Abstract
Non-surgical bleeding (NSB) is the most common clinical complication in heart failure (HF) patients supported by continuous-flow left ventricular assist devices (CF-LVADs). In this study, oxidative stress and alteration of signal pathways leading to platelet apoptosis were investigated. Thirty-one HF patients supported by CF-LVADs were divided into bleeder (n=12) and non-bleeder (n=19) groups. Multiple blood samples were collected at pre-implant (baseline) and weekly up to one-month post-implant. A single blood sample was collected from healthy subjects (reference). Production of reactive oxygen species (ROS) in platelets, total antioxidant capacity (TAC), oxidized low density lipoproteins (oxLDL), expression of Bcl-2 and Bcl-xL, Bax and release of cytochrome c (Cyt.c), platelet mitochondrial membrane potential (Δψm), activation of caspases, gelsolin cleavage and platelet apoptosis were examined. Significantly elevated ROS, oxLDL and depleted TAC were evident in the bleeder group compared to non-bleeder group (p<0.05). Platelet pro-survival proteins (Bcl-2, Bcl-xL) were significantly reduced in the bleeder group in comparison to the non-bleeder group (p<0.05). Translocation of Bax into platelet mitochondria membrane and subsequent release of Cyt.c were more prevalent in the bleeder group. Platelet mitochondrial damage, activation of caspases, gelsolin cleavage and ultimate platelet apoptosis in the bleeder group were observed. Oxidative stress and activation of both intrinsic and extrinsic pathways of platelet apoptosis may be linked to NSB in CF-LVAD patients. Additionally, biomarkers of oxidative stress, examination of pro-survivals and pro-apoptotic proteins in platelets, mitochondrial damage, caspase activation and platelet apoptosis may be used to help identify HF patients at high risk of NSB post-implant.
Keywords: Cardiac failure, Mechanical circulatory support device, Clinical complication, Signaling in platelet apoptosis
Introduction
Heart failure (HF) is the leading cause of death in the US affecting more than 5 million Americans. Despite medical advances and an annual expenditure of $34.4 billion in the US, 50% of diagnosed HF patients will succumb within 5 years [1,2]. Mechanical circulatory support (MCS) therapy has evolved into a standard therapy for patients with advanced HF [3,4] providing long-term or destination therapy (DT), as bridge to cardiac transplant (BTT), or as a platform for myocardial remodeling/recovery [5,6]. Advances in CF-LVADs have also demonstrated improved device reliability, durability, and performance. The number of HF patients receiving life-saving CF LVAD therapy has quadrupled over the last five years [7] with 6-month (90%) and 1-year (85%) survival rates [8,9]. However, the incidence of major device-related adverse events (bleeding, infection, and stroke) as well as right ventricular failure and multi-organ failure remain significant clinical problems [10–13]. The annual report from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) showed that bleeding is the most common post-operative complication in HF patients supported by CF-VADs [7].
The specific causes, pathways, and response mechanisms of NSB associated with CF LVADs has been elusive and become a high priority. Recently, it was reported that platelet glycoprotein Ibα (GPIbα) ectodomain shedding may be a key contributor [14]. Non-physiological high shear stress has been suggested to contribute to the shedding of GPIbα, GPVI and GPIIbIIIa [15–17]. Since platelets play a critical role in hemostasis and thrombosis, we hypothesize that altered platelet function and/or platelet damage may lead to bleeding complications in HF patients supported by CF-LVADs. High incidence of platelet apoptosis in CF-LVAD patients was observed in recent reports [18,19], but the involvement of relevant signaling pathways leading to platelet apoptosis in CF-LVAD patients and bleeding complication is still needed to be explored. The current study focused on the status of oxidative stress along with examination of intrinsic and extrinsic signal pathways which may influence platelet apoptosis mechanism in CF-LVAD patients with bleeding complication.
Methods
Subjects
Thirty-one NYHA class IV HF patients implanted with CF-LVAD as BTT or DT and 11 healthy volunteers (reference group) were studied. The CF-LVAD patients were divided into two groups: (i) bleeder group (n=12; NSB within one-month after device implant with bleeding defined according to INTERMACS classification) and (ii) non-bleeder group (n=19). Eleven patients received HeartMate II CF-LVAD (Thoratec Corp, Pleasanton, CA), eleven patients-the Jarvik 2000 CF-LVAD (Jarvik Heart, New York, NY), and nine patients-the HeartWare HVAD LVAD (HeartWare Inc, Framingham, MA). Among these CF-LVAD patients, eight patients also received the Levitronix CentriMag VAD (Thoratec Corp, Pleasanton, CA) for right ventricular support [HeartMate II (n=2), Jarvik 2000 (n=2), and HeartWare HVAD (n=4)].
Inclusions and Exclusions Criteria
The inclusion criteria for the selection of HF patients were: (i) ages between 18 to 70 years, (ii) undergoing CF-LVAD implantation and (iii) able to provide informed consent for the study. The inclusion criteria for the control subjects were (i) no clinical evidence of HF or other cardiovascular diseases, (ii) no history of malignancy and (iii) no inflammatory disease on careful examination and routine laboratory tests. Pregnant or breastfeeding women or women using oral contraceptives were also excluded from the study.
Ethics
The study was approved by the Institutional Review Boards (IRB). All CF-LVAD patients and healthy volunteers gave informed consent in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Anticoagulation/Antiplatelet Treatment
Anticoagulation was initiated with a titrated heparin dose with the goal for partial thromboplastin time of 40–45s once chest drainage was less than 30 mL/h for at least 4 hours. Thereafter the goal was aimed to have an anti-Xa activity level of 0.1–0.15 U/mL. The anticoagulation medication was subsequently converted to warfarin with a targeted international normalized ratio (INR) for the HeartMate II (1.8 – 2.3), the Jarvik 2000 (2.0 – 3.0) and the HeartWare HVAD (2.0 – 3.0). Antiplatelet agents were added to the anticoagulation regimen and the dosage was titrated based on measurements of platelet function using a platelet function analyzer (PFA-100®, Dade Behring, Inc, Deerfield, IL) and thrombelastogram (TEG) (TEG® 5000 Thrombelastograph® Hemostasis Analyzer System, Haemonetics Corporation, Braintree, MA). All the patients received pentoxifylline to improve red blood cell deformability in the hope of mitigating shear-induced hemolysis.
Collection and Preparation of Blood Sample
EDTA/Citrate-anticoagulated blood samples from the HF patients were collected before CF-LVAD implant surgery (baseline/pre-operative: Pre-OP) and at day 7, 14, 21 and 30 (Post-operative duration 1, 2, 3 weeks and 1 month: POD-1W, 2W, 3W and 1M) after the implant surgery. A single blood sample was collected from each of the healthy donors. All HF patient and the healthy volunteer blood samples were aliquoted and processed immediately. Platelet rich plasma (PRP) and platelet pellets were prepared from whole blood samples using established procedures [18,19].
Platelet Function Measurements
Measurements of platelet function with the PFA-100® and thromboelastography (TEG) were performed according to the manufacturer’s instructions. For PFA-100 test, data were expressed as the closure time (CT) for the collagen/adenosine diphosphate [CADP-CT (sec.)] or collagen/epinephrine [CEPI-CT (sec.)]; and for TEG parameters, TEG-maximum amplitude (TEG-MA), kinetic time (TEG-KT) and angle (TEG-Angle) were analyzed. The detailed procedures can be found in our previous reports [20].
Flow Cytometric Measurement of ROS in Platelets
To investigate the intraplatelet oxidation, platelets in PRP were loaded with 2′,7′-dichlorofluorescein (H2DCF-DA; Sigma), a cell-permeable non-fluorescent dye that is cleaved by intracellular esterases to H2DCF, rendering it membrane-impermeable, and then emits fluorescent energy in the presence of ROS. H2DCF-DA (10 μM final concentration) was incubated with PRP for 30 min at 37°C. For positive and negative controls we used ROS Inducer (Pyocyanin) and ROS Inhibitor (N-acetyl-L-cysteine) respectively. After washed in ice-cold PBS, 10,000 events were acquired immediately in a flow cytometer (FACS Calibur with sorter, Becton Dickinson, San Jose, CA, USA) and analyzed using Cell Quest software (BD, USA). Generation of ROS by the platelets resulted in green fluorescence that was recorded in fluorescence channel-1(FL1; Ex/Em = 490/525 nm) and was expressed as mean fluorescence intensity (MFI) in arbitrary unit.
Assessment of Total Antioxidant Capacity in Plasma
Total antioxidant capacity (TAC) was measured in blood plasma using a commercially available kit (Randox Laboratories, Antrim, UK) according to the manufacturer’s instruction. The OD was measured at 600 nm and the result was expressed as mmol/L.
Assessment of Oxidized Low Density Lipoprotein (oxLDL)
The concentration of oxidized low density lipoprotein (oxLDL) in plasma was measured by ELISA using a commercially available kit (Mercodia Inc, Winston Salem, NC) following the manufacturer’s instruction. Each sample was assayed in duplicate. The lowest detection limit of the kit was 1.0 mU/L.
Isolation of Mitochondrial Fraction from washed Platelets
The mitochondrial fraction of washed platelets was performed using a commercial Mitochondria Isolation Kit (Pierce Biotechnology, Inc., Pierce, Rockford, IL, USA). Briefly, washed platelets were resuspended in the Mitochondria Isolation Reagent A and lysed with Mitochondria Isolation Reagent B at 4°C. Samples were further mixed with Mitochondria Isolation Reagent C and then centrifuged at 700 x g for 10 min at 4°C. The supernatant was further subjected to centrifugation at 12 000 x g for 15 min to yield the mitochondrial pellet and the supernatant with mitochondria-free cytosolic fraction [21].
Quantitative Immunoblotting
To determine different protein expression levels; platelet extracts, mitochondrial pellet and cytosolic fraction were prepared from each patients and western blot analysis was performed according to our standard laboratory procedures. Western blot analysis was performed on the following proteins: Bcl-2, Bcl-xL, Bax, Cytochrome c, cleaved caspase-3 (Asp175) and cleaved caspase-9 (Asp330) (from cell signaling Technology, Danvers, MA) (1:1000 dilution) and cleaved gelsolin (from Sigma, St Louis, MO, USA). Immunoreactivities were detected using enhanced chemiluminescence (ECL) (Amersham Biosciences, UK) and photographic film (Denville Scientific Inc. Metuchen, NJ). Densitometric quantification of the digitized immunoreactive bands was performed using the UNSCAN-IT Gel 5.1 software (Silk Scientific, Orem, Utah). Beta actin (1:2000 dilution) (cell signaling Technology, Danvers, MA) was used to confirm equal loading conditions for total and cytosol protein expression, and the voltage-dependent anion channel (VDAC) (1:1000 dilution) (Cell Signaling Technology, Danvers, MA) was used as a mitochondrial loading control. The densitometric result was expressed as the ratio of the target protein to loading controls from the same blot individually for each patient. Thus, after obtaining a set of data (Baseline, 1W, 2W, 3W and 1M) for each patient for a particular protein of interest were statistically evaluated for each patient’s temporal change. Then each set of data were divided into two groups (bleeder vs. non-bleeder) and statistically evaluated.
Detection of Platelet Mitochondrial Damage
Platelet mitochondrial damage was measured by flow cytometry using mitochondrial membrane potential (ΔΨm)-sensitive dye, tetramethylrhodamine ethyl ester (TMRE), a commercially available MitoPT® TMRE Assay Kit (cat no: 9103, ImmunoChemistry Technologies, LLC, Bloomington, MN). CCCP (carbonyl cyanide 3-chlorophenylhydrazone) and DMSO (Dimethyl sulfoxide) were used as positive and negative controls respectively. The PRP samples were incubated with TMRE and a platelet identifying marker (CD41) in the dark at 37°C for 20 min, analyzed by flow cytometry followed by the instruction provided by the MitoPT® TMRE Assay Kit manual. Optimal emission from the TMRE lies in the fluorescence channel-2 (FL2). Appropriate emission filter was utilized to detect the presence of this potentiometric dye. TMRE measured apoptosis by monitoring the loss of fluorescence intensity and displayed as % of depolarized ΔΨm platelets.
Detection of Platelet PS Expression
Platelet-surface exposure of phosphatidylserine (PS) was determined using flow cytometry with the Annexin V-FITC Apoptosis Detection Kit (cat no: K101-100, BioVision, Inc., Milpitas, California) according to the manufacturer’s instruction with slight modification (18). In brief, PRP samples were labeled with PE-conjugated mouse anti-CD41 IgG (cat no: 555467, BD Biosciences, San Jose, California) and mixed with annexin V–FITC. Platelet apoptosis was determined by flow cytometry when both annexin V-FITC (FL1; Ex/Em = 490/525 nm) and PE-conjugated mouse anti-CD41 IgG (FL2; Ex/Em: 496/578 nm) stains on platelets were positive and was expressed as mean fluorescence intensity (MFI) in arbitrary unit.
Data Analyses
The data are presented as mean ± SD (standard deviation) or median with interquartile range (IQR). Statistical differences were determined by using χ2-test (for results presented as percentages), Student’s t-test (for results presented as mean±SD), and Mann-Whitney ‘U’ test (for median values with IQR). One-way and repeated measures ANOVA with Tukey-Kramer adjustment for multiple comparisons were used for testing statistical differences between baseline and longitudinal data. Statistical significance was assigned at p < 0.05. All analyses were performed using statistical software SAS version 9.4 (SAS Institute, Cary, NC, USA) and GraphPad (GraphPad Software Inc., La Jolla, CA, USA).
Results
Patient Population
Out of thirty-one CF-LVAD patients, 12 experienced at least one episode of NSB within one month after implantation (bleeder group). Comparative analyses of baseline demographic and clinical characteristics of the patients in the bleeder group and non-bleeder group (n=19) are summarized in Table 1. All the bleeding patients were transfused with either red cells or fresh frozen plasma or platelets. Anti-coagulation regimen at the time of bleeding was clinically optimized individually for each patient.
Table 1.
Demographic and baseline clinical characteristics of HF patients prior to CF-LVAD implantation
| Characteristics | Pre-implant HF patients (n = 31) | |
|---|---|---|
|
| ||
| Non-bleeder group (n = 19) | Bleeder group (n =12) | |
| Demography | ||
| Age in years, median (IQR) | 61 (48 – 76) | 60.0 (25 – 70) |
| Sex, n (% male) | 18 (94.7 %) | 9 (75.0 %) |
| Race | ||
| Caucasian white, n (%) | 11 (57.9 %) | 4 (33.3 %) |
| Black, n (%) | 8 (42.1 %) | 8 (66.7 %) |
| Height in meter, median (IQR) | 1.8 (1.7 – 1.9) | 1.7 (1.6 – 1.9) |
| Weight in kilograms, median (IQR) | 102 (60 – 127.9) | 80.0 (53.0 – 112.0)* |
| Body mass index (BMI) (kg/m2), median (IQR) | 31.0 (20.8 – 39.0) | 24.8 (19.5 – 34.3)* |
| Body surface area (BSA) (m2), median (IQR) | 2.3 (1.7 – 2.5) | 1.9 (1.6 – 2.4)* |
| History of smoking, n (%) | 5 (26.3 %) | 3 (25.0 %) |
| History of substance abuse | ||
| Ethyl alcohol abuse, n (%) | 3 (15.8 %) | 2 (16.7 %) |
| Drug abuse, n (%) | 5 (26.3 %) | 2 (16.7 %) |
| Vital signs | ||
| Systolic blood pressure (mmHg), mean ± SD | 97.4 ± 11.6 | 112.25 ± 14.7 |
| Diastolic blood pressure (mmHg), mean ± SD | 58.0 ± 7.2 | 74.5 ± 20.6 |
| Etiology of heart disease | ||
| Ischemic cardiomyopathy, n (%) | 8 (42.1 %) | 4 (33.3 %) |
| Non-ischemic cardiomyopathy, n (%) | 8 (42.1 %) | 4 (33.3 %) |
| Idiopathic Cardiomyopathy, n (%) | 3 (15.8 %) | 4 (33.3 %) |
| Echocardiographic parameters | ||
| Left ventricular end diastolic diameter (mm), mean ± SD | 67.7 ± 9.2 | 69.6.5 ±10.7 |
| Left ventricular ejection fraction, (%), mean ± SD | 13.8 ± 3.8 | 16.2 ± 5.8 |
Demographic and clinical parameters of non-bleeder versus bleeder groups of HF patients were statistically compared by Mann-Whitney ‘U’ test (for median values with IQR), χ2-test (for results presented as percentages) and Student’s t-test (for results presented as mean±SD) as applicable,
p<0.05 were considered significant.
Laboratory Hematology and Blood Chemistry
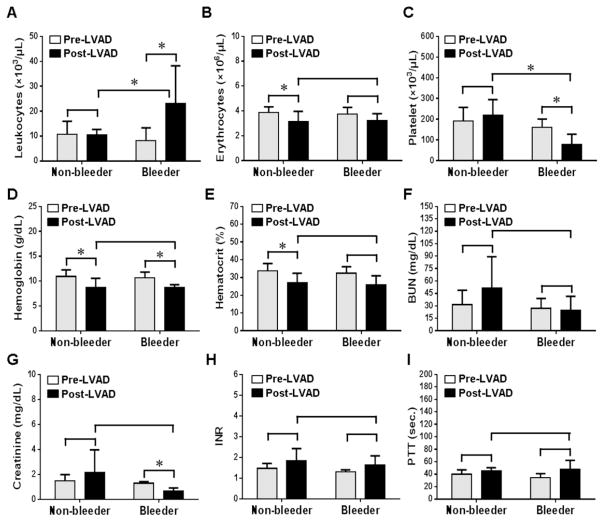
Summary of routine laboratory hematologic and blood chemistry parameters are presented in fig. 1. Post implant leukocyte count was significantly increased in the bleeder group compared to the non-bleeder group (p<0.05). Erythrocytes, hemoglobin and hematocrit were significantly decreased after CF-LAVD implant in the non-bleeder group, but a reduction in the hemoglobin level was statistically significant in the bleeder group (p<0.05). Platelet counts post-implant were significantly reduced in the bleeder group when compared to their corresponding baseline values (p<0.05). Creatinine level in the bleeder group was significantly decreased post-implant compared to baseline (p<0.05). There were no statistically discernible differences between the bleeder and non-bleeder groups for all other parameters (p>0.05) as shown in fig. 1.
Figure 1.
Comparison of routine laboratory hematology and blood chemistry between non-bleeder and bleeder groups before and after implantation of CF-LVADs. Bars represent standard deviation of mean values. *, p<0.05 considered significant.
Platelet Function Tests
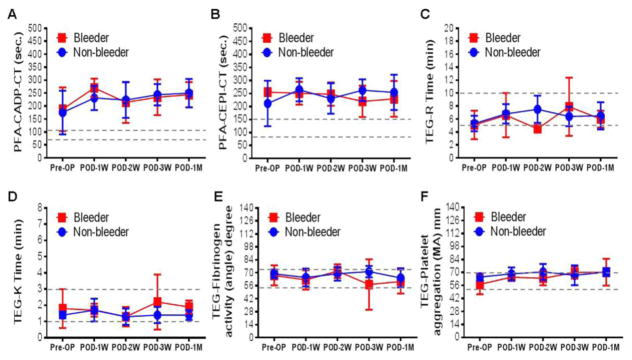
The changes in the CTs obtained with the PFA 100® and the TEG data from thromboelastography over the time period during CF-LVAD support in both the non-bleeder and bleeder groups are depicted in fig.2. The mean CTs for the CADP and CEPI cartridges before implantation were slightly higher than the normal range in both groups. There was no significant difference in the severity of the primary hemostatic defect indicated by the PFA tests between the non-bleeder and bleeder groups. The mean values of all the TEG parameters of the two groups were in the normal range.
Figure 2.
Change in platelet function and whole blood hemostasis tests parameters between non-bleeder and bleeder groups before and after CF-LVAD support during the study period.
Temporal Changes in Oxidative Stress
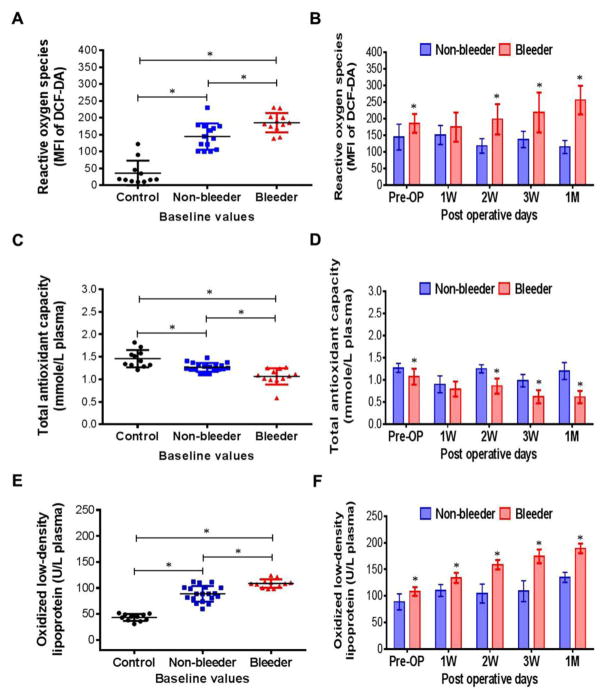
ROS generation from platelets expressed as MFI of DCF-DA indicated that ROS generation was significantly higher in all HF patients prior to CF-LVAD implantation when compared to that of the healthy subjects (Fig. 3A). Significantly lower levels of total antioxidant capacity in plasma were detected in the baseline samples of all the HF patients compared to the healthy subjects (Fig. 3C). These results suggest a pre-existing condition of oxidative stress in the HF patients. At baseline, the elevated ROS generation and depleted antioxidant capacity were significantly higher in the bleeder group than the non-bleeder group (Fig. 3A,C). The ROS levels continued to increase temporally post-implant with a concomitant decrease in total antioxidant capacity in the bleeder group (Fig. 3B,D). A higher level of plasma oxidized low-density lipoprotein, another important biomarker of oxidative stress, was also observed in the HF patients compared to the healthy subjects (Fig. 3E), which was also higher pre- and post-implant in the bleeder group and continued to increase temporally post-implant (Fig. 3F).
Figure 3.
Scatter plots and histograms indicating change in reactive oxygen species generation in platelets (A,B), total antioxidant capacity (C, D) and oxidized low-density lipoproteins (E, F) among controls, non-bleeders and bleeders at baseline and multiple time frames after CF-LVAD implantation. *p<0.05 is considered significant between two groups in Student’s t-test. When necessary, differences between means were further analyzed using the Tukey–Kramer HSD test. Least square mean±SD were obtained from statistical models and used in all graphical illustrations.
Temporal Changes in the Expression of Bcl-2 and Bcl-xL
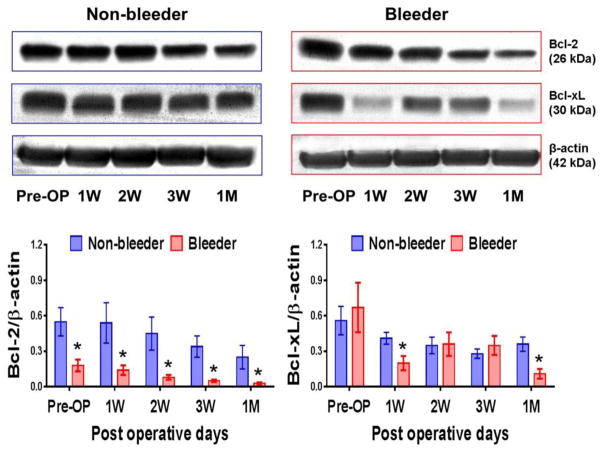
At baseline (pre-implant), the expression of the platelet pro-survival protein Bcl-2 significantly lower in the bleeder group compared to the non-bleeder group. After CF-LVAD implant, Bcl-2 protein expression in the bleeder group temporally decreased throughout the study period compared to the non-bleeder group. There is no statistical difference in the expression of other pro-survival protein Bcl-xL between these two groups at baseline. Additionally, we noticed that the expression of Bcl-xL proteins in the bleeder group remained significantly lower at one week and one month after CF-LVAD support (Fig. 4).
Figure 4.
Bcl-2 and Bcl-xL proteins in non-bleeder vs bleeder platelets at baseline and multiple time frames after CF-LVAD implantation. The expression levels of platelet Bcl-2 and Bcl-xL are shown in representative western blots. The bars in the histograms represent the mean ± SD concentrations of these proteins, expressed as the ratio to β actin. *p<0.05 is considered significant.
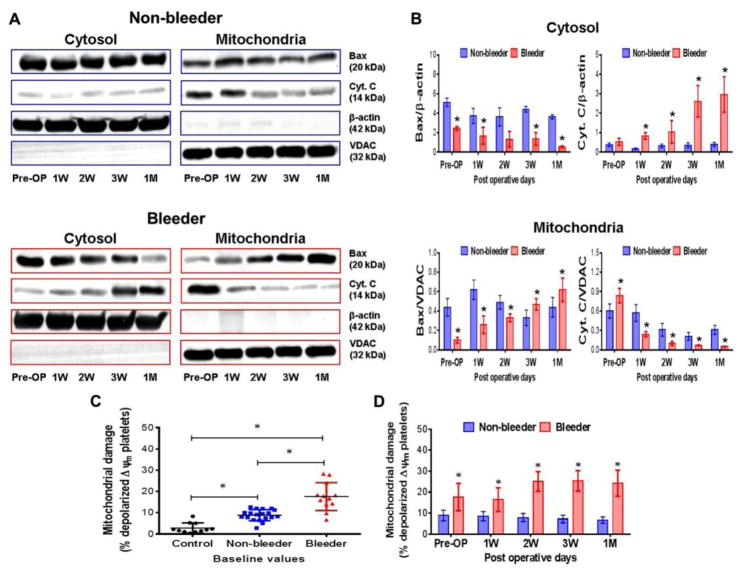
Temporal Changes in Bax Translocation, Cyt.c Release and Mitochondrial Damage
A significant reduction in the cytosolic Bax and concomitant rise in mitochondrial Bax as well as a significant decrease in mitochondrial Cyt.c and increase in cytosolic Cyt.c were observed in the bleeder group when compared to the non-bleeder group throughout the CF-LVAD support duration (Fig. 5A,B). The observation indicated that the pro-apoptotic protein Bax translocated from the cytosol into mitochondria and Cyt.c released from mitochondria into the cytosol. The flow cytometric analysis of depolarized ΔΨm platelets showed that mitochondrial damage was significantly higher in all the HF patients prior to CF-LVAD implantation when compared to the healthy subjects (Fig. 5C). Moreover the baseline level of depolarized ΔΨm platelets was significantly higher in the bleeder group compared to that in the non-bleeder group. Post-implant levels of depolarized ΔΨm platelets in the non-bleeder group did not show any significant change in comparison to the baseline level although a decreasing tendency was evident with increasing post implant time. In contrast, a consistent increase in the level of depolarized ΔΨm platelets was noted in the bleeder group (Fig. 5D); indicating platelet mitochondrial damage is more prominent in the HF patients with bleeding complication during CF-LVAD support.
Figure 5.
Changes in Bax translocation and Cyt.C release in cytosol and mitochondrial fractions (A,B) and mitochondrial damage (C,D) in non-bleeder vs bleeder platelets at baseline and multiple time frames after CF-LVAD implantation. VDAC (for the mitochondrial fraction) and β-actin (for the cytosolic fraction) were used as the respective internal controls. The bars in the histograms represent the mean ± SD. *p<0.05 is considered significant.
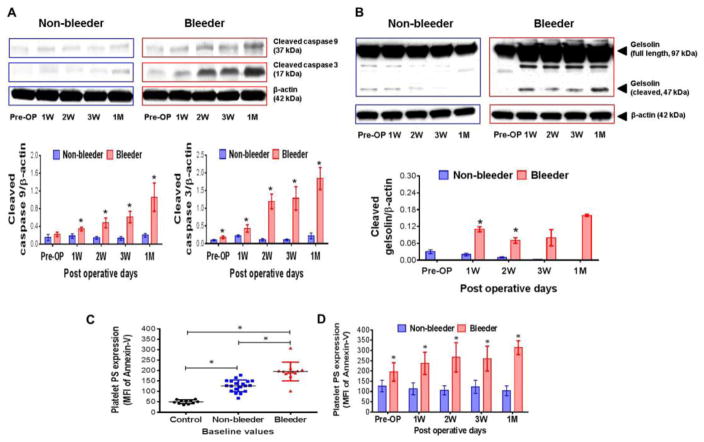
Temporal Changes in Activation of Caspases, Gelsolin Cleavage and Platelet Apoptosis
Quantification of activated 37 kDa cleaved caspase-9 and 17 kDa cleaved caspase-3 showed that activation of these caspases increased temporally in the bleeder group while no discernible changes were noticed in the non-bleeder group (Fig. 6A). Similarly, the cleavage of caspase substrate protein, gelsolin, another indicator of platelet apoptosis, increased temporally in the bleeder group but not so prominent in the non-bleeder group (Fig. 6B). Increased MFI of annexin V positive platelets demonstrated that the exposure of phosphatidylserine (PS) to the external platelet surface was significantly higher in pre-implant HF patients compared to healthy subjects, and higher in bleeders than non-bleeders (Fig. 6C). Platelet PS expression in the bleeder group increased temporally post-implant, but was unchanged in the non-bleeder group (Fig. 6D).
Figure 6.
Changes in activation of caspase-9 and -3 (A), gelsolin cleavage (B) and phosphatidyl serine (PS) expression (C,D) in non-bleeder vs bleeder platelets at baseline and multiple time frames after CF-LVAD implantation. The activated cleavage products of caspase-9, -3 and gelsolin are shown in representative western blots. The bars in the histograms represent the mean±SD concentrations of these cleaved proteins, expressed as the ratio to β actin. Platelet apoptosis is expressed as mean fluorescence intensity of annexin-V positive events and the bars in the scatter plot (C) and histogram (D) represent the mean±SD. *p<0.05 is considered significant.
Discussion
Although survival rates have improved significantly with MCS therapy over the past decade, thrombotic and bleeding complications have not been eradicated. In NYHA class IV HF patients receiving CF-LVAD therapy, clinically significant NSB resulting in10% readmission rate [22], and 30% gastrointestinal (GI) and 11% intracranial bleeding rates [4,23] have been reported. These adverse event rates were not seen with early pulsatile VADs [24,25], but are recognized as major cause of morbidity in patients supported by CF-LVADs [13,26,27]. The potential causes of bleeding are likely multi-factorial. Several studies have shown that all patients with CF-LVADs developed acquired von-Willebrand Syndrome due to a loss in high molecular weight vWF multimers, which may result in reduced vWF associated platelet activity and aggregation [23, 28–31]. However, not all CF-LVAD patients experienced NSB. The principal function of platelets is to prevent bleeding; subsequently it stands to reason that abnormal platelet function may be a contributing factor to NSB events. We recently reported platelet receptor shedding and apoptosis in CF LVAD patients that were associated with bleeding complications as well as presented the potential role of oxidative stress and platelet mitochondrial damage due to inflammation/infection [14,18–20,32]. In this present study, a potential mechanism of platelet apoptosis in CF-LVAD patients that developed NSB within one-month post-implant was investigated.
Platelets are unique blood cells that do not have a nucleus, but contain mitochondria and play an important role in hemostasis and thrombosis. Apoptosis or programmed cell death not only occurs in nucleated cells, but also were reported in anucleate platelets. The function of the apoptotic machinery remains unclear in human platelets, thought several studies have reported apoptosis-like events, at least in part, are involved in platelet activation [33,34]. Zhang et al. [35] showed that the induction of platelet apoptosis enhances its clearance in vivo by the reticuloendothelial system. Mason et al. [36] reported inactivation of pro-survival Bcl-xL reduced platelet half-life and caused thrombocytopenia. In our study, CF-LVAD patients with NSB complications had significantly reduced platelet counts (fig. 1), and no significant differences in the severity of the primary hemostatic defect as indicated by PFA 100® tests and TEG analysis were observed (Fig. 2). The events of platelet apoptosis via the intrinsic pathway may include only cytoplasmic events initiated by the increased rate of endogenous ROS [37]. Significantly higher intraplatelet ROS generation in the bleeder group was observed in our study supporting this notion. We also noticed concomitant decrease in total antioxidant capacity and elevated oxidized low density lipoproteins, suggesting that the total antioxidant capacity might not be strong enough to minimize the deleterious effect of oxidative stress.
Several studies reported a decrease in mitochondrial membrane potential, caspase activation, and phosphatidylserine exposure in platelets, leading to the speculation that platelets may undergo apoptotic cell death [38–40]. It has been demonstrated that platelets contain several apoptosis-related proteins such as Bcl-2 family proteins and a variety of caspase family proteins [38–41,35]. The Bcl-2 family proteins, such as Bcl-2 and Bcl-xL, are essential for cellular survival, and inhibition of Bcl-2/Bcl-xL induces apoptosis [35,42]. In our study, the reduced expression of the Bcl-2 and Bcl-xL proteins in platelets were evident in HF patients supported by CF LVAD, especially in the bleeder group, suggesting that their platelets may be more vulnerable to apoptotic death.
It has been shown that Bax may be sufficient to cause platelet apoptosis in the absence of Bcl-xL, indicating that Bax is redundant and plays an important role in the development of mitochondrial damage in platelets [43]. The pro-apoptotic multidomain Bcl-2 family proteins (Bak and Bax) serve as effector molecules for the mitochondrial pathway of apoptosis. Upon activation, they form pores by homooligomerization at the mitochondrial outer membrane through which apoptogenic factors such as Cyt.c is released into the cytosol [44]. Bax translocation to the mitochondria is a key event that regulates the release of Cyt.c from the mitochondria, which leads to downstream apoptotic events [45]. Similarly, in our study, patients that developed NSB exhibited Bax translocation from the cytosol into platelet mitochondria and Cyt.c release from mitochondria into the cytosol of the platelets that was not seen in the non-bleeder group (Fig. 5). Additionally, depolarized ΔΨm platelets, an indicator of mitochondrial damage, was found to be significantly elevated in the bleeder group. These findings suggest the role of mitochondria as pivotal mediators of the intrinsic pathway of apoptosis in CF-LVAD patients with NSB complications.
It is well known that the Bcl-2 proteins and caspases play a critical role as regulator and executioner in the apoptosis process. The intrinsic apoptotic pathway may be initiated by a variety of stress stimuli, which lead to the release of Cyt.c and subsequent activation of caspase-9 and -3 [21,39]. We found that the CF-LVAD support resulted in the appearance of the active form of caspase-9 (37 kDa) and caspase-3 (17 kDa) exclusively in the platelets of the bleeder group (Fig. 6). Although most cellular substrates for caspase 3 are nuclear proteins, the cytoskeleton regulatory protein gelsolin is a cytoplasmic substrate for caspase 3 [46] and is highly expressed in platelets [39]. In our current investigation, caspase 3 was activated in the bleeder group. Consequently, gelsolin would be proteolysed by active caspase 3. We examined the change in the molecular weight of gelsolin over the course of CF-LVAD support in both the non-bleeder and bleeder groups. We found that gelsolin was cleaved from its full length (97 kDa) to 47 kDa fragments after CF-LVAD implantation, which more pronounced in the bleeder group and the cleavage product was highest at one month post-implant. The post-implant time profile of gelsolin proteolysis matched that of caspase-3 activation, which suggests that gelsolin probably was a target of caspase 3 in platelets of the CF-LVAD patients with NSB complications. The dying cells upregulate surface expression of phosphatidylserine (PS), a specific death signal for phagocytosis, and reduce mitochondrial function [47]. In normal platelets, phosphatidylserine (PS) is located on the cytoplasmic surface of the membrane. However, during apoptosis, PS is translocated from the inner to the outer leaflet of the platelet membrane. In conformity with this we noticed a consistent increase in platelets PS expression in the bleeder group; indicating platelet apoptosis is more prominent in the HF patients supported by CF-LVAD who developed NSB.
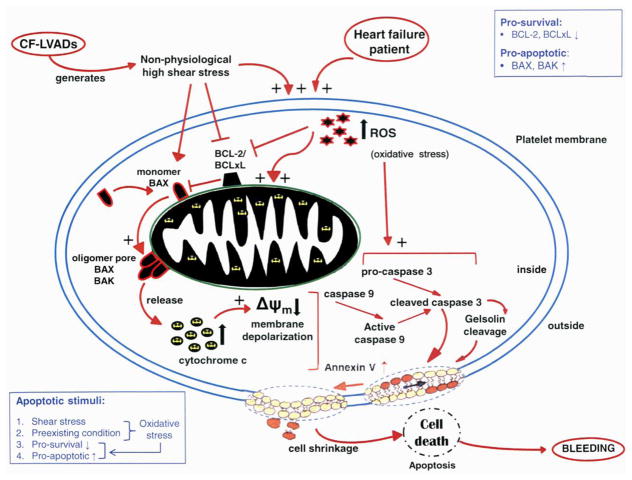
In summary, all of the CF-LVAD patients had elevated levels of oxidative stress pre-implant and worsened post-implant, for which the body’s antioxidant capacity may have been insufficient to combat the deleterious effects of ROS in the bleeder group. Based upon this study’s findings, we hypothesize that platelet mitochondrion may be one of the targets of oxidative stress, which is responsible for the inhibition of pro-survival proteins and may facilitate Bax translocation on the mitochondrial membrane and the release of Cyt.c into the platelet cytosol, as illustrated in Fig. 7. This mitochondrial damage may lead to the down regulation of the electron transport chain with intensification of ROS generation and the formation of mitochondrial permeability transition pore producing inner transmembrane potential depolarization. It may then be reasoned that extrinsic and intrinsic signal pathways play a key role in platelet apoptosis in those HF patients supported by CF-LVAD who develop NSB. In the extrinsic pathway, caspases are activated, followed by induction of Bax translocation, induction of Cyt.c release, and Δψm depolarization of platelets. In the intrinsic pathway, the target is mitochondria, and Δψm depolarization of platelets is evoked. These pathways converge into a common pathway activating caspase-3, which results in phosphatidylserine (PS) exposure and apoptosis in the NSB group. Taken together, we speculate that ROS may indirectly play an important role in NSB observed in some CF-LVAD patients.
Figure 7.
Hypothetical scheme indicating the mechanism of platelet apoptosis among CF-LVAD patients with bleeding complication.
The understanding of the role of ROS as a contributory factor in platelet apoptosis may enable development of effective medical management strategy aimed at targeting intraplatelet ROS. Therapeutic approaches may also have potential clinical utility in platelet-associated disorders involving oxidative damage. The long-term exposure to the high shear stress flow environment as well as the artificial blood contacting materials found in CF- LVADs may also contribute to platelet apoptosis. Previously, it was shown that the non-physiological high shear stress environment that occurs with CF-LVADs may be responsible for platelet dysfunction as evidenced by GPIbα, GPVI and GPIIbIIIa receptor shedding [15–17]. Analysis of the apoptosis mechanism in platelets may also provide a potential molecular mechanism(s) for understanding and regulating platelet viability during CF-LVAD support, especially in bleeder group. By reducing apoptosis signals or adding apoptosis inhibitors, platelet viability may be improved to help mitigate bleeding complication. Additionally, biomarkers of oxidative stress, estimation of pro-survivals and pro-apoptotic proteins in platelets, mitochondrial damage, caspase activation and platelet apoptosis may be used to help identify HF patients at high risk of NSB post-implant.
Acknowledgments
The described research was sponsored by the National Institutes of Health (Grant 1R01HL124170).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.WHO Fact Sheet. [Accessed May 15, 2013]. [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 5.Birks EJ, Yacoub MH, Banner NR, Khaghani A. The role of bridge to transplantation: should LVAD patients be transplanted? Curr Opin Cardiol. 2004;19:148–153. doi: 10.1097/00001573-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2011;57:1890–1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 10.Lietz K. Destination therapy: patient selection and current outcomes. J Card Surg. 2010;25:462–471. doi: 10.1111/j.1540-8191.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 11.Lahpor J, Khaghani A, Hetzer R, et al. European results with a continuous-flow ventricular assist device for advanced heart failure patients. Eur J Cardiothorac Surg. 2010;37:357–361. doi: 10.1016/j.ejcts.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 12.Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137:208–215. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25:352–356. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Mondal NK, Sorensen EN, et al. Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2014;33:71–79. doi: 10.1016/j.healun.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Mondal NK, Ding J, Gao J, Griffith BP, Wu ZJ. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: glycoprotein Ibα and glycoprotein VI. Thromb Res. 2015;135:692–698. doi: 10.1016/j.thromres.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Mondal NK, Ding J, Wu ZJ. Activated expression and shedding of platelet glycoprotein IIb/IIIa under nonphysiological shear stress. Mol Cell Biochem. 2015;409:93–101. doi: 10.1007/s11010-015-2515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ. Paradoxical Effect of Non-Physiological Shear Stress on Platelets and von Willebrand factor. Artif Organs. 2016;40:659–668. doi: 10.1111/aor.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondal NK, Sorensen EN, Hiivala NH, et al. Comparison of Intraplatelet Reactive Oxygen Species, Mitochondrial Damage and Platelet Apoptosis after Implantation of Three Continuous Flow Left Ventricular Assist Devices: HeartMate II, Jarvik 2000 and HeartWare. ASAIO J. 2015;61:244–252. doi: 10.1097/MAT.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondal NK, Sorensen EN, Hiivala NH, et al. Intraplatelet reactive oxygen species, mitochondrial damage and platelet apoptosis augment non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist device. Platelets. 2015;26:536–544. doi: 10.3109/09537104.2014.948840. [DOI] [PubMed] [Google Scholar]
- 20.Mondal NK, Sorensen EN, Feller ED, Pham SM, Griffith BP, Wu ZJ. Systemic Inflammatory Response Syndrome after Contentious-Flow Left Ventricular Assist Device Implantation and Change in Platelet Mitochondrial Membrane Potential. J Card Fail. 2015;21:564–571. doi: 10.1016/j.cardfail.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin KH, Hsiao G, Shih CM, Chou DS, Sheu JR. Mechanisms of resveratrol-induced platelet apoptosis. Cardiovasc Res. 2009;83:575–585. doi: 10.1093/cvr/cvp139. [DOI] [PubMed] [Google Scholar]
- 22.Bonde P, Dew MA, Meyer D, et al. National Trends in Readmission (REA) Rates Following Left Ventricular Assist Device (LVAD) Therapy. J Heart Lung Transplant. 2011;30:S9. http://dx.doi.org/10.1016/j.healun.2011.01.011. [Google Scholar]
- 23.Uriel N, Pak SW, Jorde UP, Jude B, et al. Acquired von Willebrand syndrome after continuous flow mechanical device support contributes to a high prevalence of bleeding during long term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 24.McBride LR, Naunheim KS, Fiore AC, Moroney DA, Swartz MT. Clinical experience with 111 thoratec ventricular assist devices. Ann Thorac Surg. 1999;67:1233–1238. doi: 10.1016/s0003-4975(99)00246-5. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy PM, Smedira NO, Vargo RL, et al. One hundred patients with the HeartMate left ventricular assist device: evolving concepts and technology. J Thorac Cardiovasc Surg. 1998;115:904–912. doi: 10.1016/S0022-5223(98)70373-3. [DOI] [PubMed] [Google Scholar]
- 26.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–3047. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 27.Harvey L, Holley CT, John R. Gastrointestinal bleed after left ventricular assist device implantation: incidence, management, and prevention. Ann Cardiothorac Surg. 2014;3:475–479. doi: 10.3978/j.issn.2225-319X.2014.08.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen D, Milano C, Thomas W, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assists device recipients. Ann Thorac Surg. 2010;90:1263–1269. doi: 10.1016/j.athoracsur.2010.04.099. [DOI] [PubMed] [Google Scholar]
- 29.Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3:675–681. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]
- 30.Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielsen LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II) J Am Coll Cardiol. 2009;53:2162–2167. doi: 10.1016/j.jacc.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 31.Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Mondal NK, Sorensen EN, Pham SM, Griffith BP, Slaughter MS, Wu ZJ. The systemic inflammatory response syndrome in end stage heart failure patients following contentious-flow left ventricular assist device implantation: The difference in plasma redox status and leukocyte activation. Artif Organs. 2016;40:434–443. doi: 10.1111/aor.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remenyi G, Szasz R, Friese P, Dale GL. Role of mitochondrial permeability transition pore in coated-platelet formation. Arterioscler Thromb Vasc Biol. 2005;25:467–471. doi: 10.1161/01.ATV.0000152726.49229.bf. [DOI] [PubMed] [Google Scholar]
- 34.Dale GL, Friese P. Bax activators potentiate coated-platelet formation. J Thromb Haemost. 2006;4:2664–2669. doi: 10.1111/j.1538-7836.2006.02211.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Nimmer PM, Tahir SK, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 36.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 37.Lopez JJ, Salido GM, Gómez-Arteta E, Rosado JA, Pariente JA. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J Thromb Haemost. 2007;5:1283–1291. doi: 10.1111/j.1538-7836.2007.02505.x. [DOI] [PubMed] [Google Scholar]
- 38.Bertino AM, Qi XQ, Li J, Xia Y, Kuter DJ. Apoptotic markers are increased in platelets stored at 37 degrees C. Transfusion. 2003;43:857–866. doi: 10.1046/j.1537-2995.2003.t01-4-00431.x. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Xia Y, Bertino AM, Coburn JP, Kuter DJ. The mechanism of apoptosis in human platelets during storage. Transfusion. 2000;40:1320–1329. doi: 10.1046/j.1537-2995.2000.40111320.x. [DOI] [PubMed] [Google Scholar]
- 40.Perrotta PL, Perrotta CL, Snyder EL. Apoptotic activity in stored human platelets. Transfusion. 2003;43:526–535. doi: 10.1046/j.1537-2995.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 41.Vanags DM, Orrenius S, Aguilar-Santelises M. Alterations in Bcl-2/Bax protein levels in platelets form part of an ionomycin-induced process that resembles apoptosis. Br J Haematol. 1997;99:824–831. doi: 10.1046/j.1365-2141.1997.4813284.x. [DOI] [PubMed] [Google Scholar]
- 42.Vogler M, Hamali HA, Sun XM, et al. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood. 2011;117:7145–7154. doi: 10.1182/blood-2011-03-344812. [DOI] [PubMed] [Google Scholar]
- 43.Kodama T, Takehara T, Hikita H, et al. BH3-only activator proteins Bid and Bim are dispensable for Bak/Bax-dependent thrombocyte apoptosis induced by Bcl-xL deficiency: molecular requisites for the mitochondrial pathway to apoptosis in platelets. J Biol Chem. 2011;286:13905–13913. doi: 10.1074/jbc.M110.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renault TT, Manon S. Bax: Addressed to kill. Biochimie. 2011;93:1379–1391. doi: 10.1016/j.biochi.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Kothakota S, Azuma T, Reinhard C, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]