Abstract
Patients with dementia due to Alzheimer’s disease (AD) have increased risk of developing delirium. This study investigated the relationship between a magnetic resonance imaging (MRI)-derived biomarker associated with preclinical AD and postoperative delirium. Participants were older adults (≥70 years) without dementia who underwent preoperative MRI and elective surgery. Delirium incidence and severity were evaluated daily during hospitalization. Cortical thickness was averaged across a published set of a priori brain regions to derive a measure known as the “AD signature.” Logistic and linear regression was used, respectively, to test whether the AD signature was associated with delirium incidence in the entire sample (N=145) or with the severity of delirium among those who developed delirium (N=32). Thinner cortex in the AD signature did not predict incidence of delirium (odds ratio=1.15, p=.38), but was associated with greater delirium severity among those who developed delirium (b=−1.2, p=.014). These results suggest that thinner cortices, perhaps reflecting underlying neurodegeneration due to preclinical AD, may serve as a vulnerability factor that increases severity once delirium occurs.
Keywords: delirium, cortical thickness, Alzheimer’s disease, biomarker, neurodegeneration, atrophy, preclinical Alzheimer’s disease, delirium severity
Graphical abstract
1. INTRODUCTION
Delirium is a well-characterized clinical disorder of attention and cognition, but its pathophysiology is not well understood. Although cognitive impairment and dementia are consistently identified as the leading risk factors for delirium, (Fong et al., 2015; Jones et al., 2016) mechanisms contributing to this increased risk remain unclear. Alzheimer’s disease (AD), the most common cause of dementia, occurs as a continuum or trajectory of pathophysiological processes and clinical symptomatology, beginning up to two decades or more before clinical symptoms appear (Dubois et al., 2016). Preclinical AD refers to the stage of the AD trajectory that is characterized by accumulating AD-related pathological changes with only subtle, if any, cognitive changes (Dubois et al., 2016). Although compelling work has identified dementia as a major risk factor for delirium (Fong et al., 2015), considerably less is known about the relationship between delirium and preclinical AD. Given that preclinical AD represents an important window for potential interventions to slow or stop progression to dementia (Dubois et al., 2016). it is important to determine whether AD is a risk factor for delirium, not only at the dementia stage but also during the preclinical timeframe. Furthermore, a better understanding of the relationship between delirium and specific features of preclinical AD could improve our pathophysiological understanding of delirium and suggest a biological mechanism for the association between dementia and delirium.
The primary pathological features of AD are beta-amyloid (Aβ) plaques, neurofibrillary tangles, and neurodegeneration (Jack et al., 2016; Jack et al., 2013). In individuals without dementia, some studies have reported an association between cerebrospinal fluid (CSF) or plasma levels of Aβ and tau and delirium (Idland et al., 2016; van den Boogaard et al., 2011; Xie et al., 2014) while others have not (Witlox et al., 2011). Similarly, some magnetic resonance imaging (MRI) studies of regional brain volume and delirium have identified an association (Gunther et al., 2012; Shioiri et al., 2016) while others have not (Cavallari et al., 2015; Root et al., 2013). To date, brain volume studies in delirium research have used mostly exploratory methods rather than a priori-defined region-of-interest measures of brain regions vulnerable to AD pathology, making inferences about the connection to AD difficult or impossible.
We have previously shown that reduction of regional cortical thickness in a specific set of brain regions of interest (ROIs)—the “AD signature”—is (1) associated with cognitive decline and progression to dementia (Bakkour et al., 2009; Bakkour et al., 2013; Dickerson et al., 2009; Dickerson et al., 2011a; Dickerson et al., 2011b, 2012, 2013; Putcha et al., 2011), (2) a better predictor of progression from mild cognitive impairment (MCI) to AD compared to entorhinal (Bakkour et al., 2009) or hippocampal volume (Dickerson et al., 2013), and (3) closely associated with AD-like CSF characteristics (Dickerson et al., 2012). Although molecular markers of Aβ and tau are needed to definitively define preclinical AD (Dubois et al., 2016), the AD signature’s close association with amyloid, tau, and conversion to MCI and dementia make it a promising preclinical AD biomarker. This study investigates for the first time the association between the AD signature and delirium by examining the AD signature preoperatively in a group of older adults who underwent major elective non-cardiac surgery and who were assessed daily for post-operative delirium during hospitalization. We hypothesized that individuals exhibiting a biomarker suggestive of preclinical AD (reduced cortical thickness in the AD signature regions) prior to surgery would be at greatest risk of delirium and that among those with delirium, thinner cortices in the AD signature would be associated with worse delirium severity.
2. METHODS
2.1. Study sample
The Successful AGing after Elective Surgery (SAGES) study is an ongoing prospective cohort study (enrollment from June 2010 and August 2013) of 560 adults aged 70 years or older who underwent elective major non-cardiac surgery and who did not have dementia at baseline. Subjects were recruited through regular review of operating room schedules for eligible elective surgeries. Of 1,052 potential participants contacted by telephone, 318 declined an interview; thus, 734 individuals were assessed for eligibility, of whom 163 were ineligible and five were eligible but refused participation, resulting in a total of 566 individuals enrolled. The response rate (percentage of estimated number eligible who were enrolled) was 70%. Of the 566 enrolled, 6 were subsequently excluded from analysis due to probable dementia at baseline. Approximately one third of SAGES participants were recruited to undergo MRI one month before surgery. The neuroimaging subset did not differ from the rest of the SAGES cohort on various relevant characteristics including age, sex, education, and surgery type. Details of the SAGES study including inclusion and exclusion criteria have been described previously (Cavallari et al., 2016; Schmitt et al., 2012; Schmitt et al., 2015). Inclusion criteria included age ≥70 years old, English speaking, and undergoing elective surgery at Beth Israel Deaconess Medical Center (BIDMC) or Brigham and Women’s Hospital (BWH). Exclusion criteria included diagnosis of dementia as assessed by initial medical record screening or reported by the patient; cognitive impairment as defined by a score ≤69 or its education-adjusted equivalent on the Modified Mini-Mental State Examination during the baseline interview or by neuropsychological testing; terminal disease; total blindness; severe deafness; and alcohol intake >5 drinks per day (men) or >4 drinks per day (women). Additional exclusion criteria for MRI included contraindications to 3-T MRI, such as pacemakers, certain stents, and implants. Written informed consent was obtained from all participants according to procedures approved by the institutional review boards of BIDMC and BWH, the two study hospitals, and Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts.
2.2. Neuroimaging protocol
For this study, all participants who had undergone MRI scans prior to surgery without dementia (N=145, n=32 with delirium) were included. We analyzed the magnetization-prepared fast gradient-echo 3D anatomical T1-weighted imaging (TR 7.9 ms, TE 3.2 ms, 15° flip angle, 32 kHz bandwidth, coronal acquisition plane with 24×19 cm field of view, 0.94 mm in-plane resolution, 1.4 mm slices, preparation time of 1100 ms with repeated saturation at the beginning of the saturation period, and an adiabatic inversion pulse 500ms before imaging), collected at the BIDMC Radiology Department on a 3T HDxt MRI (General Electric Medical Systems) scanner using a standard 8-channel head coil (Cavallari et al., 2015).
2.2.1. Cortical atrophy AD signature
T1 image volumes were examined quantitatively by a cortical surface-based reconstruction and analysis of cortical thickness, using a hypothesis-driven approach as described in multiple previous publications (Bakkour et al., 2009; Dickerson et al., 2009; Dickerson et al., 2011b, 2013). Briefly, the 3D T1-weighted acquisitions for each participant were motion-corrected and segmented into gray and white matter. Following standard procedures, selected coronal slices of each automated segmentation were visually inspected, and scans with errors in processing of the structures of interest were identified. The distribution of the quantitative volumetric data and a review of scans at either tail of the distribution and outliers were examined in greater detail. In the present analysis, no scans were identified with important errors of reconstruction of cortical surfaces. Cortical thickness measurements were obtained by calculating the distance between the white and pial surfaces across the cortical mantle. Thickness measures were then mapped to the inflated surface of each participant’s reconstructed brain. The data were smoothed on the surface using an iterative nearest-neighbor averaging procedure. The FreeSurfer software used to perform the analyses and visualization employed in this study, along with complete documentation, is freely available via the internet (http://surfer.nmr.mgh.harvard.edu).
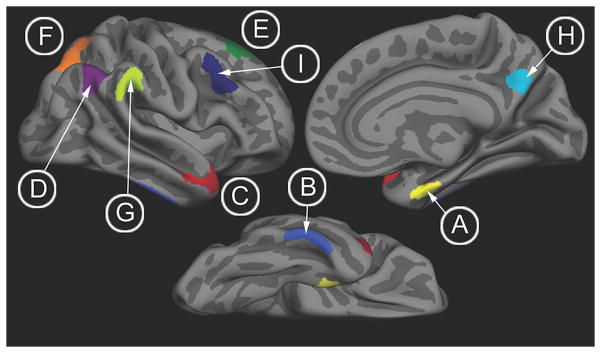
Nine bilateral AD signature ROIs (Figure 1) identified from prior analysis were mapped onto the individual participants (Bakkour et al., 2009; Dickerson et al., 2009). The derivation and validation cohorts for the development of the AD signature are entirely separate and did not overlap at all with the current study’s participants. The bilateral ROIs include: medial temporal cortex, inferior temporal gyrus, temporal pole, angular gyrus, superior frontal gyrus, superior parietal lobule, supramarginal gyrus, precuneus, and middle frontal gyrus. For each subject, mean cortical thickness within each ROI was calculated by deriving an average of all of the thickness estimates at vertices that fell within the labeled ROI. The resultant ROI measures of cortical thickness were averaged across the 2 hemispheres, and these 9 values per subject were subsequently averaged to obtain a continuous measure of cortical thickness, the “AD signature”, which was used for further statistical analysis as the primary diagnostic biomarker.
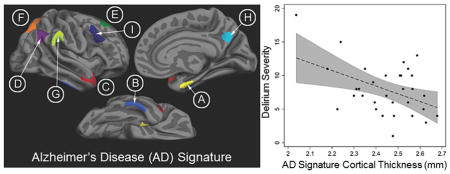
Figure 1.
Brain regions comprising the AD signature: (A, yellow) medial temporal cortex, (B, light blue) inferior temporal gyrus, (C, red) temporal pole, (D, purple) angular gyrus, (E, green) superior frontal gyrus, (F, orange) superior parietal lobule, (G, chartreuse) supramarginal gyrus, (H, aqua) precuneus, (I, dark blue) inferior frontal sulcus.
2.3. Assessment of delirium and preoperative cognitive function
Delirium incidence and severity were assessed using a structured battery on each postoperative day. Cognitive function was assessed at baseline prior to surgery.
2.3.1. Delirium Incidence
Delirium incidence was diagnosed using the Confusion Assessment Method (CAM) (Wei et al., 2008) diagnostic algorithm, supplemented with a validated chart review method (Inouye et al., 2005) to detect delirium presence or absence for each patient. The CAM was rated based on information from patient interviews performed once daily in the late morning or early afternoon at approximately the same time each day; these included a brief cognitive screen (orientation, short-term recall, sustained attention), the Delirium Symptom Interview (Albert et al., 1992), and information related to acute changes in mental status noted by nurses or family members (Schmitt et al., 2015). Study interviewers underwent intensive training and standardization (Inouye, 2003). The CAM has high sensitivity (94%) and specificity (89%) for delirium and moderate to high interrater reliability (kappa 0.7–1.0) across studies. The chart-based delirium instrument has a sensitivity of 74% and specificity of 83% (Inouye et al., 2005). The CAM plus chart combined approach is the preferred method for detecting delirium since it maximizes sensitivity; while the CAM detects the majority of delirium cases, the additional chart review increases sensitivity by identifying delirium throughout the 24-hour period (Saczynski et al., 2014).
2.3.2. Delirium severity
Delirium severity was determined using the CAM-Severity (CAM-S) long form, which is based on the 10 features from the full CAM instrument to quantify the intensity of delirium features (Inouye et al., 2014a). CAM-S demonstrates strong psychometric properties and strong associations with important clinical outcomes (Inouye et al., 2014a). When interrater reliability for CAM-S long form in the SAGES data was evaluated in 73 pairs, the overall agreement was 97% and intraclass correlation coefficient was 0.88 (Inouye et al., 2014a). Scores on the CAM-S long form range from 0 to 19, with higher scores indicating more severe delirium. Delirium severity was evaluated for each patient by assessing both CAM-S peak, the highest single CAM-S rating observed during hospitalization, and sum, the summed score across all hospital days which thus captures both delirium intensity and duration (Vasunilashorn et al., 2016).
2.3.3. Cognitive function
Cognitive abilities were assessed at baseline using a standardized neuropsychological battery including 11 tests covering cognitive domains of attention, memory, language, and executive functioning. Baseline cognitive function was assessed in two ways. First, an age-adjusted neuropsychological test battery composite measure, General Cognitive Performance (GCP), was calculated as described previously (Inouye et al., 2016; Jones et al., 2010). GCP is a continuous measure calibrated on a T-score metric (mean of 50; standard deviation [SD] of 10) to a nationally representative sample of adults aged ≥70 years (Gross et al., 2014; Langa et al., 2005). Second, all participants who scored 1.5 SDs below population means on two or more neuropsychological tests (one of which tested memory), or who scored > 2 SDs below the population mean on a measure of memory, were identified for review by a consensus panel of seven experienced clinical experts. Patients were assigned as having amnestic MCI (aMCI) using National Institute of Aging/Alzheimer’s Association (NIA-AA) criteria (Albert et al., 2011) following review of demographic data, neuropsychological testing results, Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) short-form (Jorm, 1994), activities of daily living (ADL) scores, and instrumental activities of daily living (IADL) scores.
2.4. Covariate data
Participant age and sex were obtained by interview. Vascular comorbidity was defined as presence or absence of at least one of the following pathological conditions: confirmed or history of myocardial infarction, congestive heart failure, peripheral vascular disease, diabetes (with or without end organ damage), cerebrovascular disease (carotid stenosis, history of stroke or transient ischemic attack), or hemiplegia. Depression was defined as a score ≥6 on the Geriatric Depression Scale (GDS, range 0–15). Additional demographic and surgery characteristics of the sample are described in Table 1.
Table 1.
Sample characteristics of SAGES neuroimaging sub-cohort
| Sample characteristic | SAGES MRI (N=145) | No Delirium (N=113) | Delirium (N=32) | p-value |
|---|---|---|---|---|
|
| ||||
| Age (years, mean±SD) | 76 ± 4.5 | 76±4.6 | 77±4.3 | .40 |
| Female sex (n, %) | 87, 60% | 65, 58% | 22, 69% | .25 |
| Non-white race (n, %) | 12, 8% | 10, 9% | 2, 6% | .64 |
| Education (years, mean±SD) | 15 ± 2.8 | 15±2.8 | 14±2.7 | .24 |
| GCP score (mean±SD) | 59 ± 7.1 | 60±6.7 | 55±7.4 | .002 |
| aMCI diagnosis at baseline (n, %) | 9, 6% | 4, 4% | 5, 16% | .012 |
| Geriatric Depression Scale (n, % ≥6) | 16, 11% | 12, 11% | 4, 13% | .76 |
| Vascular comorbidity (n, %)* | 57, 39% | 44, 39% | 13, 41% | .86 |
| Charlson comorbidity index (mean±SD) | 0.9±1.0 | 0.8±1.0 | 1.0±1.2 | .42 |
| Surgery type (n, %) | .64 | |||
| Orthopedic | 119, 82% | 91, 81% | 28, 88% | |
| Vascular | 8, 6% | 7, 6% | 1, 3% | |
| Gastrointestinal | 18, 12% | 15, 13% | 3, 9% | |
| Anesthesia type (n, %) | .034 | |||
| General alone | 119, 82% | 90, 80% | 29, 91% | |
| Spinal alone | 25, 17% | 23, 20% | 2, 6% | |
| General + Spinal | 1, <1% | 0, 0% | 1, 3% | |
| Surgery duration (hours, mean±SD) | 2.3 ± 1.3 | 2.3 ± 1.4 | 2.4 ± 1.0 | .54 |
| Delirium present on discharge day (n, %) | 6, 4% | 0, 0% | 6, 19% | <.001 |
| Length of hospital stay (days, mean±SD) | 5.1 ± 4.3 | 4.6 ± 2.0 | 6.9 ± 8.3 | .010 |
| ICU admission after surgery (n, %) | 9, 6% | 5, 4% | 4, 13% | .095 |
| CAM-S peak | 4±3.2 | 2±1.6 | 8±3.8 | <.001 |
| CAM-S sum | 8±10.5 | 5±3.9 | 20±16.3 | <.001 |
| AD signature (mm, mean±SD) | 2.42±0.131 | 2.42±0.126 | 2.44±0.146 | .33 |
| Angular gyrus | 2.23±0.223 | 2.22±0.221 | 2.28±0.229 | .16 |
| Middle frontal gyrus | 2.27±0.156 | 2.27±0.154 | 2.27±0.163 | .92 |
| Inferior temporal gyrus | 2.49±0.287 | 2.49±0.287 | 2.50±0.290 | .70 |
| Medial temporal | 2.58±0.343 | 2.57±0.336 | 2.62±0.367 | .44 |
| Precuneus | 2.43±0.191 | 2.42±0.186 | 2.46±0.210 | .26 |
| Superior frontal gyrus | 2.66±0.213 | 2.66±0.209 | 2.67±0.231 | .69 |
| Supramarginal gyrus | 2.45±0.200 | 2.45±0.196 | 2.45±0.217 | .98 |
| Superior parietal | 1.94±0.168 | 1.92±0.169 | 2.00±0.149 | .018 |
| Temporal pole | 2.76±0.244 | 2.77±0.227 | 2.72±0.298 | .28 |
| Calcarine cortex, control region (mm, mean±SD) | 1.71±0.14 | 1.70±0.15 | 1.73±0.13 | .36 |
P-values are for the contrast between the no delirium and delirium groups; chi square tests were used for dichotomous variables, t-tests for continuous variables with a normal distributions, and Wilcoxon rank-sum tests for continuous variables with a skewed distribution.
SAGES=Successful Aging after Elective Surgery. MRI=magnetic resonance imaging; GCP=General Cognitive Performance; aMCI=amnestic Mild Cognitive Impairment; CAM-S= Confusion Assessment Method Severity score (peak=largest single CAM-S rating observed during hospitalization [possible range 0–19]; sum=summed value of CAM-S across all hospitalization days); AD signature=average cortical thickness (mm) in 9 bilateral cortical regions of interest.
Vascular comorbidity is a dichotomous variable defined as presence or absence of at least one of the following pathological conditions: confirmed or history of myocardial infarction, congestive heart failure, peripheral vascular disease, diabetes (with or without end organ damage), cerebrovascular disease (carotid stenosis, history of stroke or transient ischaemic attack), or hemiplegia.
2.5. Statistical Models
Differences between the delirious and non-delirious groups were analyzed using t-tests or Wilcoxon rank-sum tests (for skewed data) for continuous variables and chi-square tests for dichotomous variables. For the main hypotheses, covariate-adjusted associations were estimated using multiple regression models. Type-I error probability (α) was set at 0.05. All analyses were performed in Stata MP 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). Covariates for all models included age at surgery and sex. Multiple logistic regression was used to assess the association between the AD signature and delirium incidence, and multiple linear regression was used to assess associations between the AD signature and CAM-S peak and sum. The AD signature was scaled by a factor of 10 so that the resultant odds ratio (OR) and regression coefficients (b) indicate, respectively, the odds of developing delirium or the change in CAM-S score associated with a 0.10 mm (slightly less than 1.5 SD) increase in AD signature cortical thickness. Because it is possible that subtle cognitive changes due to preclinical AD—rather than delirium—could increase scores on the CAM-S (e.g. mild memory impairment), our primary analyses of delirium severity were restricted to those 32 individuals exhibiting incident delirium. We examined two measures of multicollinearity, variance inflation factor (VIF) and tolerance, which were deemed acceptable if VIF was less than 10 and tolerance was greater than 0.1. R2 change refers to the change in R2 after the AD signature was added to the covariates-only model (only age and sex as independent variables).
2.5.1. Additional Sensitivity and Exploratory Analyses
Further analyses were performed to establish the robustness or to facilitate interpretation of statistically significant associations as follows. Linear regression offers a simple interpretation and is relatively robust for validity against nonnormality or nonlinearity; however, because CAM-S is positively skewed, we first replicated the primary linear regression model using Poisson regression. Second, we evaluated the association between the AD signature and CAM-S in the entire sample. Although there is significant unexplained heterogeneity in subsyndromal delirium (i.e. presence of one or more symptoms of delirium but not meeting criteria for delirium (Cole et al., 2013), it is possible that the AD signature may also be associated with delirium severity across the full sample. Third, to investigate whether a particular ROI was driving the effect for the association between the AD signature and delirium, separate regression models were performed for each of the 9 ROIs (average of left and right) that comprise the AD signature as well as a control region, calcarine cortex. Fourth, the association of AD signature with delirium outcomes was examined after controlling for several potential preoperative confounders: (1) preoperative cognitive function measured by baseline GCP score (Jones et al., 2016); (2) presence of aMCI to test whether observed relationships were driven by extreme phenotypes; (3) presence of vascular comorbidity (Gorelick et al., 2011; Schneider et al., 2002), and (4) presence of depression, which can exist simultaneously with or confer increased risk for both delirium and dementia (Downing et al., 2013). Finally, to reduce the influence of outliers, we Winsorized our data at the 98th percentile, a commonly used approach to handling outliers that preserves their status but reduces the influence of extreme values (Thomas and Ward, 2006).
3. RESULTS
3.1. Sample Characteristics
Participants’ demographic, cognitive, and clinical characteristics are described in Table 1. Thirty-two participants (22%) developed delirium. Nine participants (6%) had aMCI at baseline prior to surgery. The delirium group had greater rates of aMCI at baseline (16% compared to 4%) and, similarly, had lower GCP scores by about 4 points on average. As expected, the delirium group had significantly higher scores on CAM-S peak and CAM-S sum (8 and 20 points higher on average, respectively). Delirium groups also differed by hospital length of stay (patients with delirium stayed for 2 days longer on average) and anesthesia type, although the vast majority of both groups underwent general anesthesia. Groups did not differ on other demographic, clinical, or hospital-related factors.
3.2. AD Signature and Delirium Incidence
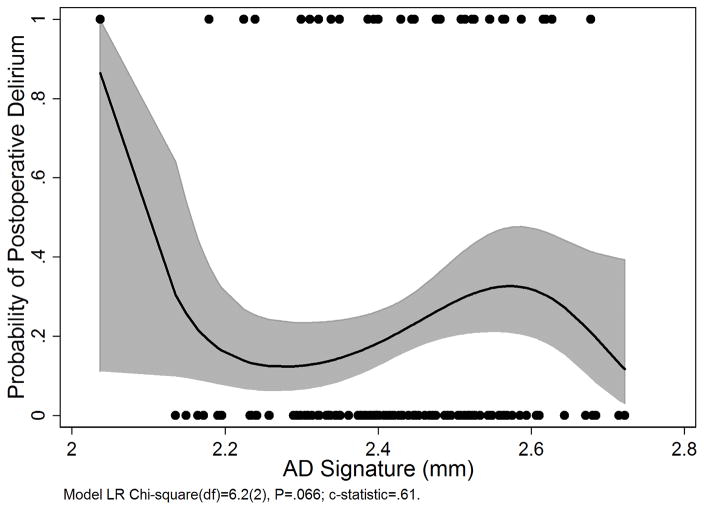
AD signature cortical thickness did not predict incidence of delirium (OR=1.15, 95% C.I. [0.8, 1.6]; Table 2 and Figure 2).
Table 2.
Association between the AD signature and delirium incidence (CAM)
| Model covariates | Odds Ratio (SE) | 95% C.I. | p-value |
|---|---|---|---|
|
| |||
| Age (years) | 1.03 (0.05) | 0.95, 1.13 | 0.40 |
| Female Sex | 1.55 (0.67) | 0.66, 3.62 | 0.32 |
| AD signature (mm) | 1.15 (0.18) | 0.84, 1.57 | 0.38 |
C.I.=Confidence Interval; AD signature=average cortical thickness (mm) in 9 bilateral cortical regions of interest; CAM= Confusion Assessment Method.
Figure 2.
Probability of developing postoperative delirium as a fractional polynomial function of the AD signature (solid black line) with 95% confidence bands (grey shaded area). Black dots are the raw AD signature values for those with (top) and without (bottom) delirium. C-statistic=.61. AD signature=average cortical thickness (mm) in 9 bilateral cortical regions of interest.
3.3. AD Signature and Delirium Severity
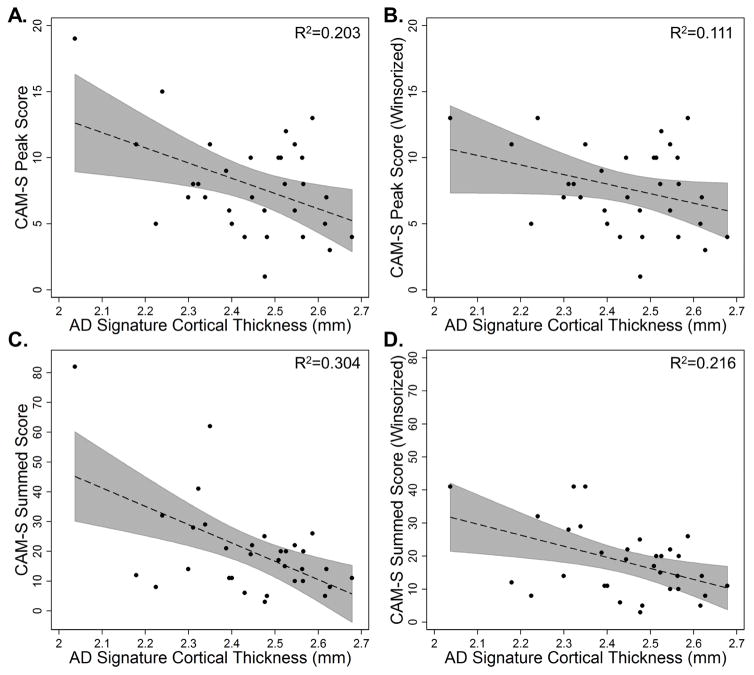
Among those who developed delirium, thinner cortex in the AD signature was associated with greater delirium severity, measured by CAM-S peak (b = −1.2, 95% C.I. [−2.2, −0.3]; Table 3A, Figure 3A) and sum (b = −6.0, 95% C.I. [−9.8, −2.1]); Table 3B, Figure 3C).
Table 3.
Association between the AD signature and delirium severity (CAM-S peak or CAM-S sum) in the delirium group only (N=32)
| Model covariates | Regression Coefficient (SE) | 95% C.I. | p-value | R2 | R2 change* |
|---|---|---|---|---|---|
|
| |||||
| A) CAM-S Peak | |||||
|
| |||||
| Age (years) | −0.03 (0.2) | −0.36, 0.29 | 0.84 | ||
| Female Sex | 0.43 (1.4) | −2.41, 3.27 | 0.76 | ||
| AD signature | −1.2 (0.5) | −2.20, −0.27 | 0.014 | 0.21 | 0.20 |
|
| |||||
| B) CAM-S Sum | |||||
|
| |||||
| Age (years) | 0.01 (0.6) | −1.31, 1.32 | 0.99 | ||
| Female Sex | −2.3 (5.6) | −13.83, 9.30 | 0.69 | ||
| AD signature | −6.0 (1.9) | −9.83, −2.13 | 0.004 | 0.31 | 0.25 |
On average, a 1/10 mm reduction in AD signature cortical thickness is associated with an increase of 1.2 points on the CAM-S peak score and an increase of 6 points on the CAM-S summed score.
C.I.=Confidence Interval; AD signature=average cortical thickness (mm) in 9 bilateral cortical regions of interest; CAM-S= Confusion Assessment Method Severity score (peak=largest single CAM-S rating observed during hospitalization [possible range 0–19]; sum=summed value of CAM-S across all hospitalization days).
R2 change refers to the change in R2 after the AD signature was added to the covariates-only model (only age and sex as independent variables).
Figure 3.
Scatter plots of AD signature cortical thickness (mm) by CAM-S peak (A and B, top) and sum (C and D, bottom) for the delirium group only (N=32). 95% confidence intervals are displayed in gray. Both uncorrected raw (A and C, left) and Winsorized data (B and D, right) are displayed. R2 for the uncorrected association between AD signature cortical thickness and CAM-S scores are reported in the top right corners.
3.4. Additional Sensitivity and Exploratory Analyses
Additional sensitivity and exploratory analysis were performed to test the robustness and further explore our main finding that the AD signature was associated with greater delirium severity in those who developed delirium. Results using Poisson regression were consistent with the primary model using linear regression (CAM-S peak incidence rate ratio = 0.9, 95% C.I. [0.8, 1.0]; CAM-S sum incidence rate ratio = 0.77, 95% C.I. [0.67, 0.89]). In the entire sample of those both with and without delirium, the AD signature was not associated with delirium severity as measured by CAM-S peak (b = −0.1, 95% C.I. [−0.6, 0.3]) or sum (b = −0.8, 95% C.I. [−2.1, 0.55]).
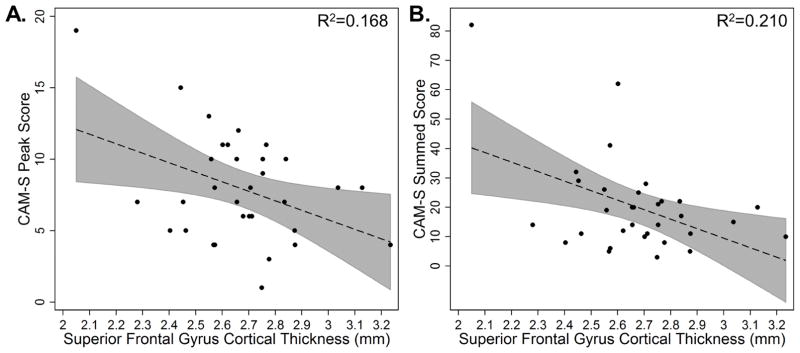
The individual ROIs comprising the AD signature were associated with a similar pattern observed for the entire AD signature: thinner cortex in the ROIs was associated with greater delirium severity (Table 4). However, only the superior frontal gyrus demonstrated a statistically significant effect for both CAM-S peak and sum (Figure 4). Other regions that were significantly associated with CAM-S sum but not peak included middle frontal gyrus, supramarginal gyrus, and superior parietal lobule. A control region, calcarine cortex, which is outside the AD signature, was not associated with delirium severity (Table 4).
Table 4.
Association between the individual ROIs comprising the AD signature and CAM-S peak in the delirium group only (N=32)
| CAM-S peak | CAM-S sum | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| AD signature ROI | Regression Coefficient (SE) | 95% C.I. | p-value | Regression Coefficient (SE) | 95% C.I. | p-value |
|
| ||||||
| Angular gyrus | −0.6 (0.3) | −1.3, 0.03 | .059 | −2.4 (1.4) | −5.2, 0.5 | .097 |
| Middle frontal gyrus | −0.5 (0.5) | −1.4, 0.4 | .27 | −4.2 (1.8) | −7.9, −0.5 | .028 |
| Inferior temporal gyrus | −0.3 (0.3) | −0.9, 0.2 | .21 | −1.7 (1.1) | −3.9, 0.5 | .13 |
| Medial temporal | −0.3 (0.2) | −0.7, 0.11 | .16 | −1.4 (0.8) | −3.0, 0.3 | .10 |
| Precuneus | −0.5 (0.3) | −1.2, 0.2 | .15 | −2.7 (1.4) | −2.0, 0.1 | .057 |
| Superior frontal gyrus | −0.7 (0.3) | −1.2, −0.09 | .026 | −3.1 (1.2) | −5.5, −0.8 | .011 |
| Supramarginal gyrus | −0.6 (0.3) | −1.3, 0.1 | .095 | −3.5 (1.3) | −6.3, −0.8 | .012 |
| Superior parietal | −0.8 (0.5) | −1.8, 0.2 | .099 | −5.8 (1.8) | −9.5, −2.1 | .004 |
| Temporal pole | −0.1 (0.2) | −0.64, 0.35 | .55 | 0.27 (1.0) | −1.8, 2.4 | .80 |
| Calcarine cortex (control region) | −0.3 (0.5) | −1.3, 0.8 | .64 | −0.2 (2.3) | −4.8, 4.5 | .95 |
On average, a 1/10 mm reduction in the cortical thickness of the individual ROIs comprising the AD signature is associated with an increase of 0.1 to 0.8 points on the CAM-S peak score and 0.27 to 9.5 points on CAM-S sum.
AD=Alzheimer’s disease; ROI=Region of Interest; SD=standard deviation; SE=standard error; C.I.=Confidence Interval. Models were corrected for age and sex. Significant results are italicized.
Figure 4.
Scatter plots of cortical thickness of the superior frontal gyrus cortical thickness (mm) by CAM-S peak (A, left) and sum (B, right) in individuals who developed delirium (N=32). 95% confidence intervals are displayed in gray. R2 for the uncorrected association between superior frontal gyrus cortical thickness and CAM-S scores are reported in the top right corners.
After controlling for preoperative cognitive function (measured by GCP), one of the strongest predictors of delirium, the AD signature was still significantly associated with delirium severity (CAM-S peak b = −0.9, 95% C.I. [−1.8, −0.05]; CAM-S sum b = −4.6, 95% C.I. [−7.9, −1.2]). As expected, GCP was also a significant predictor of delirium severity (CAM-S peak b = −0.2, 95% C.I. [−0.4, −0.05]; CAM-S sum b = −1.1, 95% C.I. [−1.7, −0.4). When either GCP or the AD signature were added to the baseline model (covariates of age and sex), GCP explained an additional 25% variance in CAM-S peak and 33% of variance in CAM-S sum (R2 change) while the AD signature explained an additional 20% variance in CAM-S peak and 25% of variance in CAM-S sum. GCP and the AD signature were only mildly correlated in the SAGES MRI total sample (Pearson’s correlation 0.12, p = .14) and were moderately (albeit not statistically significantly) correlated in the delirium subsample (Pearson’s correlation 0.29, p = .11). After controlling for aMCI, the AD signature was still significantly associated with CAM-S peak (b = −1.17, 95% C.I. [−2.2, −0.2) and sum (b = −4.895% C.I. [−8.5, −1.2]).
Neither vascular comorbidity nor depression were significant predictors of delirium severity. Including or excluding vascular comorbidity as a covariate did not affect the primary results (CAM-S peak b = −1.2, 95% C.I. [−2.2, −0.2]; CAM-S sum b = −5.8, 95% C.I. [−10.0, −1.7]). Including depression as a covariate reduced the strength of the association between the AD signature and delirium severity (CAM-S peak b = −1.0, 95% C.I. [−2.1, .05]; CAM-S sum b = −5.2, 95% C.I. [−9.4, −0.9]), but the effect sizes remained in the moderate range (R2 change—here calculated as the change in R2 after adding the AD signature to a model with age, sex, and depression—was 0.11 for CAM-S peak and 0.16 for CAM-S sum).
Winsorization affected two subjects with the highest CAM-S scores for both peak and sum. Analyses using the Winsorized dataset reduced the strength of the association between the AD signature and delirium severity, but effect sizes remained moderate (CAM-S peak b = −0.76, 95% C.I. [−1.6, 0.09], R2 change=0.11; CAM-S sum b = −3.19, 95% C.I. [−5.8, −0.5], R2 change=0.17). Scatter plots of the Winsorized data are displayed in Figure 3B and 3D.
4. DISCUSSION
With the maturation of imaging and fluid biomarkers, the study of AD has undergone a critical paradigm shift by including the preclinical phase. This has important implications for the study of delirium. While dementia is a known risk factor for delirium, a greater understanding of why and how dementia incurs this risk, and when risk begins, is needed. We found that in a sample of patients without dementia that a well-validated biomarker of AD-related neurodegeneration—the AD signature of cortical atrophy—did not predict who would develop postoperative delirium, but did predict delirium severity among those who developed delirium. This suggests that preclinical AD may serve as a vulnerability factor that increases severity once delirium is incited by noxious precipitants. These results provide support for the use of preclinical AD biomarkers, like the AD signature, in delirium research and suggest that one contributor to delirium severity is pre-existing neurodegeneration potentially due to preclinical AD.
Prior studies have found that MCI is associated with greater risk of delirium incidence and severity (Kazmierski et al., 2014; Oldham et al., 2015). Our study showed that the AD signature was not associated with delirium incidence but was associated with delirium severity even after controlling for aMCI, suggesting that a relationship between AD and delirium begins prior to MCI. Furthermore, that the association of delirium severity and the AD signature persisted even after accounting for one of the strongest predictors of delirium, baseline cognitive performance, suggests that the AD signature is not just a surrogate measure of cognitive performance. This supports the notion that a relationship between delirium and AD begins in the preclinical stage of AD, and that the association may in part be related to AD-related cortical atrophy.
When the AD signature was broken down into regional subcomponents, only the superior frontal gyrus was significantly associated with both CAM-S sum and peak. Similarly, Gunther et al. (2012) found that volume loss in this region was associated with longer duration of delirium. The AD signature accounted for more variance than the superior frontal gyrus alone (20% compared to 16% for CAM-S peak; 25% compared to 20% for CAM-S sum) suggesting that while atrophy in this frontal lobe region contributes the greatest vulnerability to delirium severity of the 9 ROIs, the AD signature is still a better predictor of delirium severity. Other regions that were significantly associated with CAM-S sum but not peak included middle frontal gyrus, supramarginal gyrus, and superior parietal lobule, further suggesting that brain vulnerability in frontal and parietal brain regions in particular may predispose individuals to greater delirium severity.
Neuroimaging studies of delirium have been mixed and investigations of underlying brain vulnerabilities are ongoing (Cavallari et al., 2016; Cavallari et al., 2015; Gunther et al., 2012; Root et al., 2013; Shioiri et al., 2016). This literature is likely mixed in part because of the heterogeneous nature of delirium and the variety of imaging analysis methods used. For instance, previous work from our group has shown that diffusion imaging biomarkers are associated with delirium incidence and severity (Cavallari et al., 2016) but brain volumes are not (Cavallari et al., 2015). In this study we examined an MRI measure that is relatively specific for AD-related neurodegeneration. Unlike other neuroimaging measures, such as voxel-based morphometry techniques, cortical thickness used in the AD signature has adequate resolution for interpretation at the individual subject level and is highly reliable within scanner systems and across manufacturers and field strengths (Dickerson et al., 2008). These features make it a reliable measure of atrophy, which could translate to potential clinical applications in individual patients, and may also explain why cortical thickness, but not brain volumes, were associated with delirium severity in this cohort. Moreover, the temporal dynamics of biomarker changes during the course of AD are still being investigated; it is possible that white matter degeneration, cortical thinning, and volumetric atrophy occur in a progressive manner and/or that different degrees of abnormality are needed to detect an association with delirium outcomes. Processes unrelated to AD might contribute to white matter abnormalities or other changes in brain structure and function, thus explaining some of the variability in the observed associations between different neuroimaging biomarkers and delirium.
A recent study of three large population-based cohort studies found that delirium affected cognitive decline independent of the neuropathologic burden of the main dementia pathologies, suggesting that additional unmeasured pathologic processes may be related specifically to delirium (Davis et al., 2017). Our null findings for the association of the AD signature and delirium as well as other studies using AD CSF biomarkers (Witlox et al., 2011) could be explained by more dominant aging-related or delirium-specific pathological processes in the examined cohorts. Future work is needed to better understand how AD pathology may interact with comorbid pathology and to identify specific types of neurological stressors that together may contribute to delirium incidence and/or severity, including those that are specific to delirium, aging- (Bakkour et al., 2013), AD and related disorders. Indeed, a combination of biomarkers will likely be most effective in predicting delirium incidence and severity, and its long-term sequelae.
Delirium affects 29–64% of hospitalized seniors and is preventable in 30–40% of cases through targeted interventions that are feasible and cost-effective (Inouye et al., 2014b). Biomarkers for delirium severity could help to identify individuals at greatest risk of developing severe cases of delirium in order to enroll them in established therapeutic or preventive interventions. This study suggests that biomarkers sensitive to preclinical AD pathology could be beneficial in this effort. It will be important to conduct future studies to determine whether delirium interventions are equally effect (or perhaps more effective) in individuals harboring MCI or preclinical AD.
4.1. Limitations
The primary limitation of this study is sample size along with its attendant risk of type 1 error. We performed sensitivity and exploratory analyses to test the robustness of our results and showed that, despite a small sample size, the association between the AD signature and delirium severity in those who experienced delirium remained when using nonlinear regression models and after controlling for baseline cognitive performance or aMCI; furthermore, this association was not detected in a control region in calcarine cortex. However, controlling for depression and Winsorization of outliers reduced the strength of some associations. While we attempted to measure and control for important baseline influences while avoiding over-controlling for variables that might be intermediaries between delirium and AD (Schisterman et al., 2009), it remains a possibility that residual or unmeasured confounding biased our results. Studies with larger sample sizes will be critical to confirm the robustness of the present findings. Furthermore, in this sample, the presence of aMCI was small (n=9), potentially contributing to the lack of association with delirium incidence; future studies using the AD signature in patients with MCI and delirium will be an important extension of this work.
CSF or PET was not collected as part of the SAGES study so it was not possible to examine protein biomarkers of AD-specific pathology directly. While the AD signature is a well-established and robust imaging biomarker of neurodegeneration in AD, it may be that other AD biomarkers (e.g., CSF or PET measures of tau or Aβ) need to be measured to obtain a complete perspective on the relationship between delirium and preclinical AD. The AD signature is strongly correlated with CSF AD biomarkers (Dickerson et al., 2012, 2013), so it is likely the effects of underlying pathology were at least partly accounted for in the present analyses. Nevertheless, because we only had a biomarker of neurodegeneration, we cannot definitively confirm presence of preclinical AD in this cohort (Dubois et al., 2016; Jack et al., 2016).
Delirium was identified with the CAM supplemented with a validated chart review method rather than DSM-5 criteria. CAM is a widely used measure of delirium and is highly correlated with DSM-5 criteria for delirium (Adamis et al., 2015); but it is possible the method for delirium detection may have affected the present findings. Additionally, delirium was not assessed on the day of surgery or after hospital discharge, thus it is also possible that some episodes of delirium were not captured, and the full duration may have been truncated in some cases. Finally, this sample was well-educated and mostly white, so the results may not be generalizable to more socioeconomic and racially diverse groups. Greater than average cognitive reserve, which can occur in all persons regardless of whether or not they have preclinical AD, may further explain why an association was not observed with delirium incidence. Furthermore, we only examined post-operative delirium and so our results may not be generalizable to other etiologies of delirium.
4.2. Conclusions
Biomarkers for severity of delirium are important to advance the field. Given that considerable evidence suggests that delirium and AD dementia are related, it is possible that AD biomarkers, even in the absence of dementia, may also be useful biomarkers for predicting greater severity of delirium. This study suggests that cortical atrophy related to AD increases brain vulnerability (decreased reserve/resilience) in the face of delirium, resulting in increased delirium severity. Greater delirium severity may be evidence of underlying brain pathology related to AD. Future studies with larger sample sizes and molecular markers for AD are needed to confirm the present results. For instance, an important next step will be to examine delirium within a multidomain biomarker model of preclinical AD including biomarkers for amyloid and tau in addition to neurodegeneration biomarkers like the AD signature (Jack et al., 2016). Future work should also investigate AD biomarkers in the context of other pathophysiological processes related to aging or other neurodegenerative process; in relation to duration of surgery, perioperative factors, and ICU stay; and in relation to short- and long-term cognitive outcomes after surgery. This study represents an important step in furthering our understanding of the complex relationship between preclinical AD and delirium.
Highlights.
Preclinical Alzheimer’s disease (AD) may be an important risk factor for delirium.
A neuroimaging biomarker—‘the AD Signature’—was evaluated in relation to delirium.
Cortical thickness in the AD Signature was not associated with delirium incidence.
In those with delirium, the AD Signature was associated with delirium severity.
Greater delirium severity may be evidence of preclinical AD-related neurodegeneration.
Acknowledgments
The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study.
Funding: This work was supported by National Institute on Aging grants: P01AG031720 (S.K.I.), K07AG041835 (S.K.I.), R24AG054259 (S.K.I.), R01AG030618 (E.R.M.), K24AG035075 (E.R.M.), R01AG051658 (E.R.M.), T32-AG02348 (A.M.R.); 3UL1TR001102 from the National Center for Advancing Translational Sciences (T.G.F.); Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., Co-Trustee (S.M.V.). T.G.T. was in part supported by the Boston Pepper Older Americans Independence Center (P30AG031679). S.K.I. holds the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the design, conduct, or reporting of this study.
Abbreviations
- Aβ
beta-amyloid
- AD
Alzheimer’s disease
- ADL
activities of daily living
- CAM
Confusion Assessment Method
- CAM-S
Confusion Assessment-Severity
- CSF
cerebrospinal fluid
- GCP
General Cognitive Performance
- IADL
instrumental activities of daily living
- IQCODE
Informant Questionnaire on Cognitive Decline in the Elderly
- NIA-AA
National Institute of Aging/Alzheimer’s Association
- OR
odds ratio
- MCI
mild cognitive impairment
- aMRI
amnestic mild cognitive impairment
- MRI
magnetic resonance imaging
- ROI
regions of interest
- SAGES
Successful AGing after Elective Surgery
- VIF
variance inflation
Footnotes
Conflict of interest:
DCA receives post-market institutional royalties for MRI inventions unrelated to the techniques used in this report. All other authors report no conflicts of interest.
5. DISCLOSURES
DCA receives post-market institutional royalties for MRI inventions unrelated to the techniques used in this report. All other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamis D, Rooney S, Meagher D, Mulligan O, McCarthy G. A comparison of delirium diagnosis in elderly medical inpatients using the CAM, DRS-R98, DSM-IV and DSM-5 criteria. Int Psychogeriatr. 2015;27(6):883–889. doi: 10.1017/S1041610214002853. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Levkoff SE, Reilly C, Liptzin B, Pilgrim D, Cleary PD, Evans D, Rowe JW. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5(1):14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72(12):1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–344. doi: 10.1016/j.neuroimage.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari M, Dai W, Guttmann CR, Meier DS, Ngo LH, Hshieh TT, Callahan AE, Fong TG, Schmitt E, Dickerson BC, Press DZ, Marcantonio ER, Jones RN, Inouye SK, Alsop DC, Group SS. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain. 2016;139(Pt 4):1282–1294. doi: 10.1093/brain/aww010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari M, Hshieh TT, Guttmann CR, Ngo LH, Meier DS, Schmitt EM, Marcantonio ER, Jones RN, Kosar CM, Fong TG, Press D, Inouye SK, Alsop DC, Group SS. Brain atrophy and white-matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging. 2015;36(6):2122–2129. doi: 10.1016/j.neurobiolaging.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MG, Ciampi A, Belzile E, Dubuc-Sarrasin M. Subsyndromal delirium in older people: a systematic review of frequency, risk factors, course and outcomes. Int J Geriatr Psychiatry. 2013;28(8):771–780. doi: 10.1002/gps.3891. [DOI] [PubMed] [Google Scholar]
- Davis DH, Muniz-Terrera G, Keage HA, Stephan BC, Fleming J, Ince PG, Matthews FE, Cunningham C, Ely EW, MacLullich AM, Brayne C Epidemiological Clinicopathological Studies in Europe Collaborative M. Association of Delirium With Cognitive Decline in Late Life: A Neuropathologic Study of 3 Population-Based Cohort Studies. JAMA psychiatry. 2017;74(3):244–251. doi: 10.1001/jamapsychiatry.2016.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39(1):10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011a;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA Alzheimer’s Disease Neuroimaging I. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011b;82(1):45–51. doi: 10.1136/jnnp.2009.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA Alzheimer’s Disease Neuroimaging I. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78(2):84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA Alzheimer’s Disease Neuroimaging I. Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-beta and tau. Front Aging Neurosci. 2013;5:55. doi: 10.3389/fnagi.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365. doi: 10.1007/s11920-013-0365-4. [DOI] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR., Jr Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement; Proceedings of the Meeting of the International Working G, the American Alzheimer’s Association on “The Preclinical State of AD; July; Washington Dc U.S.A. 2016. pp. 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823–832. doi: 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S American Heart Association Stroke Council C.o.E, Prevention C.o.C.N.C.o.C.R Intervention Council on Cardiovascular S. Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Jones RN, Fong TG, Tommet D, Inouye SK. Calibration and validation of an innovative approach for estimating general cognitive performance. Neuroepidemiology. 2014;42(3):144–153. doi: 10.1159/000357647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR, 3rd, Canonico A, Merkle K, Cannistraci CJ, Rogers BP, Gatenby JC, Heckers S, Gore JC, Hopkins RO, Ely EW Visions Investigation V.I.S.N.S. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idland AV, Wyller TB, Stoen R, Eri LM, Frihagen F, Raeder J, Chaudhry FA, Hansson O, Zetterberg H, Blennow K, Bogdanovic N, Braekhus A, Watne LO. Preclinical Amyloid-beta and Axonal Degeneration Pathology in Delirium. J Alzheimers Dis. 2016 doi: 10.3233/JAD-160461. [DOI] [PubMed] [Google Scholar]
- Inouye SK. The Confusion Assessment Method (CAM): Training Manual and Coding Guide. New Haven: Yale University School of Medicine; 2003. [Google Scholar]
- Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Jones RN. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014a;160(8):526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, Saczynski JS, Ngo LH, Alsop DC, Jones RN. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014b;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Marcantonio ER, Saczynski JS, Tommet D, Gross AL, Travison TG, Alsop DC, Schmitt EM, Fong TG, Cizginer S, Shafi MM, Pascual-Leone A, Inouye SK. Preoperative Cognitive Performance Dominates Risk for Delirium Among Older Adults. J Geriatr Psychiatry Neurol. 2016 doi: 10.1177/0891988716666380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Rudolph JL, Inouye SK, Yang FM, Fong TG, Milberg WP, Tommet D, Metzger ED, Cupples LA, Marcantonio ER. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychol. 2010;32(10):1041–1049. doi: 10.1080/13803391003662728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychological medicine. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R, Sobow T, Kloszewska I. Mild cognitive impairment with associated inflammatory and cortisol alterations as independent risk factor for postoperative delirium. Dement Geriatr Cogn Disord. 2014;38(1–2):65–78. doi: 10.1159/000357454. [DOI] [PubMed] [Google Scholar]
- Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, Burke JR, Fisher GG, Fultz NH, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Weir DR, Willis RJ. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25(4):181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- Oldham MA, Hawkins KA, Yuh DD, Dewar ML, Darr UM, Lysyy T, Lee HB. Cognitive and functional status predictors of delirium and delirium severity after coronary artery bypass graft surgery: an interim analysis of the Neuropsychiatric Outcomes After Heart Surgery study. Int Psychogeriatr. 2015;27(12):1929–1938. doi: 10.1017/S1041610215001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J Neurosci. 2011;31(48):17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root JC, Pryor KO, Downey R, Alici Y, Davis ML, Holodny A, Korc-Grodzicki B, Ahles T. Association of pre-operative brain pathology with post-operative delirium in a cohort of non-small cell lung cancer patients undergoing surgical resection. Psychooncology. 2013;22(9):2087–2094. doi: 10.1002/pon.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saczynski JS, Kosar CM, Xu G, Puelle MR, Schmitt E, Jones RN, Marcantonio ER, Wong B, Isaza I, Inouye SK. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62(3):518–524. doi: 10.1111/jgs.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO, Jr, Fong TG, Metzger E, Inouye SK, Group SS. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818 e811–810. doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EM, Saczynski JS, Kosar CM, Jones RN, Alsop DC, Fong TG, Metzger E, Cooper Z, Marcantonio ER, Travison T, Inouye SK Successful Aging after Elective Surgery Study G. The Successful Aging After Elective Surgery Study: Cohort Description and Data Quality Procedures. J Am Geriatr Soc. 2015;63(12):2463–2471. doi: 10.1111/jgs.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Bohner H, Habel U, Salloum JB, Stierstorfer A, Hummel TC, Miller C, Friedrichs R, Muller EE, Sandmann W. Risk factors for postoperative delirium in vascular surgery. Gen Hosp Psychiatry. 2002;24(1):28–34. doi: 10.1016/s0163-8343(01)00168-2. [DOI] [PubMed] [Google Scholar]
- Shioiri A, Kurumaji A, Takeuchi T, Nemoto K, Arai H, Nishikawa T. A Decrease in the Volume of Gray Matter as a Risk Factor for Postoperative Delirium Revealed by an Atlas-based Method. Am J Geriatr Psychiatry. 2016;24(7):528–536. doi: 10.1016/j.jagp.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Ward K. Economic profiling of physician specialists: use of outlier treatment and episode attribution rules. Inquiry. 2006;43(3):271–282. doi: 10.5034/inquiryjrnl_43.3.271. [DOI] [PubMed] [Google Scholar]
- van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, Pickkers P. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15(6):R297. doi: 10.1186/cc10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn SM, Marcantonio ER, Gou Y, Pisani MA, Travison TG, Schmitt EM, Jones RN, Inouye SK. Quantifying the Severity of a Delirium Episode Throughout Hospitalization: the Combined Importance of Intensity and Duration. J Gen Intern Med. 2016;31(10):1164–1171. doi: 10.1007/s11606-016-3671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witlox J, Kalisvaart KJ, de Jonghe JF, Verwey NA, van Stijn MF, Houdijk AP, Traast HS, MacLullich AM, van Gool WA, Eikelenboom P. Cerebrospinal fluid beta-amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. J Am Geriatr Soc. 2011;59(7):1260–1267. doi: 10.1111/j.1532-5415.2011.03482.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Swain CA, Ward SA, Zheng H, Dong Y, Sunder N, Burke DW, Escobar D, Zhang Y, Marcantonio ER. Preoperative cerebrospinal fluid beta-Amyloid/Tau ratio and postoperative delirium. Ann Clin Transl Neurol. 2014;1(5):319–328. doi: 10.1002/acn3.58. [DOI] [PMC free article] [PubMed] [Google Scholar]