Abstract
Background
Oral supplementation with n-3 PUFA increases the omega-3 index, a biomarker of red blood cell eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and plasma levels of biosynthesis pathway markers and potent lipid mediators involved in the resolution of inflammation among patients with peripheral arterial disease (PAD).
Objective
We aimed to quantify the association between an upstream change in the omega-3 index and downstream changes in lipid mediator production.
Methods
We conducted a secondary analysis of the OMEGA-PAD I Trial, a randomized, placebo controlled trial investigating high-dose n-3 PUFA oral supplementation in PAD patients. Eighty subjects were randomized to either 4.4g of fish oil or placebo for one month. Regression analyses using generalized estimating equation techniques were used to investigate the relationship between changes in the omega-3 index and changes in lipid mediators, pre- and post-intervention.
Results
In the fish oil group, there was a significant increase in the omega-3 index (5±1% to 9±2%, p<0.001) as well as in the plasma levels of several downstream lipid mediator pathway markers of resolution, which are involved with the regulation of leukocyte effector function and host defense. A doubling of the omega-3 index correlated with increases of 2.3-fold in 18-hydroxy-eicosapentaenoic acid (HEPE) (p<0.0001), 1.7-fold in 15-HEPE (p=0.03), 1.9-fold in 5-HEPE (p=0.04), and 3.6-fold in 4-hydroxy-docosahexaenoic acid (HDHA) (p<0.001).
Conclusion
Among subjects with symptomatic PAD who took oral fish oil supplements for one month, observed changes in the omega-3 index were strongly associated with increases in downstream mediators in the biochemical pathways of resolution.
Keywords: peripheral artery disease, fish oil, n-3 polyunsaturated fatty acids, omega-3 index, specialized pro-resolving mediators, lipid mediators, resolution of inflammation
Introduction
The atherosclerotic lesion, the hallmark of the disease process in peripheral arterial disease (PAD), represents a series of cellular and molecular responses that are the result of excess inflammation1, 2. In recent years, it has become appreciated that specific biochemical signals, rather than a passive decrescendo of inflammatory cytokines, leads to the resolution of inflammation. This work has demonstrated that a unique class of specialized pro-resolving lipid mediators (SPMs) drives the process of resolution3. SPMs are derived from the omega-3 polyunsaturated fatty acids (n-3 PUFA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are found in marine oils. It has long been recognized that fish oils have beneficial actions in cardiovascular disease4–6 and more recent work in the field of resolution biology has implicated SPMs as having a paramount role in a variety of inflammatory diseases7–12, many of which may be heralded by a resolution deficit.
The process by which n-3 PUFA impacts inflammation begins with the incorporation of EPA and DHA into cellular membranes. Once incorporated, n-3 PUFA can affect the activity of membrane proteins and physical membrane characteristics and, once released by intracellular phospholipases, they can be converted into a wide variety of bioactive lipid mediators13. Alternatively, circulating unesterified n-3 PUFA have been found to arrive at a site of inflammation where direct conversion to bioactive SPMs occurs14. Several families of structurally and functionally distinct SPMs have been identified including the E-series resolvins (RvE) derived from EPA via P450 metabolism or aspirin-acetylated cyclooxygenase (COX-2), and the D-series resolvins, protectins and maresins derived from DHA via lipoxygenase (LOX) or aspirin acetylated COX-23. More specifically, the formation of the RvE begins via the action of acetylated COX-2 or cytochrome P450 on EPA, which results in an intermediate known as 18-hydroperoxy eicosapentaenoic acid (HpEPE)15. 18-HpEPE is subsequently reduced by a peroxidase to form 18-hydroxy eicosapentaenoic acid (HEPE); this is followed by lipoxygenation by the enzyme 5-LOX to form a hydroperoxide, which is then transformed to an epoxide that undergoes enzymatic hydrolysis to form RvE115. The RvE have been found to act through G-protein coupled receptors to have potent anti-inflammatory and pro-resolving action16–19.
The omega-3 index is a validated biomarker used to define the red blood cell (RBC) content of EPA and DPA, thus it reflects the interplay between oral intake and metabolism of n-3 PUFA20. Erythrocyte fatty acid composition consists almost exclusively of phospholipids with little biologic variability between individuals21. Identifying the percentage contribution of EPA and DHA to total identified RBC fatty acids allows precise reflection of plasma and tissue levels of EPA and DHA22. However, the relationship between blood levels of these n-3 PUFA and the downstream biochemical pathways producing SPMs is poorly understood.
The physiological impact of oral supplementation of n-3 PUFA for each individual is variable depending on baseline characteristics such as prior dietary intake and hereditary metabolic factors20. In the OMEGA-PAD I Trial, we recently demonstrated the impact of n-3 PUFA supplementation on altering biochemical SPM pathways in PAD patients23. We aimed here to investigate if changes in lipid mediator pathways are associated with changes in the omega-3 index in a cohort of PAD subjects undergoing short-term (one month) fish oil supplementation.
Methods
Study Population, Intervention and Protocol
This study was a secondary data analysis of the randomized, double-blinded, and placebo-controlled OMEGA-PAD I Trial; the methods of the OMEGA-PAD study have been previously published23, 24. Briefly, the study included 80 patients aged 50 and older with symptomatic lower extremity PAD, who presented to vascular surgery clinic at the Veterans Affairs Medical Center in San Francisco. Subjects were excluded from the trial if they were already taking a fish oil supplement. Subjects were randomized to 2.2g oral n-3 PUFA (Pro-Omega; Nordic Naturals, Watsonville, CA, USA) twice daily (totaling 4.4g/day) or a matched placebo for one month. Each ProOmega capsule contained 325mg of EPA and 225mg of DHA. Treatment corresponded to four capsules twice daily, totaling 2.6g of EPA and 1.8g of DHA daily.
Lipidomics
Lipid mediators in plasma samples were measured by liquid-chromatography-tandem mass spectrometry (LC-MS/MS) as previously described25, 26.
Omega-3 Fatty Acid Measurements
Packed RBCs were stored at −80°C until assayed for n-3 PUFA content of EPA and DHA, the omega-3 index, analyzed according as previously described27.
Statistical analysis
All statistical analyses were performed using Stata version 13.0 (StataCorp, College Station, Texas). The intervention group was compared to the placebo group for homogeneity of demographic and baseline clinical variables using the chi-squared test for categorical variables and Student’s t-test for continuous variables, with statistical significance defined as α ≤ 0.05. Regression analyses using generalized estimating equation (GEE) techniques were used to investigate the relationship between changes in the omega-3 index and changes in lipid mediators, pre- and post-intervention. Measurements taken from the same person (“within-person”) are likely to be more highly correlated than those taken from different people (“between-person”). GEE accommodates the additional variability introduced by these correlated data by estimating the correlation structure from data in order to adjust standard errors in the model, while still averaging over all subjects. While a simpler analysis of the mean changes between two groups would also address this within-person correlation, GEE techniques provide very similar results while allowing for inclusion of subjects who have only one of two measurements for either lipid mediators or omega-3 index (subjects missing even one of the four measurements would be excluded in direct comparison of means changes). Because the distribution of lipid mediators and the omega-3 index tend to be skewed, all measurements were log-transformed. Log base 2 was used to provide an estimate for the fold increase in a lipid mediator when the omega-3 index is doubled.
Results
Eighty subjects with intermittent claudication participated in the study, with 40 randomized to the fish oil group and 40 randomized to the placebo group. Data on this cohort have been published previously23, and a summary of baseline characteristics is included in Table 1. The two groups were well balanced at baseline, with the only substantive differences between groups being prior history of coronary artery disease and use of a beta-blocker medication.
Table 1.
Baseline Characteristics of Patients.
| General Characteristics | Fish Oil (n=40) | Placebo (n=40) | P Value |
|---|---|---|---|
| Age (years) | 68±7 | 69±9 | 0.41 |
| Male sex, % | 39 (98) | 39 (98) | 1.0 |
| Index ABI | 0.72±0.02 | 0.71±0.02 | 0.74 |
| BMI, kg/m2 | 27.1±0.68 | 28.5±0.76 | 0.17 |
| Omega-3 Index, % | 5.2±1.7 | 4.6±1.4 | 0.13 |
| Walking Speed (score, 0–100) | 22±23 | 30±27 | 0.18 |
| Comorbidities | |||
| Coronary artery disease, % | 13 (33) | 22 (55) | 0.04* |
| Hypertension, % | 38 (95) | 35 (88) | 0.24 |
| Hyperlipidemia, % | 32 (80) | 36 (90) | 0.21 |
| Diabetes mellitus, % | 11 (28) | 14 (35) | 0.52 |
| Medications | |||
| Aspirin, % | 24 (60) | 31 (78) | 0.09 |
| Statin, % | 31 (78) | 37 (93) | 0.06 |
| β-Blocker, % | 14 (35) | 28 (70) | 0.002* |
| ACE inhibitor, % | 16 (40) | 18 (45) | 0.65 |
| PAD Risk Factors | |||
| History of smoking, % | 38 (95) | 36 (90) | 0.40 |
| Total cholesterol, mg/dL | 175±8 | 161±6 | 0.15 |
| Triglycerides, mg/dL | 157±16 | 150±11 | 0.71 |
| HDL, mg/dL | 45±2 | 44±2 | 0.77 |
| LDL, mg/dL | 100±7 | 87±5 | 0.12 |
| HbA1c, % | 6.2±0.2 | 6.1±0.2 | 0.85 |
| Serum creatinine, mg/dL | 1.06±0.07 | 1.14±0.06 | 0.42 |
| Baseline Inflammation | |||
| hsCRP, mg/L | 4.34±0.74 | 4.24±0.65 | 0.91 |
| Baseline Lipid Mediators (pg/mL) | |||
| 18-HEPE | 32±65 | 19±38 | 0.32 |
| 15-HEPE | 25±47 | 14±31 | 0.26 |
| 12-HEPE | 155±359 | 129±298 | 0.75 |
| 5-HEPE | 46±160 | 37±84 | 0.77 |
| 10,17-diHDHA | 40±56 | 29±53 | 0.42 |
| 4-HDHA | 13±27 | 22±59 | 0.42 |
Values as “mean±SD” or “n(%)”. ABI indicated ankle-brachial index; ACE, angiotensin-converting enzyme; BMI, body mass index; HDL, high-density lipoprotein; HbA1c, hemoglobin A1c; HDHA, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; PAD, peripheral artery disease.
P-values calculated using chi-square tests for frequencies and t-tests for continuous variables.
P<0.05.
In the fish oil group, as expected, the omega-3 index significantly increased (5 ±1% to 9 ±2%; p<0.001) while no change was observed in the placebo group (p=0.49) between baseline and post-intervention. In plasma, a significant increase was observed in biosynthesis pathway markers of SPMs generated from n-3 PUFA (Figure 1), specifically 18-HEPE (32 ±65 to 383 ±359; p<0.00001), 15-HEPE (25 ±47 to 180 ±276; p=0.001), 5-HEPE (46 ±160 to 173 ±236; p=0.001), and 4-hydroxy docosahexaenoic acid (HDHA) (13 ±27 to 100 ±139; p=0.001), in the fish oil cohort (Table 2). The change in omega-3 index was positively correlated with changes in plasma lipid mediators, with a doubling of the omega-3 index corresponding to a 2.3-fold increase in 18-HEPE (p <0.0001), a 1.7-fold increase in 15-HEPE (p=0.03), a 1.9-fold increase in 5-HEPE (p=0.04), and a 3.6-fold increase in 4-HDHA (p<0.0001) (Table 3). To examine whether a decrease in n-6 PUFA products compensated for the increase in n-3 PUFA products, we investigated the relationship between the change in the omega-3 index and the production of n-6 PUFA pathway markers (Table 3). In contrast to the n-3 PUFA products, no direct correlation was found between the change in omega-3 index and the measured levels of downstream n-6 PUFA mediators.
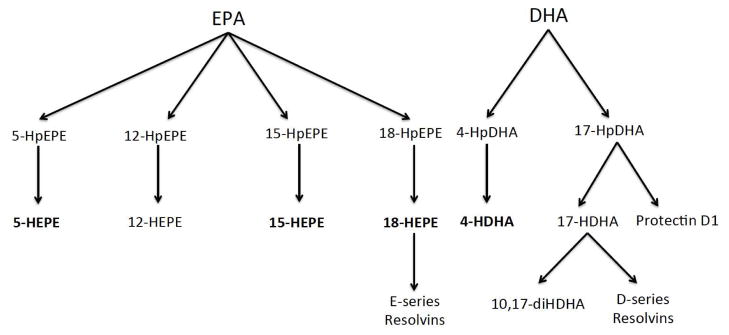
Figure 1.
Biosynthetic pathway of identified pathway markers and specialized pro-resolving mediators. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid.
Table 2.
Changes in Lipid Mediator Profiles with n-3 PUFA Supplementation.
| Lipid MediatorƗ | Fish Oil | Placebo | P Value (Between Groups) | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | P Value (Within Group) | Pre | Post | P Value (Within Group) | ||
| 18-HEPE | 32±65 | 383±359 | <0.00001 | 19±38 | 16±43 | 0.76 | <0.00001 |
| 15-HEPE | 25±47 | 180±276 | 0.001 | 14±31 | 24±48 | 0.35 | 0.003 |
| 12-HEPE | 155±359 | 1134±4072 | 0.13 | 129±298 | 108±270 | 0.75 | 0.14 |
| 5-HEPE | 46±160 | 173±236 | 0.001 | 37±84 | 33±69 | 0.86 | 0.002 |
| 10,17- diHDHA | 40±56 | 53±63 | 0.19 | 29±53 | 21±35 | 0.11 | 0.06 |
| 4-HDHA | 13±27 | 100±139 | 0.001 | 22±59 | 10±37 | 0.31 | 0.006 |
Values are mean±SD in units of pg/mL.
HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid.
Table 3.
Omega-3 Index as predictor for change in lipid mediators.
| Lipid MediatorƗ | Odds Ratio* | 95% CI | P Value |
|---|---|---|---|
| EPA Products (n-3 PUFA mediators) | |||
| 18-HEPE (n=39) | 2.3 | (1.7, 3.0) | <0.0001 |
| 15-HEPE (n=36) | 1.7 | (1.1, 2.9) | 0.03 |
| 12-HEPE (n=37) | 2.8 | (0.7, 10.8) | 0.13 |
| 5-HEPE (n=28) | 1.9 | (0.8, 1.5) | 0.04 |
| DHA Products (n-3 PUFA mediators) | |||
| 10,17-diHDHA (n=36) | 1.0 | (0.7, 1.4) | 0.95 |
| 4-HDHA (n=28) | 3.6 | (2.8, 4.8) | <0.0001 |
| AA Products (n-6 PUFA mediators) | |||
| 20-HETE (n=22) | 0.9 | (0.5, 1.5) | 0.62 |
| 15-HETE (n=37) | 0.6 | (0.4, 1.0) | 0.40 |
| 12-HETE (n=57) | 1.3 | (0.6, 1.1) | 0.11 |
| 5-HETE (n=29) | 0.7 | (0.4, 1.4) | 0.31 |
ORs calculated from regression models using GEE techniques. ORs are interpreted as follows: for each lipid mediator, the OR represents the percent increase when the omega-3 index is doubled.
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; HETE, hydroxyeicosatetraenoic acid.
Next, we separately investigated the relationship between the RBC EPA and DHA content with the production of each of the n-3 plasma lipid mediators that were detectable in our initial dataset. Similar to the results of the investigation using the omega-3 index, changes in RBC EPA and RBC DHA were positively correlated with changes in plasma lipid mediator production (Table 4). A doubling of the RBC EPA content corresponded to a 1.5-fold increase in 18-HEPE (p<0.0001), a 1.3-fold increase in 15-HEPE (p=0.03), and a 1.4-fold increase in 5-HEPE (p=0.008). A doubling of the RBC DHA content corresponded to a 4.5-fold increase in 4-HDHA (p<0.0001).
Table 4.
Red blood cell content of EPA and DHA as predictor for change in lipid mediators.
| Lipid MediatorƗ | RBC EPA | ||
|---|---|---|---|
| Odds Ratio* | 95% CI | P Value | |
| EPA Products (n-3 PUFA mediators) | |||
| 18-HEPE (n=39) | 1.5 | (1.4, 1.7) | <0.0001 |
| 15-HEPE (n=36) | 1.3 | (1.0, 1.6) | 0.03 |
| 12-HEPE (n=37) | 1.5 | (0.9, 2.6) | 0.14 |
| 5-HEPE (n=28) | 1.4 | (1.1, 1.7) | 0.008 |
| RBC DHA | |||
| DHA Products (n-3 PUFA mediators) | |||
| 10,17-diHDHA (n=36) | 0.96 | (0.6, 1.5) | 0.87 |
| 4-HDHA (n=28) | 4.5 | (3.3, 6.2) | <0.0001 |
ORs calculated from regression models using GEE techniques. ORs are interpreted as follows: for each lipid mediator, the OR represents the percent increase when the omega-3 index is doubled.
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid.
Discussion
We have demonstrated a direct correlation between changes in the omega-3 index and changes in levels of downstream n-3 PUFA metabolites in a group of individuals with PAD who took fish oil supplements for one month. Specifically, we have found that a change in the omega-3 index was significantly associated with changes in plasma 18-HEPE, 15-HEPE, 5-HEPE, and 4-HDHA. The omega-3 index is a validated and easily measured biomarker that allows for precise reflection of recent intake of n-3 PUFA22. While one may assume that the change in each individual’s production of downstream metabolites would vary substantially with oral supplementation of n-3 PUFA based on hereditary factors and prior dietary consumption, we demonstrate that changes in select downstream metabolic pathways are closely associated with an individual’s omega-3 index. Thus, changes in the omega-3 index have been found to strongly correlate with certain SPM pathway markers in our PAD population.
SPMs are generated locally by a variety of cell types and act to lessen inflammation and increase resolution via decreased production of inflammatory cytokines, increased production of anti-inflammatory cytokines, and increased clearance of cellular debris15. We show that a doubling of the omega-3 index is associated with a 2.3-fold increase in 18-HEPE, the precursor to the potent E-series resolvins3, 7, which have been shown to have an important role in limiting macrophage-induced pro-inflammatory actions28. A doubling of the omega-3 index is associated with a 1.7-fold increase in 15-HEPE, a product of EPA metabolism, which is known to decrease neutrophil migration during inflammation29. 5-HEPE, which is associated with a 1.9-fold increase with doubling of the omega-3 index, has been found to play a role in endocrine function30, and 4-HDHA, which is associated with a 3.6-fold increase with doubling of the omega-3 index, is thought to decrease oxygen-induced retinopathy31. Not all lipid mediators investigated were significantly associated with a change in the omega-3 index. For example, 12-HEPE approached, but did not reach, significance. This could be attributed to the fact that plasma levels of 12-HEPE are higher at baseline than the other investigated mediators. In order to demonstrate a significant impact on the production of 12-HEPE with increased omega-3 index, a larger sample size may be necessary. Additionally, there exists competition in the metabolic pathways of lipid mediator generation. It is possible that the enzymes needed for production of some of the investigated pathway markers and SPMs have been out-competed, leading to production of alternate downstream mediators, even in the setting of a significant change in the omega-3 index. The precise pro-resolving mechanism for each of the lipid mediators has yet to be elucidated and is a field of active investigation.
Furthermore, we examined whether a correlation existed between the change in omega-3 index and the production of downstream n-6 PUFA mediators. We aimed to determine whether a decrease in n-6 products compensated for the increase in n-3 products, but no significant association was found. This is likely due to the fact that n-6 PUFA make up a much larger proportion of plasma and tissue total fatty acids. In order to impact downstream n-6 mediator generation, a significantly larger impact would likely be necessary in the upstream n-6 PUFA.
Finally, we investigated how the individual content of RBC EPA and RBC DHA compared to the respective levels of EPA and DHA metabolites. Similar to the omega-3 index, RBC content of EPA and DHA were each directly associated with their respective metabolites. The metabolic factors that determine the kinetics of the n-3 (and n-6) PUFA metabolites are incompletely understood at present.
The precise physiologic implications from an increased omega-3 index and downstream lipid mediators are currently being investigated. It has been shown that an increase in the omega-3 index is associated with a decrease in heart rate, blood pressure, and triglycerides32, 33. The omega-3 index has also been suggested to be a risk marker for coronary heart disease and sudden cardiac death34, 35. It is likely that, at least in part, the beneficial effects observed from an increase in the omega-3 index are attributable to the associated increase in the downstream bioactive lipid mediators. For example, in the OMEGA-REMODEL study which demonstrated that treating patients who have had a recent acute myocardial infarction with high dose n-3 PUFA led to a decrease in adverse left ventricle remodeling36. The beneficial actions of n-3 PUFA seen in this study could be a result of the decreased inflammatory and pro-resolving actions of SPMs. Further research regarding the precise relationship between upstream n-3 PUFA and downstream SPMs in clinically relevant populations has great potential for elucidating the beneficial effects of these potent lipid mediators.
Limitations
Limitations of this study include the fact that it was a secondary data analysis based on a relatively small cohort. Additionally, not all lipid mediators that were investigated could be detected in subject plasma in the original study, so the relationship between the omega-3 index and these mediators could not be analyzed. Specifically, this is true for 17-HDHA, the precursor to the D-series resolvins. This could be due to the sensitivity of the equipment used for lipid mediator analysis, and should be improved with enhancement in equipment, internal standards, and technique. Finally, the patient population investigated in this study included primarily Caucasian males, which may not be representative of the broader PAD population.
Conclusion
Prior to this work, no other studies have investigated the association between an upstream measurement of n-3 PUFA intake, the omega-3 index, and the downstream changes that occurred in important lipid mediators as a result of oral n-3 PUFA supplementation in the PAD population. We have demonstrated standardized effect variables quantifying the association between 18-HEPE, 15-HEPE, 5-HEPE, and 4-HDHA and a change in omega-3 index that occur during one-month of oral supplementation with high dose n-3 PUFA. We will continue to examine the relationship between changes in the omega-3 index, downstream SPMs and markers of vascular inflammation in patients with PAD in a follow-up trial investigating the effects of a three-month treatment period with high-dose n-3 PUFA (OMEGA-PAD II; NCT01979874).
Highlights.
Fish oil supplementation increases the omega-3 index and lipid mediator production
An association between the omega-3 index and several lipid mediators is established
Markers of resolution pathways can be estimated using the omega-3 index
Acknowledgments
Funding Sources
This work was supported by start-up funds from the University of California San Francisco and the Northern California Institute for Research and Education. The project described was supported by Award Number KL2RR024130 from the National Center for Research Resources, Award Number 1K23HL122446-01 from the National Institute of Health/NHLBI, and a Society for Vascular Surgery Seed Grant and Career Development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding organizations were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2 TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors would also like to thank Nordic Naturals for donation of the treatment and placebo capsules.
Footnotes
Conflicts of Interest
The authors declare no financial conflicts of interest.
Author Contributions: The study was designed and implemented by S. M. Grenon and M.S. Schaller. Statistical analyses were conducted by N. Hills. W.S. Harris performed the fatty acid analysis. The manuscript was written by M.S. Schaller with expert contributions and critical revisions by all of the authors, each of whom has approved the final version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (dart) Lancet (London, England) 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 5.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin e after myocardial infarction: Results of the gissi-prevenzione trial. Gruppo italiano per lo studio della sopravvivenza nell’infarto miocardico. Lancet (London, England) 1999;354:447–455. [PubMed] [Google Scholar]
- 6.Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clinical cardiology. 2009;32:365–372. doi: 10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18s e-series resolvins in human leukocytes and murine inflammation. The Journal of clinical investigation. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, Mahanian M, Weitzman R, Hayden EY, Rosenthal MJ, Nemere I, Ringman J, Teplow DB. 1alpha,25-dihydroxyvitamin d3 and resolvin d1 retune the balance between amyloid-beta phagocytosis and inflammation in alzheimer’s disease patients. Journal of Alzheimer’s disease: JAD. 2013;34:155–170. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Hjorth E, Vedin I, Eriksdotter M, Freund-Levi Y, Wahlund LO, Cederholm T, Palmblad J, Schultzberg M. Effects of n-3 fa supplementation on the release of proresolving lipid mediators by blood mononuclear cells: The omegad study. Journal of lipid research. 2015;56:674–681. doi: 10.1194/jlr.P055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Recchiuti A, Codagnone M, Pierdomenico AM, Rossi C, Mari VC, Cianci E, Simiele F, Gatta V, Romano M. Immunoresolving actions of oral resolvin d1 include selective regulation of the transcription machinery in resolution-phase mouse macrophages. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:3090–3102. doi: 10.1096/fj.13-248393. [DOI] [PubMed] [Google Scholar]
- 12.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris WS. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacological research. 2007;55:217–223. doi: 10.1016/j.phrs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. Journal of immunology (Baltimore, Md: 1950) 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chemical reviews. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin e1. The Journal of experimental medicine. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin e1 selectively interacts with leukotriene b4 receptor blt1 and chemr23 to regulate inflammation. Journal of immunology (Baltimore, Md: 1950) 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 18.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins rve1 and rvd1 attenuate inflammatory pain via central and peripheral actions. Nature medicine. 2010;16:592–597. 591–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. Rve1 protects from local inflammation and osteoclastmediated bone destruction in periodontitis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 20.von Schacky C. Omega-3 index and cardiovascular health. Nutrients. 2014;6:799–814. doi: 10.3390/nu6020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clinical biochemistry. 2010;43:338–340. doi: 10.1016/j.clinbiochem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Harris WS. The omega-3 index: From biomarker to risk marker to risk factor. Current atherosclerosis reports. 2009;11:411–417. doi: 10.1007/s11883-009-0062-2. [DOI] [PubMed] [Google Scholar]
- 23.Grenon SM, Owens CD, Nosova EV, Hughes-Fulford M, Alley HF, Chong K, Perez S, Yen PK, Boscardin J, Hellmann J, Spite M, Conte MS. Short-term, high-dose fish oil supplementation increases the production of omega-3 fatty acid-derived mediators in patients with peripheral artery disease (the omega-pad i trial) Journal of the American Heart Association. 2015;4:e002034. doi: 10.1161/JAHA.115.002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grenon SM, Owens CD, Alley H, Chong K, Yen PK, Harris W, Hughes-Fulford M, Conte MS. N-3 polyunsaturated fatty acids supplementation in peripheral artery disease: The omega-pad trial. Vascular medicine (London, England) 2013;18:263–274. doi: 10.1177/1358863X13503695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellmann J, Zhang MJ, Tang Y, Rane M, Bhatnagar A, Spite M. Increased saturated fatty acids in obesity alter resolution of inflammation in part by stimulating prostaglandin production. Journal of immunology. 2013;191:1383–1392. doi: 10.4049/jimmunol.1203369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short-term physical inactivity impairs vascular function. The Journal of surgical research. 2014;190:672–682. doi: 10.1016/j.jss.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. Jama. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M. 18-hepe, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. The Journal of experimental medicine. 2014;211:1673–1687. doi: 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ternowitz T, Fogh K, Kragballe K. 15-hydroxyeicosatetraenoic acid (15-hete) specifically inhibits ltb4-induced chemotaxis of human neutrophils. Skin pharmacology: the official journal of the Skin Pharmacology Society. 1988;1:93–99. doi: 10.1159/000210754. [DOI] [PubMed] [Google Scholar]
- 30.Kogure R, Toyama K, Hiyamuta S, Kojima I, Takeda S. 5-hydroxy-eicosapentaenoic acid is an endogenous gpr119 agonist and enhances glucose-dependent insulin secretion. Biochemical and biophysical research communications. 2011;416:58–63. doi: 10.1016/j.bbrc.2011.10.141. [DOI] [PubMed] [Google Scholar]
- 31.Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE. 5-lipoxygenase metabolite 4-hdha is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Science translational medicine. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skulas-Ray AC, Kris-Etherton PM, Harris WS, West SG. Effects of marine-derived omega-3 fatty acids on systemic hemodynamics at rest and during stress: A dose-response study. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2012;44:301–308. doi: 10.1007/s12160-012-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. The American journal of clinical nutrition. 2011;93:243–252. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. The New England journal of medicine. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 35.Harris WS. The omega-3 index: Clinical utility for therapeutic intervention. Current cardiology reports. 2010;12:503–508. doi: 10.1007/s11886-010-0141-6. [DOI] [PubMed] [Google Scholar]
- 36.Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: The omega-remodel randomized clinical trial. Circulation. 2016;134:378–391. doi: 10.1161/CIRCULATIONAHA.115.019949. [DOI] [PMC free article] [PubMed] [Google Scholar]