Abstract
Introduction
Cobalamin C disease is a multisystemic disease with variable manifestations and age of onset. Genotype-phenotype correlations are well-recognized in this disorder. Here, we present a large cohort of individuals with cobalamin C disease, several of whom are heterozygous for the c.482G>A pathogenic variant (p.Arg161Gln). We compared clinical characteristics of individuals with this pathogenic variant to those who do not have this variant. To our knowledge, this study represents the largest single cohort of individuals with the c.482G>A (p.Arg161Gln) pathogenic variant.
Methods
A retrospective chart review of 27 individuals from 21 families with cobalamin C disease who are followed at our facility was conducted.
Results
13 individuals (48%) are compound heterozygous with the c.482G>A (p.Arg161Gln) on one allele and a second pathogenic variant on the other allele. Individuals with the c.482G>A (p.Arg161Gln) pathogenic variant had later onset of symptoms and easier metabolic control. Moreover, they had milder biochemical abnormalities at presentation which likely contributed to the observation that 4 individuals (31%) in this group were missed by newborn screening.
Conclusion
The c.482G>A (p.Arg161Gln) pathogenic variant is associated with milder disease. These individuals may not receive a timely diagnosis as they may not be identified on newborn screening or because of unrecognized, late onset symptoms. Despite the milder presentation, significant complications can occur, especially if treatment is delayed.
Keywords: Cobalamin C, Genotype-phenotype, Hydroxocobalamin, R161Q, Late-onset
1. INTRODUCTION
Cobalamin C disease (methylmalonic aciduria (MMA) and homocystinuria, cblC type; OMIM # 277400) is the most common disorder of intracellular cobalamin metabolism. From newborn screening (NBS) data, the incidence has been estimated to be 1:100,000 in the state of New York 1. In 2006, Lerner-Ellis et al. identified the gene responsible for cobalamin C disease, MMACHC (OMIM # 609831)2. As a result of pathogenic variants in this gene, there is impairment of the synthesis of adenosylcobalamin and methylcobalamin, which are cofactors for the methylmalonyl-CoA mutase and methionine synthase enzymes respectively. As a consequence, elevated total plasma homocysteine and methylmalonic acid with low plasma methionine is observed in this disorder.
The presentation in cobalamin C disease is variable and can involve different organ systems3,4. In 1997, Rosenblatt described two distinct phenotypes with differing severity and age of onset: early-onset and late-onset disease5. The early-onset form of the disorder presents in the first year of life with feeding difficulties, hypotonia, ophthalmological (e.g. maculopathy and retinopathy), neurological, and hematological complications. The late-onset disease presents after four years of age, mainly with neuropsychiatric symptoms, including cognitive decline, myelopathy, gait disturbances and psychiatric symptoms. Renal (e.g. hemolytic uremic syndrome) and pulmonary complications have also been reported6.
Cobalamin C disease is one of the secondary targets in newborn screening7 and is typically detected through elevated levels of propionylcarnitine (C3). Newborn screening facilitates early diagnosis and initiation of treatment with parenteral hydroxocobalamin. Other agents like betaine, folic acid, and carnitine may also be prescribed. A special diet, including protein restriction, is not necessary and not recommended8,9. The long term outlook of cobalamin C disease in the era of newborn screening is yet to be comprehensively explored. Despite early treatment, some complications, such as ophthalmological abnormalities and neurodevelopmental delay have been reported, especially in early onset disease 10,11.
Since the MMACHC gene was identified, several pathogenic variants have been reported. The most common pathogenic variant reported, c.271dupA (p.Arg91LysfsX14), results in a frameshift and premature protein termination and accounts for ~40% of alleles in individuals of European ancestry12–14. Similarly, there are other pathogenic variants which are more common in certain ethnic groups like c.331C>T (p.Arg111Ter) in Louisiana Cajuns and French Canadians14 and the c.609G>A (p.Trp203Ter) in Chinese individuals15. Studies have suggested a genotype-phenotype correlation in individuals with cobalamin C disease. For example, the c.271dupA (p.Arg91LysfsX14), and c.331C>T (p.Arg111Ter) pathogenic variants, either in a homozygous or compound heterozygous state, are associated with early onset disease, whereas the c.394 C>T (p.Arg132Ter), which is associated with higher levels of MMACHC mRNA transcript, has been associated with late onset disease12–14. Similarly, the c.482G>A(p.Arg161Gln) pathogenic variant has been associated with late onset disease, even if compound heterozygous with pathogenic variants predicting severe phenotype 2,6,13–15. Although several patients with the c.482G>A (p.Arg161Gln) pathogenic variant have been reported, this study represents the largest single cohort of individuals with this variant.
For this study, we performed a retrospective chart review of the large, local cohort of individuals with molecularly confirmed cobalamin C disease followed in the metabolic clinic at Texas Children’s Hospital. We reviewed molecular diagnosis, metabolites, treatment, and outcomes.
2. MATERIALS AND METHODS
We conducted a retrospective review of electronic and paper medical records for all individuals with cobalamin C disease who are followed in the metabolic clinic at Texas Children’s Hospital. Nearly half of our individuals are compound heterozygous for the c.482G>A (p.Arg161Gln) pathogenic variant, and therefore, those who are compound heterozygous for this variant (referred to in the text and table 2 as R161Q group; n=13, from 8 families) were analyzed separately from the other individuals (referred to in the text and table 2 as non-R161Q group; n=14, out of 13 families). Statistical analysis was performed with a two-tailed unpaired t-test for variables with normal distribution and Mann–Whitney U test for variables that were not normally distributed. Chi square test was used to compare proportions. P value ≤ 0.004 was used as the threshold for significance to account for multiple testing for variables presented in table 2. Variables in published studies were compared to this study with the Kruskal-Wallis test followed by Dunn’s post-test. This study was approved by the Baylor College of Medicine Institutional Review Board (IRB) (protocol H-33596).
Table 2.
R161Q vs. non-R161Q Groups
| Variable | R161Q (n=13) | Non-R161Q (n=14) | p value* |
|---|---|---|---|
| Age range at last follow up (years) | 0.92–35 (mean 7.8, median 5.2) | 1–26.6 (mean 7.6, median 4.3) | 0.48 |
| Total plasma homocysteine level on presentation a (micromol/L) | 32.2–76.3 (mean 59.1, median 60.3)(n=10) | 32.5–280 (mean 172, median 166) (n=10) | 0.0006 |
| Methionine level on presentation b (micromol/L) | 7–52 (mean 29.9, median 26.1) (n=8) | 2.8–31 (mean 14, median 11) (n=9) | 0.02 |
| Methylmalonic acid level on presentation c (micromol/L) | 8–37 (mean 20.6, median 21.2)(n=10) | 0.4–311 (mean 62.4, median 10.2)(n=8) | 0.2 |
| Most recent hydroxocobalamin dose (mg/kg/day) | 0.01–0.6 (mean=0.1, median=0.06) | 0.03–1.5 (mean 0.6, median 0.4) | 0.0002 |
| Number of subjects on betaine | 2/13 (15%) | 11/14 (79%) | 0.001 |
| Number of subjects on carnitine | 5/13(38%) | 11/14 (79%) | 0.03 |
| Most recent total plasma homocysteine level a (micromol/L) | 5.5–48.4 (mean 16.9, median 11.2) | 11.2–109 (mean 40.5, median 30.5) | 0.0013 |
| Most recent methionine level b (micromol/L) | 11–46.1 (mean 24, median 21.8) | 7.8–79.7 (mean 30.5, median 27.5) | 0.15 |
| Most recent methylmalonic acid level c (micromol/L) | 0.1–44.9 (mean 6.4, median 0.5) | 0.3–9.09 (mean 2.3, median 1.2) | 0.13 |
| Subjects with abnormal eye exam | 2/8 (25%) | 7/13 (54%) | 0.2 |
| Subjects with developmental delay, learning difficulties, or intellectual disability | 5/13(38%) | 11/14 (79%) | 0.03 |
p value for all variables in this table was set at 0.004 to correct for multiple testing.
typical reference range 4–14 micromoles/L with slight variations in some tests,
typical reference range 11–50 micromoles/L with slight variations in some tests,
typical reference range 0–0.5 micromoles/L with slight variations in some tests
3. RESULTS
Thirty-one individuals with cobalamin C disease are followed at our clinic, and this represents approximately 84% of all individuals with inherited cobalamin metabolism disorders in the clinic. Results of molecular testing were unavailable for four individuals, and thus they were excluded from the analysis. In the remaining 27 individuals who were included in our analysis, there was an equal distribution of males and females (52% and 48% respectively). 19 (70%) had newborn screening, but the screen was normal in 5 of them. These 5 individuals were diagnosed either because of a positive family history (subjects 7, 8, 9, 11) or symptomatic presentation (subject 18). Of the remaining 8 individuals who were born before expanded newborn screening was initiated, two were diagnosed because of family history, and the remaining individuals (n=6) were diagnosed symptomatically. The age range for individuals in the cohort at their last follow up visit was 11 months-35 years (median = 4.7 years, mean = 7.7 years). Additional characteristics are summarized in Table 1.
Table 1.
Patient Cohort characteristics
| Variable | Value |
|---|---|
| Number of subjects | 27 |
| Male: female ratio (%) | 52:48 |
| Age range at last follow up (years) | 0.92–35 (mean 7.7, median 4.7) |
| Ascertainment | Family history: 6 (22%), Positive newborn screening: 14(52%); Symptomatic; 7(26%) |
| Propionylcarnitine (C3) level on presentation a (micromol/L) (n=11) | 2.067–22.2 (mean 9.6, median 7.6) |
| Total plasma homocysteine level on presentation b (micromol/L) (n=20) | 32.2–280 (mean 115.4, median 75.6) |
| Methionine level on presentation c (micromol/L) (n=17) | 2.8–52 (mean 21.5, median 17.5) |
| Methylmalonic acid level on presentation d (micromol/L) (n=18) | 0.4–311 (mean 39.2, median 18.2) |
| Most recent hydroxocobalamin dose | 0.42–20 mg/day (mean 6.7, median 3.85) (0.01–1.5 mg/kg/day; mean 0.4, median 0.2) |
| Most recent betaine dose (mg/kg/day) (n=13) | 56–307 (mean 161.4, median 150) |
| Other medications | Carnitine (16/27 or 60%), Folic acid (5/27 or 18%) |
| Most recent total plasma homocysteine level b (micromol/L) | 5.5–109 (mean 29, median 17.5) |
| Most recent methionine level c (micromol/L) | 7.8–79.7 (mean 27.4, median 24) |
| Most recent methylmalonic acid level d (micromol/L) | 0.1–44.9 (mean 4.3, median 0.99) |
| Subjects with abnormal eye exam | 7/21 or 33% |
| Subjects with developmental delay, learning difficulties, or intellectual disability | 16/27 or 59% |
Typical reference range 0–0.87 micromole/L with slight variations in some tests,
typical reference range 4–14 micromoles/L with slight variations in some tests,
typical reference range 11–50 micromoles/L with slight variations in some tests,
typical reference range 0–0.5 micromoles/L with slight variations in some tests.
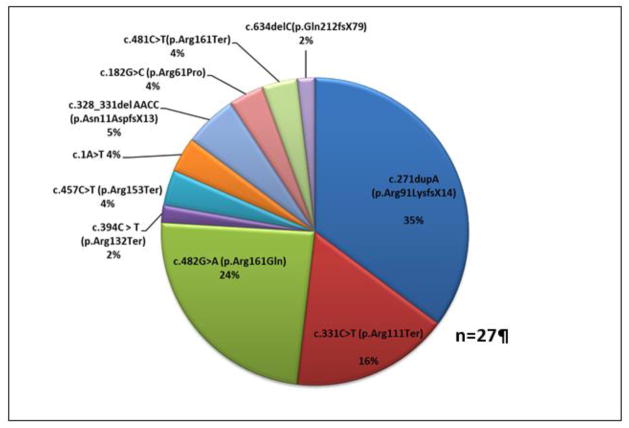
The allele frequency for the common pathogenic variant c.271dupA (p.Arg91LysfsX14) was 35% in our cohort (Figure 1). Interestingly, homozygosity for this variant was not detected in our cohort. The second most common pathogenic variant was c.482G>A (p.Arg161Gln), with an allele frequency of 24%. 10 out of 13 individuals in the R161Q group have the c.271dupA (p.Arg91LysfsX14)/c.482G>A (p.Arg161Gln), genotype. Most individuals in the R161Q group are Hispanic (7/13 or 54%), one reported Hispanic and Caucasian background, and the remaining 5 individuals (all siblings) are of mixed ethnicity (including Chinese, Caucasian, and Native American). Two individuals in our cohort (subjects 5 and 14) are heterozygous for a variant of unknown significance c.182G>C (p.Arg61Pro) that has not been previously reported, and both showed clear biochemical evidence of cobalamin C disease, although relatively mild. c.634delC (p.Gln212fsX79) is another novel variant in this cohort. It is predicted to cause a frameshift and extension of the C-terminus of the protein (Supplementary Table S1).
Figure 1. MMACHC Allelic Frequency.
Allele frequency of MMACHC variants in this cohort (n=27) is shown.
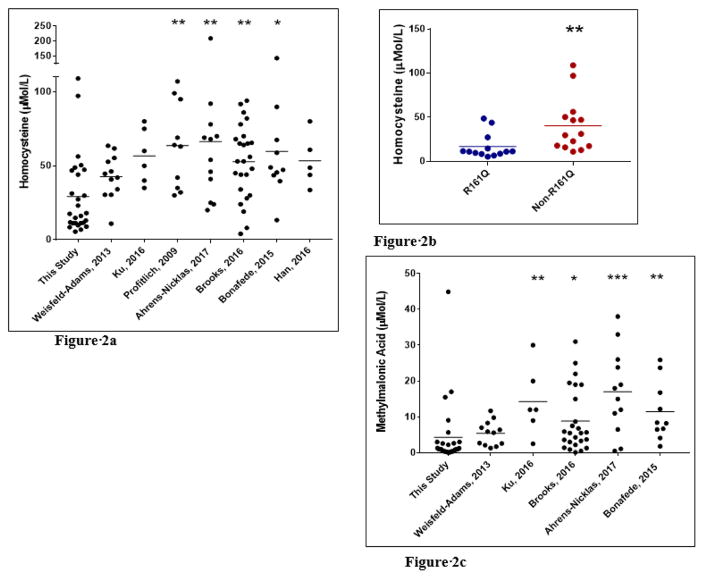
Comparisons of total plasma homocysteine, methionine, and methylmalonic acid levels in our cohort versus several large published studies10,11,16–20 demonstrated lower total plasma homocysteine and methylmalonic acid levels in our cohort as compared to these previously published studies (Figures 2a, Figure 2c) whereas methionine levels were comparable (not shown). The high frequency of the c.482G>A(p.Arg161Gln) pathogenic variant in our cohort contributes to this observation as 13/27 (48%) of our individuals are compound heterozygous for this variant compared to 4/86 (5%) individuals in these published studies (combined)8, 9, 14–18.
Figure 2. Comparison of Homocysteine and Methylmalonic Acid (MMA) Levels Between Patient Cohorts.
(A) Total plasma homocysteine levels in individuals with cobalamin C disease from this study compared to several large published studies is shown, demonstrating lower mean level in this report. (B) Total plasma homocysteine levels in individuals from this cohort with or without c.482G>A (p.Arg161Gln) pathogenic variant is shown, demonstrating that individuals harboring this pathogenic variant exhibit lower mean level. (C) Mean methylmalonic acid levels are lower in this study compared to published studies whereas methionine levels are comparable (not shown). * p<0.05, **p<0.01, ***p<0.001.
As expected, there were significant differences in presentation and clinical severity between the two genotype groups within our cohort (Table 2). In the R161Q group, only two individuals were symptomatic at the time of diagnosis (subjects 4 and 10). These two individuals did not have newborn screening and presented late (one presented at 18 years of age with spastic diplegia and the other presented at 3.5 years of age with vomiting, dehydration, and acidosis). All others who were diagnosed by a positive newborn screening or family history remained asymptomatic at their most recent follow up visit, except for one individual with learning difficulties and three others with mild speech related problems (Table 2 and Supplementary Table S1). In the non-R161Q group, 8 individuals (57%) developed symptoms in the first year of life. Five of these had newborn screening; 4 were symptomatic before initiation of treatment and the fifth individual was hospitalized at 5 weeks of age because of poor feeding and altered consciousness while on treatment. Of the remaining 6 individuals who presented after the first year of life, one did not have newborn screening and presented at 14 months of age with failure to thrive, developmental delay, and metabolic decompensation (subject 23). The other 5 who also did not have symptoms in the first year of life were treated early because of a positive newborn screening or family history. However, one of these individuals (subject 27) developed early maculopathy (genotype c.331C>T (p.Arg111Ter)/c.457C>T (p.Arg153Ter)) at three years of age, and three others were noted to have developmental delay or learning difficulties (Supplementary Table S1). Maculopathy was also noted in two additional individuals in the non-R161Q group who presented early, one was homozygous for the c.328_331del AACC (p.Asn11AspfsX13) pathogenic variant (subject 24) and the other homozygous for the c.481C>T(p.Arg161Ter) pathogenic variant (subject 25).
Finally, comparisons of the biochemical analytes between individuals in the R161Q group versus all others, as summarized in table 2, demonstrated significantly lower total plasma homocysteine levels at presentation (32.2–76.3 micromole/L; mean=59.1, median=60.3 vs. 32.5–280 micromole/L; mean=172, median=166; p value= 0.0006) and at last follow up (5.5–48.4 micromole/L; mean=16.9, median=11.2 vs. 11.2–109 micromole/L; mean=40.5, median=30.5; p value = 0.0013) in the R161Q group. The differences in methylmalonic acid and methionine levels were not significant. We also observed significant differences in the treatment regimen between the two groups. Those in the R161Q group receive a lower dose of hydroxocobalamin (0.01–0.6 mg/kg/day, mean=0.1, median=0.06 vs. 0.03–1.5 mg/kg/day, mean=0.6, median=0.4; p value= 0.0002). Moreover, fewer individuals in this group were prescribed betaine (2/13 or 15% vs 11/14 or 79%; p value = 0.001) (Table 2).
4. DISCUSSION
Cobalamin C disease is a relatively common, panethnic disorder. Some of the reported pathogenic variants are more common in certain ethnicities, such as the c.271dupA(p.Arg91LysfsX14) in Europeans12–14, c.331C>T (p.Arg111Ter) in Louisiana Cajuns and French Canadians14 and the c.609G>A(p.Try203Ter) in Chinese15. The c.482G>A (p.Arg161Gln) missense variant is observed in different ethnicities and is thought to result from a recurrent mutation at a CpG hotspot15. At least 30 individuals have been reported with this pathogenic variant in the literature, including cases diagnosed by positive newborn screening or symptomatic presentation2, 6, 10–15, 21–27.
Arginine at position 161 is highly conserved22. This residue is located in a cavity near the corrin ring and does not make direct contact with cobalamin28,29. It has been proposed that this arginine residue is important for binding of glutathione 29, with glutathione serving as an electron donor in the dealkylation reaction of adenosylcobalamin and methylcobalamin. In one study30, Froese et al. showed that MMACHC is a highly thermolabile protein but is stabilized with addition of hydroxocobalamin. On the other hand, MMACHC-R161Q was only moderately stabilized by hydroxocobalamin30, with more than twice the amount of hydroxocobalamin required to stabilize the mutant protein compared to the wild type. These results suggested that the c.482G>A (p.Arg161Gln) pathogenic variant causes decreased stability of the mutant protein and less stabilization by cobalamin30. In a more recent study, Gherasim et al. showed that the MMACHC-R161Q mutant protein is associated with lower protein stability, decreased dealkylation, impaired glutathione binding and increased production of reactive oxygen species28. In this study, the authors compared two missense pathogenic variants involving this arginine residue; c.482G>A (p.Arg161Gln) and c.481C>G (p.Arg161Gly). The latter was less stable and very susceptible to aggregation at normal body temperature compared to intermediate stability of the c.482G>A (p.Arg161Gln). This observation may explain early disease onset with the c.481C>G (p.Arg161Gly) as compared to late onset phenotypes with c.482G>A (p.Arg161Gln). Another observation in this study is both of these pathogenic variants do not significantly affect the decyanation activity, and the authors suggested that individuals with these pathogenic variants might respond to cyanocobalamin.28 The mild clinical and biochemical phenotype associated with the c.482G>A (p.Arg161Gln) pathogenic variant could be explained by findings in these two studies, including intermediate stability of the mutant protein and the stabilization by an increase in cobalamin concentration. Moreover, Learner-Ellis et al. showed higher expression of the c.482G>A(p.Arg161Gln) mutant protein compared to the c.271dupA (p.Arg91LysfsX14) pathogenic variant12.
The late onset form of cobalamin C disease has been much less commonly reported as compared to the severe, early onset form, which represents around 90% of the cases5,31. This is most likely due to underdiagnosis of the late-onset form because of multiple factors. One consideration is the possibility of missing mild disease with newborn screening. Five individuals in our cohort had normal newborn screening and were diagnosed because of positive family history (4 from one family; subjects 7, 8, 9, 11) or symptomatic presentation (single individual, subject 18). The four individuals with positive family history have the c.271dupA (p.Arg91LysfsX14)/c.482G>A (p.Arg161Gln) genotype. In the state of Texas, two newborn screening tests are performed, one at 24–48 hours of life and one at 7–14 days of life. The current primary analyte is C3 with the C3/C2 ratio used as a secondary analyte. The first newborn screen is considered positive if C3 is elevated (current elevated cutoff is 8.58 micromol/L), or if it is borderline (current borderline cutoff is 6.75 micromol/L) and the C3/C2 ratio is elevated (current elevated cutoff is 0.27). The second screen is considered positive if C3 is elevated (current elevated cutoff is 5.39 micromol/L), or if C3 value is ≥ 3 micromol/L and the C3/C2 ratio is elevated (current elevated cutoff is 0.2). Two of the four individuals who were missed had elevated C3/C2 ratio on the second screen, but C3 level did not reach 3 micromol/L. The remaining two individuals had normal ratios on the second screen, but they were treated prior to the second screen being collected, and in one of them, the ratio was elevated in the first screen.
In the fifth individual who has the c.271dupA (p.Arg91LysfsX14)/c.331C>T (p.Arg111Ter), genotype, the C3/C2 ratio was also elevated with C3 level below the cutoff. These results question the sensitivity of newborn screening in identifying infants with cobalamin C disease, especially those with genotypes predicting mild disease. Nogueira et al.32 showed that since the implementation of expanded newborn screening in 2004 in Portugal, all individuals with cobalamin C disease identified were homozygous for the c.271dupA (p.Arg91LysfsX14) pathogenic variant. They hypothesized that this finding may be related to low birth prevalence of late onset forms or the challenges of identifying these infants through newborn screening. In another example, an individual with cobalamin C disease and genotype of c.482G>A (p.R161Q)/c.608G>A (p.W203X) had C3 below the cutoff on the first and second newborn screening tests but was identified during work-up for low carnitine levels25. The authors hypothesized that this case was missed because the variants predicted mild disease and because low plasma carnitine obscured elevations in C3. The authors suggested that C3/C2 ratio can be used as a marker for cobalamin C disease because this ratio was elevated on the first and second newborn screens whereas C3 was not above the cutoff. Interestingly, we had similar observations in 3 of those missed by newborn screening in our cohort who also had the c.482G>A (p.R161Q) pathogenic variant. Besides C3/C2 ratio, low methionine could be an additional secondary analyte and was demonstrated to be associated with improved specificity and positive predictive value in one study 1.
Late onset cobalamin C disease has been recognized for more than three decades since Shinnar and Singer reported an adolescent female with cobalamin C disease who presented with dementia and myelopathy33. Phenotypes associated with this form include psychiatric symptoms, cognitive impairment, myelopathy, as well as thromboembolic events6. In spite of the clear phenotypes associated with late onset cobalamin C disease, these phenotypes may not be recognized as presenting features of an inborn error of metabolism. Moreover, genotypically affected individuals can be asymptomatic. For example, a case of cobalamin C disease with a genotype of c.482G>A (p.Arg161Gln)/c.81+1G>A was detected in an asymptomatic mother because of low carnitine in her newborn with subsequent maternal testing showing biochemical evidence of a cobalamin disorder22. While individuals with the c.482G>A (p.Arg161Gln) missense variant appear to have milder disease compared to other genotypes, lack of treatment or even late initiation of treatment can lead to a less favorable course with significant morbidity6. An example is subject 4 in this study, who in spite of treatment, is wheelchair-bound due to spastic paraplegia. This individual’s presentation demonstrates the importance of early diagnosis and treatment even in individuals with milder, presumably late onset disease. This example also indicates the need to refine screening programs to better identify these individuals.
Similar to other previously reported cases with the c.482G>A (p.Arg161Gln) pathogenic variant 11,27,34, individuals with this variant in our cohort had normal eye exam (eye exam available in 8/13 individuals), except for one with myopic astigmatism requiring glasses and another with ptosis, high hyperopia, and crowded discs, but this individual also has a confirmed diagnosis of myotonic dystrophy as well as cobalamin C disease (Supplementary Table S1). Even in the non-R161Q group, the prevalence of ophthalmological manifestations is lower than what has been previously published. One explanation for this observation is that none of the individuals in our group are homozygous for the c.271dupA(p.Arg91LysfsX14) pathogenic variant, a genotype characterized by early onset maculopathy 11,34.
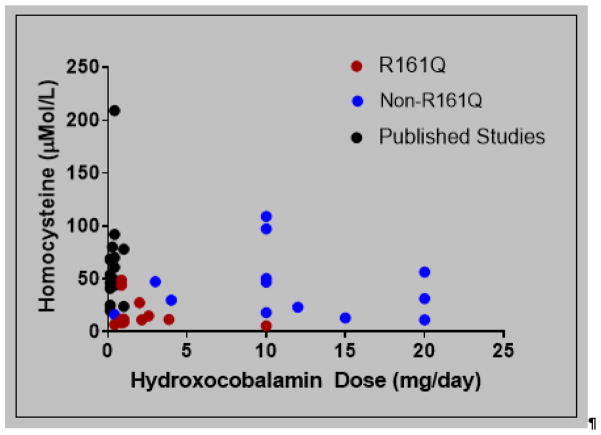
One of the observations in this study is that those in our cohort have lower total plasma homocysteine and methylmalonic acid levels compared to several published studies10,11,16–20. The high frequency of the c.482G>A (p.R161Q) pathogenic variant in this study contributes to this observation. In fact, the median total plasma homocysteine levels at last follow up visit in the R161Q group in our cohort was normal (11.2 micromole/L; normal range 4–14 micromole/L). Another possible contributing factor to lower total plasma homocysteine levels in our cohort may be the use of doses of hydroxocobalamin greater than 1 mg per day (Figure 3). The dose range of hydroxocobalamin for individuals in the R161Q group was 0.01–0.6 mg/kg/day (mean = 0.1, median= 0.06). In contrast, most of those in the non-R161Q group are treated with daily doses of 10 mg or higher (range 0.03–1.5 mg/kg/day). As hydroxocobalamin is given parentally, we use highly concentrated preparations (20–30 mg/mL), which we have found to be helpful in improving compliance. Besides receiving higher doses of hydroxocobalamin, other agents such as betaine and carnitine are more frequently utilized in the non-R161Q group. Even with this intense treatment regimen, mean and median total plasma homocysteine levels at last follow up were higher in this group as compared to the R161Q group as shown in figure 2b. Methionine levels were comparable between the two groups in our study as well as with published studies. One possible explanation is the use of betaine in severely affected individuals, which increases methionine levels.
Figure 3. Homocysteine Levels as a Function of Hydroxocobalamin Dose.
Lower doses of hydroxocobalamin (mg/day) are required for metabolic control of individuals within the R161Q group from our cohort. The total plasma homocysteine levels for individuals with cobalamin C disease from other published reports (unstratified by genotype) trend higher than our cohort and are mostly on the standard 1 mg/day hydroxocobalamin dose.
The effect of hydroxocobalamin dose escalation on metabolic control has been reported previously. Carrillo-Carrasco et al.35 presented one individual with worsening metabolic control in whom progressively increasing the hydroxocobalamin dose from 1 to 20 mg per day resulted in dose-dependent reduction in plasma methylmalonic acid by 80% and of total homocysteine by 55%, with an increase in the methionine level by twofold. In addition to metabolic control, there was subjective improvement in long-term memory. Similarly, Van Hove et al. presented two siblings with thrombotic microangiopathic nephropathy, in whom increasing the dosage of hydroxocobalamin from 1 to 5 mg daily resulted in reduced homocysteine levels and control of their renal disease36. Matos et al. showed variable biochemical and clinical responses to hydroxocobalamin dose escalation in five individuals with early onset disease, with improvements in communication skills and behavior in a subset of them 37.
In spite of early initiation of treatment, even when started prenatally, some complications like ophthalmological abnormalities and neurodevelopmental delay can still occur in those with early onset disease 4,11,31,38. This is related to the underlying complex and not completely understood pathophysiology of different complications seen in cobalamin C disease 4,39. However, early treatment does reduce morbidity and mortality in individuals with the early onset form4. Growth failure, feeding difficulties, vascular, and renal complication all appear to improve on therapy 4,31. Lowering homocysteine levels could prevent or resolve vascular complications 36,40. Most individuals with late onset disease showed significant clinical and biochemical response to treatment 6,38,39. Therefore, early treatment with the aim of achieving metabolic control should be initiated in all with cobalamin C disease. More intense treatment (i.e. high dose of hydroxocobalamin supplemented with betaine) may be needed in those with genotypes predicting severe disease.
One of the limitations in our study is the retrospective nature of data collection. Individuals were assessed by different providers, including different ophthalmologists, and this may create subjective variability. Also, there was no formal neuropsychological assessment available for many in our cohort. Finally, our dataset includes three sets of siblings, including one family with 5 siblings. Thus, shared genetic background within these families may also be contributing to the phenotypes observed.
5. CONCLUSION
In conclusion, here we present a large cohort of individuals with cobalamin C disease. Our study reemphasizes the genotype-phenotype correlation in individuals with cobalamin C disease. Those with the c.482G>A (p.Arg161Gln) pathogenic variant have milder disease as indicated by later onset of symptoms, less prominent biochemical abnormalities on presentation, and easier control as evident by the biochemical profile on last follow up, the severity of treatment regimen, and fewer complications. These individuals may not be diagnosed appropriately either because they are not identified on newborn screening or because of lack of awareness of late onset symptoms. The milder phenotype associated with the c.482G>A (p.Arg161Gln) may impact counselling of individuals with this pathogenic variant, as complications known to occur in the severe form of the disease, like developmental delay and maculopathy, appear to be much less common.
Supplementary Material
Highlights.
The c.482G>A (p.Arg161Gln) pathogenic variant in MMACHC is associated with mild disease.
The diagnosis of cobalamin C disease has been missed by newborn screening in some individuals with the p.Arg161Gln pathogenic variant.
Despite being associated with a milder phenotype, individuals with the p.Arg161Gln can have significant complications especially if treatment is delayed.
Acknowledgments
M.A is supported by the Genzyme-ACMG Foundation Medical Genetics training Award in Medical Biochemical Genetics for the year 2016. R.M. is supported by Michael Geisman- Osteogenesis Imperfecta Foundation (OIF) Fellowship Award. L.C.B. is supported by NIH 5K08DK106453. B.H.G. is supported by NIH R01GM098387.
Footnotes
Disclosure
All the authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weisfeld-Adams JD, Morrissey MA, Kirmse BM, et al. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Mol Genet Metab. 2010;99:116–123. doi: 10.1016/j.ymgme.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner-Ellis JP, Tirone JC, Pawelek PD, et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat Genet. 2006;38:93–100. doi: 10.1038/ng1683. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis. 2012;35:91–102. doi: 10.1007/s10545-011-9364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrillo-Carrasco N, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. II. Complications, pathophysiology, and outcomes. J Inherit Metab Dis. 2012;35:103–114. doi: 10.1007/s10545-011-9365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenblatt DS, Aspler AL, Shevell MI, Pletcher BA, Fenton WA, Seashore MR. Clinical heterogeneity and prognosis in combined methylmalonic aciduria and homocystinuria (cblC) J Inherit Metab Dis. 1997;20:528–538. doi: 10.1023/a:1005353530303. [DOI] [PubMed] [Google Scholar]
- 6.Huemer M, Scholl-Bürgi S, Hadaya K, et al. Three new cases of late-onset cblC defect and review of the literature illustrating when to consider inborn errors of metabolism beyond infancy. Orphanet J Rare Dis. 2014;9:161. doi: 10.1186/s13023-014-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR. Newborn Screening: Toward a Uniform Screening Panel and System—Executive Summary. Pediatrics. 2006;117(Supplement 3):S296–S307. doi: 10.1542/peds.2005-2633I. [DOI] [PubMed] [Google Scholar]
- 8.Manoli I, Myles JG, Sloan JL, et al. A critical reappraisal of dietary practices in methylmalonic acidemia raises concerns about the safety of medical foods. Part 2: Cobalamin C deficiency (cblC) Genet Med Off J Am Coll Med Genet. 2016;18:396–404. doi: 10.1038/gim.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huemer M, Diodato D, Schwahn B, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. 2017;40:21–48. doi: 10.1007/s10545-016-9991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisfeld-Adams JD, Bender HA, Miley-Åkerstedt A, et al. Neurologic and neurodevelopmental phenotypes in young children with early-treated combined methylmalonic acidemia and homocystinuria, cobalamin C type. Mol Genet Metab. 2013;110:241–247. doi: 10.1016/j.ymgme.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Brooks BP, Thompson AH, Sloan J, et al. Ophthalmic Manifestations and Long-Term Visual Outcomes in Patients with Cobalamin C Deficiency. Ophthalmology. 2016;123:571–582. doi: 10.1016/j.ophtha.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerner-Ellis JP, Anastasio N, Liu J, et al. Spectrum of mutations in MMACHC, allelic expression, and evidence for genotype–phenotype correlations. Hum Mutat. 2009;30:1072–1081. doi: 10.1002/humu.21001. [DOI] [PubMed] [Google Scholar]
- 13.Nogueira C, Aiello C, Cerone R, et al. Spectrum of MMACHC mutations in Italian and Portuguese patients with combined methylmalonic aciduria and homocystinuria, cblC type. Mol Genet Metab. 2008;93:475–480. doi: 10.1016/j.ymgme.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Morel CF, Lerner-Ellis JP, Rosenblatt DS. Combined methylmalonic aciduria and homocystinuria (cblC): Phenotype–genotype correlations and ethnic-specific observations. Mol Genet Metab. 2006;88:315–321. doi: 10.1016/j.ymgme.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Liu M-Y, Yang Y-L, Chang Y-C, et al. Mutation spectrum of MMACHC in Chinese patients with combined methylmalonic aciduria and homocystinuria. J Hum Genet. 2010;55:621–626. doi: 10.1038/jhg.2010.81. [DOI] [PubMed] [Google Scholar]
- 16.Han B, Cao Z, Tian L, et al. Clinical presentation, gene analysis and outcomes in young patients with early-treated combined methylmalonic acidemia and homocysteinemia (cblC type) in Shandong province, China. Brain Dev. 2016;38:491–497. doi: 10.1016/j.braindev.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Ahrens-Nicklas RC, Whitaker AM, Kaplan P, et al. Efficacy of early treatment in patients with cobalamin C disease identified by newborn screening: a 16-year experience. Genet Med. 2017 Feb; doi: 10.1038/gim.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Profitlich LE, Kirmse B, Wasserstein MP, Diaz GA, Srivastava S. High prevalence of structural heart disease in children with cblC-type methylmalonic aciduria and homocystinuria. Mol Genet Metab. 2009;98:344–348. doi: 10.1016/j.ymgme.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Ku CA, Ng JK, Karr DJ, et al. Spectrum of ocular manifestations in cobalamin C and cobalamin A types of methylmalonic acidemia. Ophthalmic Genet. 2016;37:404–414. doi: 10.3109/13816810.2015.1121500. [DOI] [PubMed] [Google Scholar]
- 20.Bonafede L, Ficicioglu CH, Serrano L, et al. Cobalamin C Deficiency Shows a Rapidly Progressing Maculopathy With Severe Photoreceptor and Ganglion Cell Loss. Invest Ophthalmol Vis Sci. 2015;56:7875–7887. doi: 10.1167/iovs.15-17857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodamer OA, Rosenblatt DS, Appel SH, Beaudet AL. Adult-onset combined methylmalonic aciduria and homocystinuria (cblC) Neurology. 2001;56:1113. doi: 10.1212/wnl.56.8.1113. [DOI] [PubMed] [Google Scholar]
- 22.Lin HJ, Neidich JA, Salazar D, et al. Asymptomatic Maternal Combined Homocystinuria and Methylmalonic Aciduria (cblC) Detected through Low Carnitine Levels on Newborn Screening. J Pediatr. 2009;155:924–927. doi: 10.1016/j.jpeds.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Sun W, Yang Y, Jia J, Li C. A clinical and gene analysis of late-onset combined methylmalonic aciduria and homocystinuria, cblC type, in China. J Neurol Sci. 2012;318:155–159. doi: 10.1016/j.jns.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Rahmandar MH, Bawcom A, Romano ME, Hamid R. Cobalamin C Deficiency in an Adolescent With Altered Mental Status and Anorexia. Pediatrics. 2014;134:e1709–21714. doi: 10.1542/peds.2013-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahrens-Nicklas RC, Serdaroglu E, Muraresku C, Ficicioglu C. Cobalamin C Disease Missed by Newborn Screening in a Patient with Low Carnitine Level. JIMD Rep. 2015;23:71–75. doi: 10.1007/8904_2015_429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai AC-H, Morel CF, Scharer G, et al. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A:2430–2434. doi: 10.1002/ajmg.a.31932. [DOI] [PubMed] [Google Scholar]
- 27.Gizicki R, Robert M-C, Gómez-López L, et al. Long-term Visual Outcome of Methylmalonic Aciduria and Homocystinuria, Cobalamin C Type. Ophthalmology. 2014;121:381–386. doi: 10.1016/j.ophtha.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Gherasim C, Ruetz M, Li Z, Hudolin S, Banerjee R. Pathogenic Mutations Differentially Affect the Catalytic Activities of the Human B12-processing Chaperone CblC and Increase Futile Redox Cycling. J Biol Chem. 2015;290:11393–11402. doi: 10.1074/jbc.M115.637132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froese DS, Krojer T, Wu X, et al. Structure of MMACHC Reveals an Arginine-Rich Pocket and a Domain-Swapped Dimer for Its B12 Processing Function. Biochemistry (Mosc) 2012;51:5083–5090. doi: 10.1021/bi300150y. [DOI] [PubMed] [Google Scholar]
- 30.Froese DS, Healy S, McDonald M, et al. Thermolability of mutant MMACHC protein in the vitamin B12-responsive cblC disorder. Mol Genet Metab. 2010;100:29–36. doi: 10.1016/j.ymgme.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer S, Huemer M, Baumgartner M, et al. Clinical presentation and outcome in a series of 88 patients with the cblC defect. J Inherit Metab Dis. 2014;37:831–840. doi: 10.1007/s10545-014-9687-6. [DOI] [PubMed] [Google Scholar]
- 32.Nogueira C, Marcão A, Rocha H, et al. Molecular picture of cobalamin C/D defects before and after newborn screening era. J Med Screen. 2017;24:6–11. doi: 10.1177/0969141316641149. [DOI] [PubMed] [Google Scholar]
- 33.Shinnar S, Singer HS. Cobalamin C Mutation (Methylmalonic Aciduria and Homocystinuria) in Adolescence. N Engl J Med. 1984;311:451–454. doi: 10.1056/NEJM198408163110707. [DOI] [PubMed] [Google Scholar]
- 34.Weisfeld-Adams JD, McCourt EA, Diaz GA, Oliver SC. Ocular disease in the cobalamin C defect: A review of the literature and a suggested framework for clinical surveillance. Mol Genet Metab. 2015;114:537–546. doi: 10.1016/j.ymgme.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Carrillo-Carrasco N, Sloan J, Valle D, Hamosh A, Venditti CP. Hydroxocobalamin dose escalation improves metabolic control in cblC. J Inherit Metab Dis. 2009;32(6):728–731. doi: 10.1007/s10545-009-1257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Hove JLK, Damme-Lombaerts RV, Grünewald S, et al. Cobalamin disorder Cbl-C presenting with late-onset thrombotic microangiopathy: Late-Onset Cobalamin C Disorder. Am J Med Genet. 2002;111:195–201. doi: 10.1002/ajmg.10499. [DOI] [PubMed] [Google Scholar]
- 37.Matos IV, Castejón E, Meavilla S, et al. Clinical and biochemical outcome after hydroxocobalamin dose escalation in a series of patients with cobalamin C deficiency. Mol Genet Metab. 2013;109:360–365. doi: 10.1016/j.ymgme.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Huemer M, Simma B, Fowler B, Suormala T, Bodamer OA, Sass JO. Prenatal and postnatal treatment in cobalamin C defect. J Pediatr. 2005;147:469–472. doi: 10.1016/j.jpeds.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Martinelli D, Deodato F, Dionisi-Vici C. Cobalamin C defect: natural history, pathophysiology, and treatment. J Inherit Metab Dis. 2011;34:127–135. doi: 10.1007/s10545-010-9161-z. [DOI] [PubMed] [Google Scholar]
- 40.Profitlich L, Kirmse B, Wasserstein MP, Diaz G, Srivastava S. Resolution of cor pulmonale after medical management in a patient with cblC-type methylmalonic aciduria and homocystinuria: a case report. Cases J. 2009;2:8603. doi: 10.4076/1757-1626-2-8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boxer AL, Kramer JH, Johnston K, Goldman J, Finley R, Miller BL. Executive dysfunction in hyperhomocystinemia responds to homocysteine-lowering treatment. Neurology. 2005;64:1431–1434. doi: 10.1212/01.WNL.0000158476.74580.A8. [DOI] [PubMed] [Google Scholar]
- 42.Heil SG, Hogeveen M, Kluijtmans LAJ, et al. Marfanoid features in a child with combined methylmalonic aciduria and homocystinuria (CblC type) J Inherit Metab Dis. 2007;30:811. doi: 10.1007/s10545-007-0546-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.