outcome after relapse for those with CR/PR at end of front-line treatment is an interesting observation that emphasizes the need for more sensitive testing to identify the complete remission as these patients probably had residual disease below the level of detection leading to subsequent relapse. Additionally, patients receiving aggressive salvage chemotherapy were more likely to proceed to autoHCT. This is more likely based upon patient selection factors than effectiveness of salvage therapy, as patients undergoing aggressive salvage chemotherapy were younger (median age 61) than patients receiving non-aggressive treatment (median age 72). IPI at diagnosis is still prognostic of outcome post-relapse, with age as the only individual measure with prognostic power (p = 0.035).
Timing of relapse after front-line therapy has substantial prognostic importance and clinical trials need to stratify for this variable in randomization designs or account for it in interpretation of non-randomized results (Gisselbrecht, et al 2010, Hamadani, et al 2014). Relapse after 12 months from diagnosis was less common (27% of relapsed/refractory DLBCL) and 80% of these patients responded to salvage chemotherapy. Patients who relapsed after 12 months and underwent autoHCT had OS4 from autoHCT of 64%, approaching a similar outcome to de novo DLBCL.
We did not identify an association between COO and post-relapse outcomes, but our finding is limited by the small number of patients for which COO information was available - none of which was ascertained by gene expression. While COO at time of diagnosis has established prognostic value, there is little data in rituximab era on the prognostic value of COO at the time of relapse. The Bio-CORAL study indicated that COO retains its prognostic value at relapse but this is not supported by other studies (Gu, et al 2012, Moskowitz, et al 2005, Nyman, et al 2008, Thieblemont, et al 2011). Impact of auto transplant, several different types of salvage chemotherapy, targeted treatment, such as lenalidomide and ibrutinib, with preferential activity in non-GCB DLBCL could be potential mechanisms for these findings (Hernandez-Ilizaliturri, et al 2011, Thieblemont, et al 2017, Wilson, et al 2015). Newer technologies resulting in more precise identification of COO will help clarify this in future studies. (Vose 2011)
Strengths of this study include a cohort that was prospectively enrolled at diagnosis as opposed to cases ascertained at time of relapse that included a methodology for collecting post-diagnosis outcomes. Patients were followed throughout multiple therapeutic interventions that were treated across a wide spectrum of practice settings without the structure or selection biases of a therapeutic clinical trial in most cases. The size of the study allows for reasonable confidence in the outcomes of several larger subsets. Nonetheless, this study lacks regional and ethnic diversity, and the absence of a validation population dictates that published outcomes of similar subsets from other series would be helpful in further defining this problem.
In conclusion, this analysis of patterns of failure and treatment with subsequent outcomes in DLBCL patients confirm this as an unmet clinical need or, more precisely, as a collection of needs largely unmet. This analysis provides historical benchmarks for future clinical trials targeting these populations of patients. For the purpose of grouping relapsed/refractory DLBCL patients into distinct subsets for future study or analysis we recommend that categories include refractory (including transient responders) to any line therapy, those of age/criteria precluding aggressive salvage therapy, those with CNS relapse, and those with first relapse more than 12 months from diagnosis.
Acknowledgments
This study was supported in part by National Cancer Institute, USA (grant P50CA97274). This study was supported by a grant from Kite Pharma Inc.
Footnotes
This study was presented in part at the Annual Meeting of the American Society of Hematology in December 2015 in Orlando, FL.
UF designed the study, collected data, analysed data and wrote the manuscript.
MM, BL, CT designed the study, collected data, analysed data, contributed to manuscript writing and reviewed and approved the manuscript.
GT, DI, IM, WM, SS, TL, YL, SA, GN, TH, JC collected data and reviewed and approved the manuscript.
References
- Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790–795. doi: 10.1002/ajh.24086. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. For the International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Christian BA, Kuruvilla JG, Smith SM, Porcu P, Maddocks KJ, Ruppert AS, Byrd JC, Grever MR, Blum KA. Updated Results of a Phase I Study of Ibrutinib and Lenalidomide in Patients with Relapsed and Refractory B-Cell Non-Hodgkin’s Lymphoma. Blood. 2015;126:3983–3983. [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Thieblemont C, de Guibert S, Dupuis J, Ribrag V, Bouabdallah R, Morschhauser F, Navarro R, Le Gouill S, Haioun C, Houot R, Casasnovas O, Holte H, Lamy T, Broussais F, Payrard S, Hatteville L, Tilly H. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol. 2016;173:722–730. doi: 10.1111/bjh.13992. [DOI] [PubMed] [Google Scholar]
- Costa LJ, Maddocks K, Epperla N, Reddy NM, Karmali R, Umyarova E, Bachanova V, Costa C, Glenn M, Chavez J, Calzada O, Lansigan F, Nasheed H, Barta S, Zhou Z, Jaglal M, Chhabra S, Hernandez-Ilizaliturri F, Xavier AC, Mehta A, Peker D, Forero-Torres A, Al-Mansour Z, Evens AM, Cohen JB, Flowers CR, Fenske TS, Hamadani M. Diffuse Large B-Cell Lymphoma with Primary Treatment Failure: Ultra-high Risk Features and Benchmarking for Experimental Therapies. American Journal of Hematology. 2016;92:161–170. doi: 10.1002/ajh.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, Rubinger M, Buckstein R, Imrie KR, Federico M, Di Renzo N, Howson-Jan K, Baetz T, Kaizer L, Voralia M, Olney HJ, Turner AR, Sussman J, Hay AE, Djurfeldt MS, Meyer RM, Chen BE, Shepherd LE. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32:3490–3496. doi: 10.1200/JCO.2013.53.9593. [DOI] [PubMed] [Google Scholar]
- Crump M, Neelapu S, Farooq U, Van Den Neste E, Kuruvilla J, Ahmed MA, Link BK, Hay AE, Cerhan JR, Zhu L, Boussetta S, Feng L, Maurer MJ, Navale L, Wiezorek JS, Go WY, Gisselbrecht C. Outcomes in refractory aggressive diffuse large b-cell lymphoma (DLBCL): Results from the international SCHOLAR-1 study. J Clin Oncol. 2016;34(suppl) doi: 10.1182/blood-2017-03-769620. abstr 7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese MD, Griffiths RI, Gleeson ML, Dalvi T, Li J, Mikhael JR, Deeter R, Dreyling M. Second-line therapy in diffuse large B-cell lymphoma (DLBCL): treatment patterns and outcomes in older patients receiving outpatient chemotherapy. Leuk Lymphoma. 2016;58:1094–1104. doi: 10.1080/10428194.2016.1228924. [DOI] [PubMed] [Google Scholar]
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- Gerecitano JF, Roberts AW, Seymour JF, Wierda WG, Kahl BS, Pagel JM, Puvvada S, Kipps TJ, Anderson MA, Dunbar M, Zhu M, Gressick L, Wagner L, Kim SY, Heitner Enschende S, Humerickhouse RA, Davids MS. A Phase 1 Study of Venetoclax (ABT-199 / GDC-0199) Monotherapy in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Blood. 2015;126:254–254. [Google Scholar]
- Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Briere J, Moskowitz CH, Schmitz N. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- Gu K, Weisenburger DD, Fu K, Chan WC, Greiner TC, Aoun P, Smith LM, Bast M, Liu Z, Bociek RG, Bierman PJ, Armitage JO, Vose JM. Cell of origin fails to predict survival in patients with diffuse large B-cell lymphoma treated with autologous hematopoietic stem cell transplantation. Hematol Oncol. 2012;30:143–149. doi: 10.1002/hon.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- Hamadani M, Hari PN, Zhang Y, Carreras J, Akpek G, Aljurf MD, Ayala E, Bachanova V, Chen AI, Chen YB, Costa LJ, Fenske TS, Freytes CO, Ganguly S, Hertzberg MS, Holmberg LA, Inwards DJ, Kamble RT, Kanfer EJ, Lazarus HM, Marks DI, Nishihori T, Olsson R, Reddy NM, Rizzieri DA, Savani BN, Solh M, Vose JM, Wirk B, Maloney DG, Smith SM, Montoto S, Saber W, Alpdogan O, Cashen A, Dandoy C, Finke R, Gale R, Gibson J, Hsu JW, Janakiraman N, Laughlin MJ, Lill M, Cairo MS, Munker R, Rowlings PA, Schouten HC, Shea TC, Stiff PJ, Waller EK. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1729–1736. doi: 10.1016/j.bbmt.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, Pileri SA, Malik F, Macon WR, Goy A, Witzig TE, Czuczman MS. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011;117:5058–5066. doi: 10.1002/cncr.26135. [DOI] [PubMed] [Google Scholar]
- Hitz F, Connors JM, Gascoyne RD, Hoskins P, Moccia A, Savage KJ, Sehn LH, Shenkier T, Villa D, Klasa R. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Ann Hematol. 2015;94:1839–1843. doi: 10.1007/s00277-015-2467-z. [DOI] [PubMed] [Google Scholar]
- Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, Spitzer G, Palanca-Wessels MC, Kennedy DA, Levine P, Yang J, Bartlett NL. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125:1394–1402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- Kansara RR, Savage KJ, Villa D, Shenkier T, Gerrie AS, Klasa R, Hitz F, Scott DW, Slack GW, Gascoyne RD, Connors JM, Sehn LH. Outcome in Unselected Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL) Following R-CHOP When Stem Cell Transplantation Is Not Feasible. Blood. 2014;124:3069–3069. [Google Scholar]
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DA, Morton KE, Toomey MA, Rosenberg SA. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Navale L, Aycock JS, Wiezorek J, Go WY. Phase 1 Clinical Results of the ZUMA-1 (KTE-C19-101) Study: A Phase 1–2 Multi-Center Study Evaluating the Safety and Efficacy of Anti-CD19 CAR T Cells (KTE-C19) in Subjects with Refractory Aggressive Non-Hodgkin Lymphoma (NHL) Blood. 2015;126:3991–3991. [Google Scholar]
- Maddocks K, Christian B, Jaglowski S, Flynn J, Jones JA, Porcu P, Wei L, Jenkins C, Lozanski G, Byrd JC, Blum KA. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125:242–248. doi: 10.1182/blood-2014-08-597914. [DOI] [PubMed] [Google Scholar]
- Morschhauser F, Flinn I, Advani RH, Diefenbach CS, Kolibaba K, Press OW, Sehn LH, Chen AI, Salles G, Tilly H, Cheson BD, Assouline S, Dreyling M, Hagenbeek A, Zinzani PL, Yalamanchili S, Lu D, Jones C, Jones S, Chu Y-W, Sharman JP. Updated Results of a Phase II Randomized Study (ROMULUS) of Polatuzumab Vedotin or Pinatuzumab Vedotin Plus Rituximab in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Blood. 2014;124:4457–4457. doi: 10.1016/S2352-3026(19)30026-2. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Zelenetz AD, Kewalramani T, Hamlin P, Lessac-Chenen S, Houldsworth J, Olshen A, Chaganti R, Nimer S, Teruya-Feldstein J. Cell of origin, germinal center versus nongerminal center, determined by immunohistochemistry on tissue microarray, does not correlate with outcome in patients with relapsed and refractory DLBCL. Blood. 2005;106:3383–3385. doi: 10.1182/blood-2005-04-1603. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Fanale MA, Shah BD, Advani RH, Chen R, Kim S, Kostic A, Liu T, Peng J, Forero-Torres A. A Phase 1 Study of Denintuzumab Mafodotin (SGN-CD19A) in Relapsed/Refactory B-Lineage Non-Hodgkin Lymphoma. Blood. 2015;126:182–182. [Google Scholar]
- Nagle SJ, Woo K, Schuster SJ, Nasta SD, Stadtmauer E, Mick R, Svoboda J. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol. 2013;88:890–894. doi: 10.1002/ajh.23524. [DOI] [PubMed] [Google Scholar]
- Nyman H, Jantunen E, Juvonen E, Elonen E, Bohm J, Kosma VM, Enblad G, Karjalainen-Lindsberg ML, Leppa S. Impact of germinal center and non-germinal center phenotypes on overall and failure-free survival after high-dose chemotherapy and auto-SCT in primary diffuse large B-cell lymphoma. Bone Marrow Transplant. 2008;42:93–98. doi: 10.1038/bmt.2008.92. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, Lopez-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M MabThera International Trial G. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- Qu J, Maurer MJ, Cerhan JR, Novak AJ, Habermann TM, Macon WR, Feldman AL, Witzig TE, Thompson CA, Syrbu S, Link BK, Farooq U, Ansell S. Similar Phenotypes Demonstrated upon Initial Diagnosis and at Time of Recurrence in Relapsed DLBCL. Blood. 2016;128:5299–5299. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. https://www.R-project.org. [Google Scholar]
- Ribrag V, Armand P, Kuruvilla J, Michot J-M, Moskowitz CH, Marinello P, Snyder E, Balakumaran A, Shipp MA, Zinzani PL. An open-label, multicohort Phase Ib trial of pembrolizumab (MK-3475) for advanced hematologic malignancies: KEYNOTE-013. Journal for Immunotherapy of Cancer. 2015;3:P169–P169. [Google Scholar]
- Rovira J, Valera A, Colomo L, Setoain X, Rodriguez S, Martinez-Trillos A, Gine E, Dlouhy I, Magnano L, Gaya A, Martinez D, Martinez A, Campo E, Lopez-Guillermo A. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann Hematol. 2015;94:803–812. doi: 10.1007/s00277-014-2271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles GA, Czuczman MS, Assouline SE, Flinn IW, Sehn LH, Patel M, Sangha R, Tilly H, Advani R, Casasnovas O, Press OW, Yalamanchili S, Kahn R, Lu D, Chai A, Chu Y-W, Morschhauser F. Final Results Of a Phase I Study Of The Anti-CD79b Antibody-Drug Conjugate DCDS4501A In Relapsed Or Refractory (R/R) B-Cell Non-Hodgkin Lymphoma (NHL) Blood. 2013;122:4400–4400. [Google Scholar]
- Sarkozy C, Coiffier B. Primary refractory diffuse large B cell lymphoma in the rituximab era. Curr Opin Oncol. 2015;27:377–383. doi: 10.1097/CCO.0000000000000209. [DOI] [PubMed] [Google Scholar]
- Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. doi: 10.1182/blood-2014-05-577189. [DOI] [PubMed] [Google Scholar]
- Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, MacPherson N, O’Reilly S, Spinelli JJ, Sutherland J, Wilson KS, Gascoyne RD, Connors JM. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- Sud R, Friedberg JW. Salvage therapy for relapsed or refractory diffuse large B-cell lymphoma: impact of prior rituximab. Haematologica. 2008;93:1776–1780. doi: 10.3324/haematol.2008.000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer, New York: 2000. [Google Scholar]
- Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, Rosenwald A, Jack A, Sundstrom C, Cogliatti S, Trougouboff P, Boudova L, Ysebaert L, Soulier J, Chevalier C, Bron D, Schmitz N, Gaulard P, Houlgatte R, Gisselbrecht C. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol. 2011;29:4079–4087. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- Thieblemont C, Tilly H, Gomes da Silva M, Casasnovas RO, Fruchart C, Morschhauser F, Haioun C, Lazarovici J, Grosicka A, Perrot A, Trotman J, Sebban C, Caballero D, Greil R, van Eygen K, Cohen AM, Gonzalez H, Bouabdallah R, Oberic L, Corront B, Choufi B, Lopez-Guillermo A, Catalano J, Van Hoof A, Briere J, Cabecadas J, Salles G, Gaulard P, Bosly A, Coiffier B. Lenalidomide Maintenance Compared With Placebo in Responding Elderly Patients With Diffuse Large B-Cell Lymphoma Treated With First-Line Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J Clin Oncol. 2017 doi: 10.1200/JCO.2017.72.6984. JCO2017726984. [DOI] [PubMed] [Google Scholar]
- Van Den Neste E, Schmitz N, Mounier N, Gill D, Trneny M, Milpied N, Radford JA, Ketterer N, Shpilberg O, Dührsen U, Ma D, Briere J, Thieblemont C, Salles GA, Moskowitz C, Glass B, Gisselbrecht C. Outcomes in Diffuse Large B-Cell Lymphoma (DLBCL) Patients Relapsing after Autologous Stem Cell Transplantation (ASCT): An Analysis of Patients Included in the Coral Study. Blood. 2015;126:517–517. [Google Scholar]
- Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, Milpied N, Radford J, Ketterer N, Shpilberg O, Duhrsen U, Ma D, Briere J, Thieblemont C, Salles G, Moskowitz CH, Glass B, Gisselbrecht C. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51:51–57. doi: 10.1038/bmt.2015.213. [DOI] [PubMed] [Google Scholar]
- Viardot A, Goebeler M-E, Hess G, Neumann S, Pfreundschuh M, Adrian N, Zettl F, Libicher M, Sayehli C, Stieglmaier J, Zhang A, Nagorsen D, Bargou RC. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–1416. doi: 10.1182/blood-2015-06-651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose JM. Relapsed diffuse large B-cell lymphoma: clinical utility of cell of origin. J Clin Oncol. 2011;29:4065–4066. doi: 10.1200/JCO.2011.37.5733. [DOI] [PubMed] [Google Scholar]
- Walter HS, Rule SA, Dyer MJS, Karlin L, Jones C, Cazin B, Quittet P, Shah N, Hutchinson CV, Honda H, Duffy K, Birkett J, Jamieson V, Courtenay-Luck N, Yoshizawa T, Sharpe J, Ohno T, Abe S, Nishimura A, Cartron G, Morschhauser F, Fegan C, Salles G. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–419. doi: 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wagner-Bartak N, Zhou S, Fowler N, Dela Rosa ML, Zhao D, Romaguera JE, Neelapu SS, Hagemeister FB, Fanale MA, Oki Y, Shah JJ, Thomoas SK, Hosing CM, Zhang L, Badillo M, Chen W, Cai Q, Zou D, Champlin RE, Fayad LE, Lee HJ, Wang M. Lenalidomide Plus Rituximab for Relapsed or Refractory Diffuse Large B-Cell, Follicular and Transformed Lymphoma: Final Data Analysis of a Phase 2 Trial. Blood. 2015;126:3969–3969. [Google Scholar]
- Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, Lih CJ, Williams PM, Shaffer AL, Gerecitano J, de Vos S, Goy A, Kenkre VP, Barr PM, Blum KA, Shustov A, Advani R, Fowler NH, Vose JM, Elstrom RL, Habermann TM, Barrientos JC, McGreivy J, Fardis M, Chang BY, Clow F, Munneke B, Moussa D, Beaupre DM, Staudt LM. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig TE, Reeder CB, LaPlant BR, Gupta M, Johnston PB, Micallef IN, Porrata LF, Ansell SM, Colgan JP, Jacobsen ED, Ghobrial IM, Habermann TM. A Phase II Trial of the Oral mTOR Inhibitor Everolimus in Relapsed Aggressive Lymphoma. Leukemia. 2011a;25:341–347. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, Bouabdallah R, Haioun C, Tilly H, Guo P, Pietronigro D, Ervin-Haynes AL, Czuczman MS. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011b;22:1622–1627. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]