Abstract
Background
Propionic acidemia is a rare metabolic disorder caused by a deficiency of propionyl- CoA carboxylase, the enzyme converting propionyl-CoA to methylmalonyl-CoA that subsequently enters the citric acid cycle as succinyl-CoA. Patients with propionic acidemia cannot metabolize propionic acid, which combines with oxaloacetate to form methylcitric acid. This, with the defective supply of succinyl-CoA, may lead to a deficiency in citric acid cycle intermediates.
Purpose
The objective of this study was to determine whether supplements with glutamine (400 mg/kg per day), citrate (7.5 mEq/kg per day), or ornithine α-ketoglutarate (400 mg/kg per day) (anaplerotic agents that could fill up the citric acid cycle) would affect plasma levels of glutamine and ammonia, the urinary excretion of Krebs cycle intermediates, and the clinical outcome in 3 patients with propionic acidemia.
Methods
Each supplement was administered daily for four weeks with a two week washout period between supplements. The supplement that produced the most favorable changes was supplemented for 30 weeks following the initial study period and then for a 2 year extension.
Results
The urinary excretion of the Krebs cycle intermediates, α-ketoglutarate, succinate, and fumarate increased significantly compared to baseline during citrate supplementation, but not with the other two supplements. For this reason, citrate supplements were continued in the second part of the study. The urinary excretion of methylcitric acid and 3-hydroxypropionic acid did not change with any intervention. No significant changes in ammonia or glutamine levels were observed with any supplement. However, supplementation with any anaplerotic agents normalized the physiological buffering of ammonia by glutamate, with plasma glutamate and alanine levels significantly increasing, rather than decreasing with increasing ammonia levels. No significant side effects were observed with any therapy and safety labs (blood counts, chemistry and thyroid profile) remained unchanged. Motor and cognitive development was severely delayed before the trial and did not change significantly with therapy. Hospitalizations per year did not change during the trial period, but decreased significantly (p<0.05) in the 2 years following the study (when citrate was continued) compared to the 2 years before and during the study.
Conclusions
These results indicate that citrate entered the Krebs cycle providing successful anaplerotic therapy by increasing levels of the downstream intermediates of the Krebs cycle: α-ketoglutarate, succinate and fumarate. Citrate supplements were safe and might have contributed to reduce hospitalizations in patients with propionic acidemia.
Keywords: Propionic acidemia, organic acidemia, outcome, sodium citrate, anaplerosis, clinical trial
INTRODUCTION
Propionic acidemia is an autosomal recessive disorder caused by deficiency of propionyl CoA carboxylase (EC 6.4.1.3), the enzyme that converts propionyl CoA to methymalonyl CoA with the help of the cofactor biotin [1]. This conversion, which occurs in mitochondria, is part of the pathway for degradation of the amino acids isoleucine, methionine, threonine, and valine, odd chain fatty acids, and cholesterol [1]. Propionic acid also originates from the catabolism of the nucleotides thymine and uracil and from bacterial production of propionate from pyruvate in the gut [1]. Propionyl CoA is eventually converted into succinyl CoA and enters the citric acid (Krebs) cycle for energy production. Propionyl CoA carboxylase is composed of two distinct subunits: α and β, either of which can be defective in propionic acidemia [1]. As a result of defective propionyl CoA carboxylase, propionyl CoA accumulates and combines with oxaloacetate, another intermediate of the citric cycle, to form methylcitric acid, the diagnostic metabolite of propionic acidemia (Fig. 1). Most cases of propionic acidemia present with lethargy progressing to coma from 16 h to weeks after birth, depending on the severity of the enzyme impairment caused by the genetic lesion [1]. Patients can have severe hyperammonemia associated or not with metabolic acidosis [1, 2]. Even when patients are rescued from the hyperammonemic coma, the prognosis is poor since patients can develop life-threatening complications such as pancreatitis or cardiomyopathy [2, 3]. These complications, whose mechanism is unknown, cause severe morbidity and mortality even in optimally treated patients limiting the benefits of early diagnosis by newborn screening programs [2, 3].
Figure 1. Anaplerotic therapy in propionic acidemia.
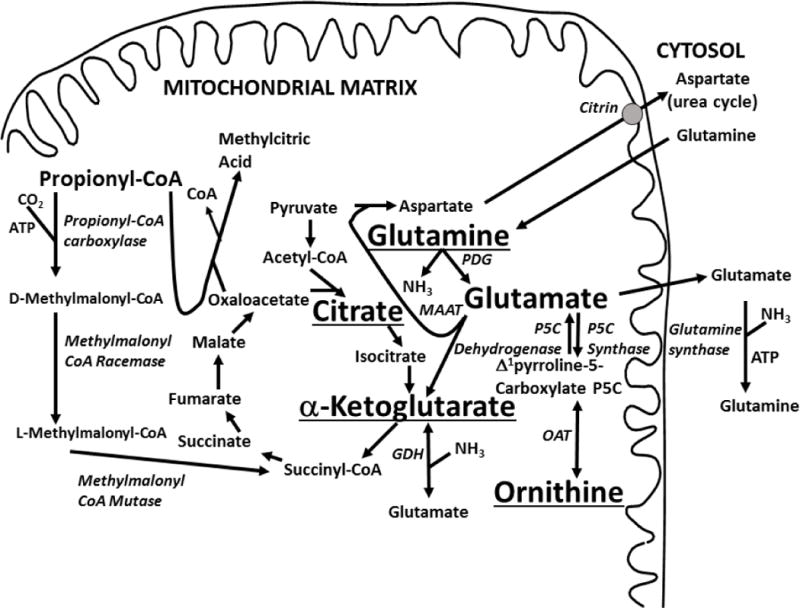
The supplements used in this study (ornithine alpha-ketogluratate, glutamine and citrate) are shown underlined in respect to propionic acid metabolism and the tricarboxylic acid cycle. Propionyl CoA is normally carboxylated by propionyl CoA carboxylase to become D-methylmalonyl CoA. With the action of methymalonyl CoA racemase and mutase, this produces succinyl CoA that can enter the Krebs cycle and contribute to energy metabolism. In propionic acidemia, propionyl CoA accumulates and condenses with oxaloacetate to produce methylcitric acid. The decrease in oxaloacetate and succinyl CoA can impair the Krebs cycle, reducing the concentration of alpha-ketoglutarate. This can be repleted to the expense of glutamine and glutamate. Ornithine can be converted to glutamate by the action of two enzymes, ornithine amino transferase and Δ1pyrroline-5-carboxylate dehydrogenase. GDH: glutamate dehydrogenase; MAAT: mitochondrial aspartate amino transferase; OAT: ornithine amino transferase; PDG: phosphate-dependent glutaminase.
The mechanism at the basis of these long-term complications is unknown. It might be related to toxic effects of metabolites accumulating as a result of the metabolic block (including chronic hyperammonemia) and/or to decreased energy production (by inhibition of the Krebs cycle). In propionic acidemia, glutamine levels are low even in well-controlled patients and decrease (rather than increase) with hyperammonemia [4]. We have proposed that this glutamine paradox, also seen by other groups [5–7], could be due to a dysfunctional Krebs cycle, with deficiency of α-ketoglutarate [4]. In propionic acidemia, glutamate dehydrogenase, normally a cataplerotic enzyme favoring the exit of α-ketoglutarate as glutamate [4], works in reverse generating α-ketoglutarate from glutamate [4]. Low levels of glutamate favor the release of ammonia from glutamine to generate glutamate, explaining the association between high ammonia and low glutamine levels (Fig. 1).
Propionic acid is important in the anaplerosis (filling-up) of the Krebs cycle as indicated by the clinical improvement of patients with fatty acid oxidation disorders and pyruvate carboxylase deficiency treated with heptanoin, an odd-chain fatty acid that is metabolized to propionic acid and converted to succinyl-CoA [4, 8–10]. Since such a mechanism is so effective in replenishing the Krebs cycle, its complete absence in patients with propionic acidemia, coupled with the sequestration of oxaloacetate by propionyl-CoA to form methylcitrate, should result in a severe deficiency of all intermediates of the citric acid cycle. In muscle, α-ketoglutarate is the intermediate with the lowest concentration (0.05 mmol/kg) after oxaloacetate (0.012 mmol/kg, [11, 12]). α-Ketoglutarate concentration further declines with exercise [11] and is regenerated from glutamine and glutamate [11, 12]. If glutamine/glutamate and α-ketoglutarate are too low, such as in propionic acidemia [4], the process might become ineffective in generating energy, possibly contributing to hypotonia and progressive organ failure.
Supplements can replenish substrates to the Krebs cycle. In patients with argininosuccinic aciduria, citrate, the intermediate with the highest concentration in the Krebs cycle (0.362 mmol/kg in muscle, [11, 13]), can generate cytoplasmic aspartate to increase conjugation with citrulline and the urinary excretion of the water-soluble argininosuccinic acid [14, 15]. This action does not require entry of citrate into mitochondria and this therapy is not routinely used given its modest efficacy and possible side effects (metabolic alkalosis, [14]). Citrate in combination with aspartate stabilized the metabolic control of one patient with pyruvate carboxylase deficiency [16] and increased plasma glutamine levels in another patient [9]. Both patients had severe neurological compromise and citrate effects on the overall outcome were difficult to assess given the concomitant use of other medications/supplements [9, 16]. Other natural supplements include glutamate, glutamine, and α-ketoglutarate. Glutamate is mostly (>90%) metabolized by the splanchnic bed, while more than 50% of oral glutamine can reach the circulation and increase glutamate production [17]. Glutamine has been used extensively in the treatment of very low birth weight infants in whom it is extremely safe (up to 0.6 g/kg/day) and prevents protein catabolism [18]. Glutamine (2 g/m2 BID) decreases narcotic requirements and the number of days of intravenous hyperalimentation in cancer patients receiving chemotherapy [19]. α-Ketoglutarate is available as a salt or in combination with the positively charged amino acids arginine and ornithine. The salt form and arginine α-ketoglutarate are widely available as supplements, but only limited clinical trials have been reported [20]. Ornithine α-ketoglutarate has been used since the early 1960s and is more effective than α-ketoglutarate or ornithine alone in increasing glutamine levels in healthy subjects [20]. This is because ornithine can be converted into glutamate through ornithine amino transferase and Δ1pyrroline-5-carboxylate dehydrogenase (Fig. 1) [20, 21]. Ornithine α-ketoglutarate improves the nutritional status in patients with burns and in children receiving total parental nutrition [22, 23]. The combination of ornithine and α-ketoglutarate can spare endogenous glutamine (increasing its plasma levels), feed directly into the Krebs cycle, and participate in the synthesis of urea cycle amino acids [21]. Since in propionic acidemia there is a direct correlation between plasma levels of glutamine/glutamate and levels of the urea cycle amino acids ornithine and arginine [4], the simultaneous administration of ornithine and α-ketoglutarate could have a synergistic effect. Both glutamine and ornithine α-ketoglutarate are extremely well tolerated in humans at doses up to 0.6 g/kg/day and have not caused any known adverse reaction. They are freely available as nutritional supplements to the general public and are marketed mostly as daily supplements for body builders or for general health. The interaction of these supplements (citrate, glutamine and alpha-ketoglutarate) with the Krebs cycle and the metabolism of propionic acid is summarized in Fig. 1.
Here we evaluate the effect of anaplerotic supplements (citrate, glutamine, or ornithine α-ketoglutarate) on biochemical parameters and outcome of patients with propionic acidemia.
PATIENTS AND METHODS
The study was approved by the University of Utah Institutional Review Board and parents signed an informed consent prior to any study procedure. The trial is registered at ClinicalTrials.gov under identifier NCT00645879. Three patients with propionic acidemia followed at the University of Utah since birth were enrolled in this study. Their ages at time of enrollment ranged from 5.9 to 11 years of age. Their diagnosis was confirmed by enzyme assay in fibroblasts or white blood cells showing absent activity (0% of controls) of propionyl-CoA carboxylase and normal activities of pyruvate carboxylase and methylcrotonyl–CoA carboxylase. They all presented within 1 month of age with acute hyperammonemia and required intubation and admission to the intensive care unit. Their developmental quotient/IQ ranged from moderately to severely delayed with the gross motor area being the most affected. Each patient in the 2 years prior to the trial required 2 – 6 hospital admissions per year to control metabolic decompensation or intercurrent illnesses.
Prior to beginning and at the conclusion of the study, each patient received a cognitive evaluation using the Stanford-Binet Intelligence Scales (SB5), Fifth Edition and a motor evaluation. The motor activity assessment included hand-held myometry studies, time-function tests, and an accelerometry evaluation.
The study occurred in two parts. In the first part, individual supplements (ornithine α-ketoglutarate, glutamine, and citrate) were evaluated for their ability to raise plasma glutamine levels, or to cause other biochemical changes as compared to baseline (before the study and during wash-out periods). In the second part, the diet of the patients was supplemented with the supplement judged to produce the most favorable changes (citrate) for 30 weeks. Doses of supplements (ornithine α-ketoglutarate, glutamine, and disodium citrate) were based on patients’ weights at weeks 0, 6, 12, and 18 and were not changed during the corresponding treatment period (4 weeks or 30 weeks). Ornithine α-ketoglutarate 400 mg/kg (2 mmol/kg ornithine, 1 mmol/kg α-ketoglutarate) per day was started at week 0 and continued until week 4. Week 4–6 was a washout period with no supplements for 2 weeks. Glutamine 400 mg/kg (2.74 mmol/kg) per day was started at week 6 and continued through week 10. A second washout period occurred from week 10–12 with no supplements for 2 weeks. At week 12, disodium citrate (7.5 mEq/kg, 2.5 mmol/kg) was started and continued through week 16. The final washout period occurred from week 14–16. At week 18, citrate was started at the same dosing level and continued through week 48 when measurements were repeated, the study was officially concluded, and patients were offered to continue citrate supplements on a clinical basis. All patients continued citrate supplements for more than 2 years following the conclusion of the study.
Study visits occurred each week starting at baseline and continued until week 16, with the exception of two week washout periods. During the final treatment period, week 18–48, study visits occurred every 6 weeks. Each study visit included a physical exam along with anthropometric and vital sign measurements. Diet records were collected and analyzed during the final visit of each trial period (weeks 4, 10, 16, 48). Concomitant medications and therapies were collected and recorded.
After the initial evaluation, parents were given the nutritional supplement to mix in the child’s metabolic formula containing all amino acids except propiogenic amino acids (isoleucine, methionine, threonine and valine). Patients were also receiving a measured amount of natural proteins from regular food. All patients had gastrostomy tubes and received part or all their daily nutrition through bolus or continuous feeds. Following the initial study period, lab results were drawn periodically at clinic visits 2–4 h after feeding. There were no changes in the diet (percent of calories from fats, carbohydrates, natural protein and special formulas) during the study period except for adjustments for the changing body weight.
Study labs included plasma amino acids, acylcarnitine profile, ammonia, lactic acid, urine acylglycines, and urine organic acids (as first void morning urine). Biochemical tests were performed at ARUP laboratories of the University of Utah. Plasma amino acids were analyzed by ion-exchange chromatography with post-column ninhydrin detection (Biochrom 30 amino acid analyzer) [24]. Urinary organic acids were analyzed by gas chromatography-mass spectrometry (HP/Agilent) [25]. Plasma acylcarnitine levels and profile were determined by tandem mass spectrometry (Waters Quattro Premiere) using stable isotope standards for quantitation. Analytes not routinely reported (methylcitrate, propionylglycine in urine) were consistently quantified in each sample.
Plasma amino acids were compared before and after treatment with anaplerotic agents. Baseline study labs (week 0, 6, 12, and 18) were averaged and compared to those obtained during treatment with ornithine α-ketoglutarate (n=4), glutamine (n=4) and citrate (n=4). Data were compared using analysis of variance with p<0.05 as a statistical cutoff. Regression analysis was used to correlate different biochemical parameters. Calculations were performed using Microsoft Excel and SigmaPlot software, version 13.0.
RESULTS
Patients and Safety
Table 1 summarizes the clinical and anthropometric data of patients with propionic acidemia. Three patients participated in this study (age at the beginning of the study ranged from 5.9 to 11 years). Anthropometric measures (weight, height, head circumference, and BMI) did not change significantly from the beginning to the end of the study. BMI improved in all 3 patients during the study, with percentiles that increased in two patients who were underweight at the beginning of the study (from the 22nd to the 50th and from the 3rd to the 46th percentile, respectively) and decreased in a third patient who was overweight (from the 95th to the 91st percentile), though these improvements were not statistically significant.
Table 1. Characteristics of patients with propionic acidemia.
Measurements taken at baseline and at the conclusion of the study period. Results reported as means ± SD.
| Patient | 1 | 2 | 3 | Average: | |
|---|---|---|---|---|---|
|
|
|||||
| Gender | F | F | M | ||
| Race | Hispanic | White | White | ||
| Age (yr) | Before | 5.9 | 11 | 6.8 | 7.9 ±2.7 |
| After | 7.3 | 12.2 | 7.8 | 9.1±2.7 | |
|
| |||||
| Weight, kg (centile) | Before | 15.6 (2) | 45.2 (80) | 20.2 (18) | 27±15.9 (33±41) |
| After | 19.7 (9) | 48.9 (74) | 27.2 (67) | 31.9±15.2 (50±36) |
|
|
| |||||
| Height, cm (centile) | Before | 104.5 (2) | 136.5 (14) | 122 (57) | 121±23 (24±29) |
| After | 112.5 (1) | 143.3 (10) | 132 (80) | 128±22 (30±43) |
|
|
| |||||
| Head circumference, cm (centile) | Before | 47.2 (<3) | 51 (10) | 49.5 (3) | 49.2 ± 1.9 |
| After | 48 (<3) | 51.5 (10) | 50 (3) | 49.8±1.8 | |
|
| |||||
| Body mass Index, kg/m2 (centile) | Before | 14.3 (22) | 24.3 (95) | 13.6 (3) | 17.4 ± 6 (40±48.6) |
| After | 15.6 (50) | 23.8 (91) | 15.6 (46) | 18.3±4.7 (62±25) |
|
| Mutation | Not Done |
PCCB c.429+3_+6delAAGT c.1218_1231delins12 (p.G407Rfs*14) |
PCCA c.1284+1G>A c.2119-1G>C |
||
Safety laboratory studies (hemoglobin, white blood cell counts, platelets, transaminases, alkaline phosphatase, total proteins, albumin, free T4, TSH, amylase, lipase) also did not change during treatment with ornithine α-ketoglutarate, glutamine or citrate. Serum lactate levels also did not change significantly during the study, with very few values above the normal range. As noted in Figure 2A, ammonia levels did not change significantly during the trial as did the urinary excretion of lactate and pyruvate (Figure 2B). Overall, routine lab values were not significantly affected by any of the treatments indicating overall safety of the supplements.
Figure 2. Effect of anaplerotic therapy on (A) plasma levels of glutamic acid (Glu), Glutamine (Gln) their sum (Glu+Gln and ammonia and (B) selected urinary organic acids in patients with propionic acidemia.
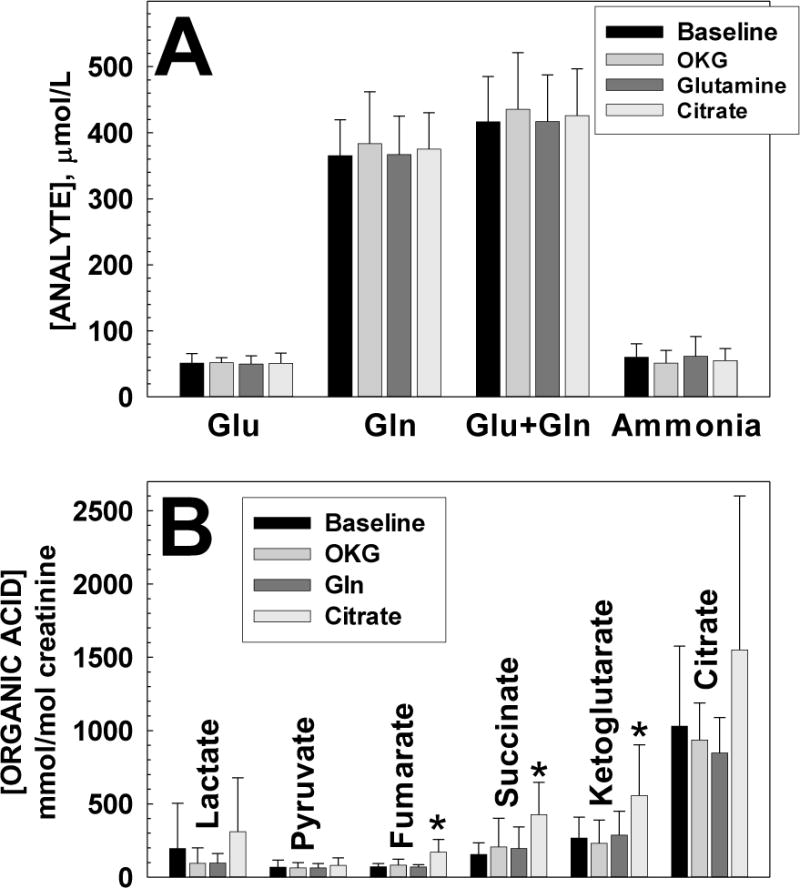
Each value is the average ± SD of at least 12 observations. *p< 0.05 versus baseline.
Amino Acids and Organic Acids
In the first part of the study, patients with propionic acidemia received three different supplements (ornithine α-ketoglutarate (OKG), glutamine (Gln) and citrate) for 4 weeks with blood and urine testing every week. Values obtained in the presence of supplements were compared to baseline (before the initiation of the study and during wash-out periods). None of the treatments significantly increased [glutamine] or [glutamine] + [glutamate] concentration (Figure 2A). Ammonia levels were mildly elevated in all patients (mean value 56.6±21.2 μmol/L, normal for age 21–50 μmol/L) and none of the supplements was effective in normalizing them (Figure 2A). The administration of OKG and glutamine provided extra amino groups, yet there was no increase in ammonia levels (Figure 2A) indicating that the extra nitrogen generated from them was normally disposed.
Since there was no change in glutamine or ammonia levels, we looked at the urinary excretion of Krebs cycle intermediates (urine organic acids) for evidence that the supplements entered mitochondria. The urinary excretion of ketoglutarate did not increase significantly during treatment with ornithine α-ketoglutarate or glutamine, but did increase significantly during citrate therapy (p<0.01) (Figure 2B). The urinary levels of fumarate and succinate also increased significantly during citrate treatment compared to baseline (p<0.001), but not with the two other supplements. Levels of lactate, pyruvate, and citrate also increased during citrate treatment, though this increase was not statistically significant compared to baseline.
Some of the supplements might have affected the accumulation of toxic metabolites in propionic acidemia. The urinary excretion of methylcitrate, the characteristic metabolite of propionic acidemia, 3-hydroxy propionic acid and propionylglycine remained elevated and did not change significantly with any supplement (Fig. 3A,B,C). In addition, the plasma concentration of propionyl (C3-) carnitine remained extremely elevated (normal 0–0.83 μmol/L) and was not modified by any of the therapies (Fig. 3D).
Figure 3. Effect of anaplerotic therapy on the urinary excretion of methylcitrate (A), 3-hydroxy-propionate (B), propionylglycine (C), and ketone bodies (sum of 30hydroxybutyric acid and acetoacetate) (D) in patients with propionic acidemia.
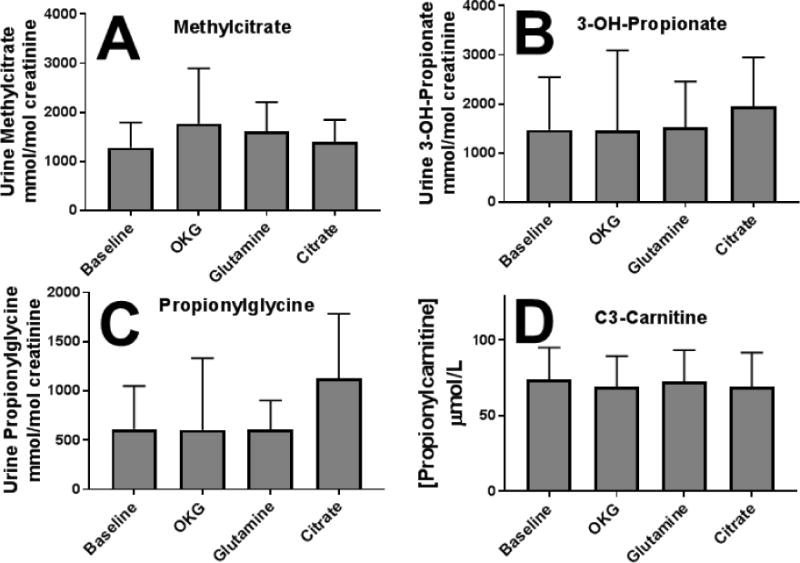
Each value is the average ± SD of at least 12 observations. There were no statistically significant difference between any treatment and baseline.
In summary, citrate, but not glutamine nor ornithine α-ketoglutarate significantly increased the urinary excretion of ketoglutarate, succinate and fumarate, all metabolites produced after citrate entry into mitochondria and its modification by enzymes of the Krebs cycle. At the same time, there was no statistically significant increase in the excretion of lactate and pyruvate, which accumulate when the Krebs cycle is dysfunctional nor a change in the classic metabolites that accumulate in propionic acidemia. Thus, citrate was supplemented in the second part of the study for 30 weeks.
To evaluate changes in plasma amino acids, the results of both parts of the study were grouped together and are summarized in Tables 3 and 4 and Figure 4. Table 3 shows the average values of the 22 amino acids collected at baseline and during each treatment period. The majority of amino acids remained unchanged throughout the study. As expected, ornithine levels increased significantly (p<0.001 compared to baseline) during ornithine α-ketoglutarate supplementation. This indicated that the chemical was successfully absorbed in the gut. However, ketoglutarate excretion in urine did not increase during the ornithine α-ketoglutarate treatment period (Figure 2B) indicating that α-ketoglutarate was either metabolized prior to entry into mitochondria or did not enter the Krebs cycle in sufficient amounts to increase its urinary excretion or that of downstream metabolites.
Table 3. Plasma Amino Acids (μmol/L) in patients with propionic acidemia.
Values are averages ± SD of at least 12 observations.
| Amino Acid | Normal | Baseline | Citrate | Glutamine | OKG |
|---|---|---|---|---|---|
| Alanine | 240–600 | 349±85 | 331±91 | 328±79 | 362±65 |
| Arginine | 40–160 | 42.8±11.7 | 36.5±10.2 | 45.3±21.4 | 41.4±10.9 |
| Asparagine | 15–40 | 31.2±13.1 | 27.6±9.9 | 26±9.5 | 29.2±5.0 |
| Aspartate | 0–20 | 11.1±4.6 | 9.9±2.8 | 9.7±2.5 | 11.7±2.8 |
| Citrulline | 10–60 | 19.6±4.2 | 17.5±4.9 | 21.4±7.9 | 21.9±4.8 |
| Cysteine | 7–70 | 34±7.5 | 29.7±6.9 | 33.5±7.1 | 32.1±7 |
| Glutamine | 410–700 | 365±55 | 375±49 | 367±55 | 391±74 |
| Glutamate | 10–120 | 51.2±14.2 | 50.7±15.6 | 49.7±12.6 | 52±7.3 |
| Glycine | 140–490 | 1268±177 | 1173±244 | 1249±229 | 1376±158 |
| Histidine | 50–130 | 76.9±20.3 | 67.7±22.1 | 60.8±20.2 | 71.9±20.4 |
| Isoleucine | 30–130 | 20.7±10.6 | 20.6±9.5 | 20.7±11 | 23.3±9.5 |
| Leucine | 60–230 | 69.5±35.7 | 60.8±21.7* | 64.3±22.6 | 50.1±15.8 |
| Lysine | 80–250 | 227±76 | 226±68 | 244±103 | 224±82 |
| Methionine | 17–53 | 12.6±3 | 13.5±3.6 | 13.9±3.9 | 12.7±2.9 |
| Ornithine | 20–135 | 40.7±16.9 | 32.6±16 | 37.3±12.7 | 94.3±39.2* |
| Phenylalanine | 30–80 | 42.6±9.5 | 41±7 | 39.4±7.9 | 34.3±5.2* |
| Proline | 110–500 | 252±92 | 308±79 | 264±64 | 284±70 |
| Serine | 60–200 | 142±22 | 132±29 | 132±33 | 147± 7 |
| Taurine | 25–80 | 67.8±15.6 | 60.8±8.8 | 61.2±7.1 | 66.5±10.2 |
| Threonine | 60–220 | 65.3±15.2 | 74±26 | 55.4± 4.2 | 54.1±8.7* |
| Tyrosine | 30–120 | 79.8±20.8 | 67.4±20 | 80.1±21 | 66.1±17.6 |
| Valine | 140–350 | 60.7±25.6 | 67.8±28.2 | 64±26.6 | 55.5± 4.8 |
p < 0.05 (or better) versus baseline. Values outside the normal range are indicated in bold.
Table 4. Correlation of ammonia levels with plasma amino acids in patients with propionic acidemia during the clinical trial.
Statistical significance was calculated using analysis of variance. R2 and p are indicated.
| Amino Acid | R2 | p |
|---|---|---|
| Alanine | 0.07* | 0.041 |
| Arginine | 0.29* | <0.001 |
| Asparagine | 0.01 | 0.380 |
| Citrulline | 0.13* | 0.006 |
| Cysteine | 0.01 | 0.388 |
| Glutamine | 0.02 | 0.282 |
| Glutamine + Glutamate | 0.05 | 0.112 |
| Glutamine + Glutamate + Alanine | 0.09* | 0.026 |
| Glutamate | 0.12* | 0.008 |
| Glycine | 0.06 | 0.074 |
| Histidine | 0.02 | 0.240 |
| Isoleucine | 0.01 | 0.369 |
| Leucine | 0.11* | 0.012 |
| Lysine | 0.52* | <0.001 |
| Methionine | 0.02 | 0.278 |
| Ornithine | 0.05 | 0.108 |
| Phenylalanine | 0.10* | 0.014 |
| Proline | 0.006 | 0.582 |
| Serine | 0.02 | 0.246 |
| Threonine | 0.04 | 0.148 |
| Tyrosine | 0.13* | 0.007 |
| Valine | 0.005 | 0.608 |
p <0.05 or better (indicated in bold).
Figure 4. Correlation of ammonia levels with selected plasma amino acids in patients with propionic acidemia.
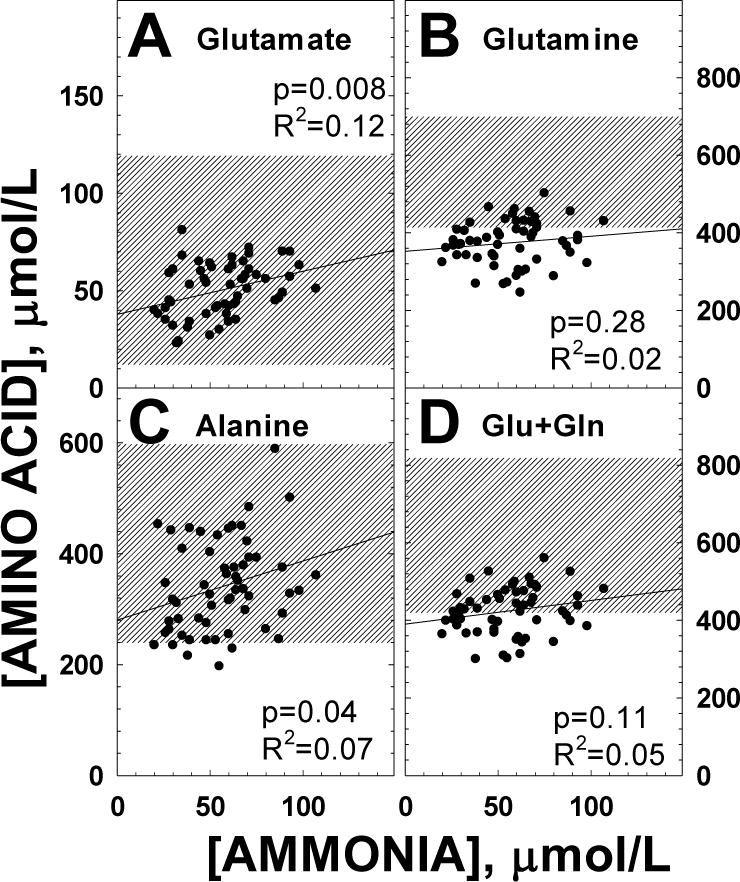
Correlation of ammonia levels with select plasma amino acids in patients with propionic acidemia during the clinical trial. Data were analyzed by linear regression with results indicated in the figure. The shaded area represents the normal range of variation.
Some amino acids (leucine, phenylalanine and threonine) decreased significantly compared to baseline (Table 3) during supplementation with ornithine α-ketoglutarate (p<0.01, p=0.014, and p=0.037, respectively) and leucine decreased significantly (p=0.02) during citrate supplementation. These changes were relatively small and probably reflected mild changes in protein restriction during the trial. As expected, glycine levels were significantly above the normal range and did not change significantly with any treatment. Similarly, levels of isoleucine, methionine and valine were below normal reflecting their restriction in medical foods and did not change with any intervention.
In propionic acidemia, glutamine levels are low even in well-controlled patients and decrease (rather than increase) with hyperammonemia [4]. This might be due to low levels of α-ketoglutarate favoring release of nitrogen from glutamine and glutamate to generate the Krebs cycle intermediate [4]. Since all supplements used in the study could have potentially increased α-ketoglutarate production and reversed this relationship, we explored whether the negative correlation between ammonia and glutamine/glutamate and alanine were still observed during the study. As shown in Figure 4 and Table 4, glutamine levels did not decrease with increasing ammonia levels (panel B) and levels of glutamate (A) and alanine (C) significantly increased (even though the correlation was minimal), rather than decreased with increasing ammonia levels. This suggests that these therapies might have re-established, at least in part, ketoglutarate levels and the physiological mechanism of ammonia buffering by the centrilobular hepatocytes.
In propionic acidemia, levels of ammonia positively correlated with an increase in the amino acids leucine, tyrosine and phenylalanine [4]. During this clinical trial we observed the same trend (Table 4, Figure 5C,E,F). Since these amino acids are essential and provided both by natural proteins and the special formula that patients with propionic acidemia consume, these results suggest that ammonia levels correlated to overall protein intake. In addition to these large neutral amino acids, arginine, citrulline, and lysine increased with ammonia levels (Figure 5A,B,D), with lysine rising in many occasions above the normal range as ammonia increased (Figure 5D).
Figure 5. Correlation of ammonia levels with selected plasma amino acids in patients with propionic acidemia.
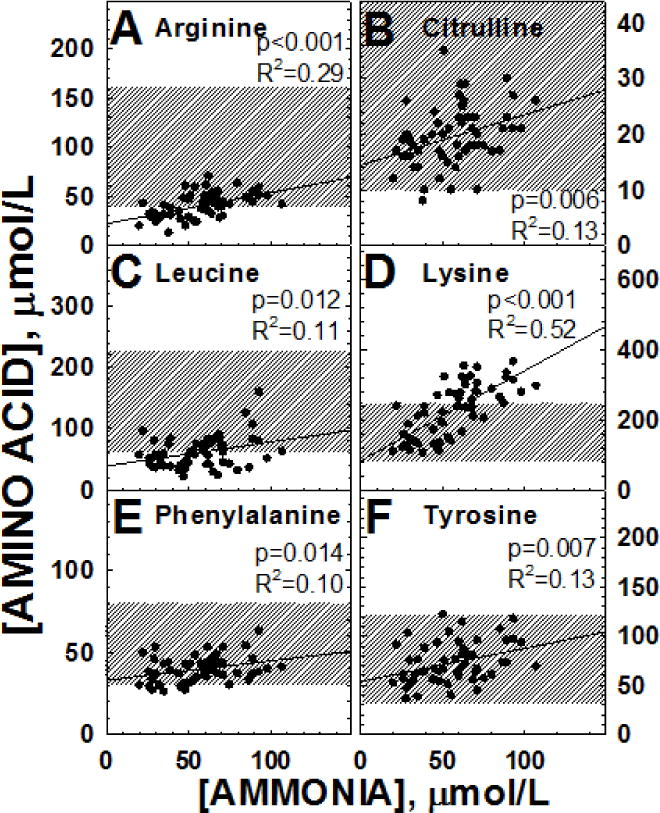
Data were analyzed by linear regression with results indicated in the figure. The shaded area represents the normal range of variation. Note that several lysine levels were above the normal range.
There was no significant correlation between ammonia levels and the remaining amino acids (Table 4).
Cognitive and Motor Assessment
The cognitive assessment (Table 5) indicated that all patients had severely compromised intellectual abilities before the study. There were no changes in nonverbal IQ, verbal IQ, or full scale IQ from the beginning to the end of the study in patients 1 or 2. Patient 3 was too severely affected and unable to complete the evaluation at the beginning and end of the study.
Table 5.
Cognitive Assessment using the Stanford Binet Fifth Edition in patients with propionic acidemia.
| Nonverbal IQ | Verbal IQ | Full Scale IQ | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pt. | Before (Percentile) | After (Percentile) | Before (Percentile) | After (Percentile) | Before (Percentile) | After (Percentile) |
| 1 | 42 (<0.1) | 42 (<0.1) | 43 (<0.1) | 43 (<0.1) | 40 (<0.1) | 40 (<0.1) |
| 2 | 42 (<0.1) | 42 (<0.1) | 43 (<0.1) | 43 (<0.1) | 40 (<0.1) | 40 (<0.1) |
| 3 | Child not sufficiently cooperative | |||||
|
| ||||||
| Average: | 42 (<0.1) | 42 (<0.1) | 43 (<0.1) | 43 (<0.1) | 40 (<0.1) | 40 (<0.1) |
Table 6 shows the results from the motor assessment of patients 1 and 2. Patient 3 was unable to perform the tasks of the assessment. The hand-held myometry composite score increased for both patient 1 and 2 during the course of the study. Results of the time function tests were variable between the patients. Time required to walk/run 10 meters and to climb 4 stairs improved for patient 1, but the time to rise from supine to stand worsened. Patient 2 was unable to complete all testing because she did not have her walker during the final day of testing. The time to climb four stairs worsened for patient 2 during the study. Accelerometry studies showed that both patient 1 and 2 became less active from the beginning to the end of the study with decreases in average steps in 7 days, average minutes of activity in 7 days, and increase in average % of inactivity in 7 days.
Table 6. Evaluation of muscle activity in patients with propionic acidemia.
Results reported at baseline and at the conclusion of the study.
| Pt: | 1 | 2 | ||
|---|---|---|---|---|
| Hand-Held Myometry | Composite Score (lbs) | Before After |
10.85 14.7 |
12.6 12.85 |
| Time Function Test | 10 meter walk/run (sec) | Before After |
7.1 5 |
14.6 NA** |
| Time to rise from supine to stand (sec) | Before After |
3.3 4.2 |
NA** NA** |
|
| Time to climb 4 stairs (sec) | Before After |
4.3 2.7 |
12.9 14.7 |
|
| Accelerometry Tests | Average steps in 7 days | Before After |
7679 5599 |
2192 1891 |
| Average minutes of activity in 7 days | Before After |
496 485 |
247 227 |
|
| Average % of inactivity in 7 days | Before After |
65.5 66.3 |
82.8 84.21 |
Patient 3 was unable to complete the evaluation of muscle activity.
Patient 2 did not bring her walker on the final day of testing and was unable to perform these tasks.
Hospitalizations
To evaluate the effect of treatment on overall health, we counted the number of days that our patients spent in the hospital before, during and after the clinical trial while they were continuing on the supplements (Table 7). Hospitalization days were normalized per year to account for the shorter time span during the study compared to the 2 years before and after the study. The cause of hospitalizations was related mostly to respiratory problems, pancreatitis, or viral infections. In no case were adverse events deemed related to treatment, but rather to the underlying medical condition or intercurrent illnesses. Patients with propionic acidemia did not have any change in the number of days in the hospital during the study. However, the average number of days/year that patients were hospitalized significantly decreased during the 2 years after the study, while the patients continued to supplement with open-label citrate, as compared to the 2 years prior to the study (p=0.04) or during the study (p=0.03). This suggests that citrate therapy might be clinically effective, but might require prolonged time before generating measurable changes. The number of ER visits not leading to hospital admissions during and after the study was also lower than the number of ER visits per year during the 2 years prior to the study, though the changes were not statistically significant.
Table 7. Hospitalizations in patients with propionic acidemia before, during and after anaplerotic therapy.
Average number of days hospitalized and ER visits in patients with propionic acidemia two years prior to study, during study, and two years following study. The number of days spent in the hospital was normalized per year.
| Pt: | Two years prior to study | During study | Two years following study | |||
|---|---|---|---|---|---|---|
| Days Hospitalized | ER visits | Days Hospitalized | ER visits | Days Hospitalized | ER visits | |
| 1 | 19 | 3.5 | 10 | 2.1 | 4.5 | 0.5 |
| 2 | 25 | 0.5 | 32 | 0 | 0 | 0.5 |
| 3 | 2 | 0 | 5.5 | 0 | 1 | 0 |
|
| ||||||
| Average±SD | 15.3±11.9 | 1.3±1.9 | 15.8±14.2 | 0.7±1.2 | 1.8±2.4* | 0.3±0.3 |
| Total: | 46 | 4 | 47.5 | 2.1 | 5.5 | 1 |
p <0.05 versus prior to study using analysis of variance.
DISCUSSION
The objective of this study was to determine whether citrate, glutamine, or ornithine α-ketoglutarate were safe and effective in raising plasma glutamine and reducing plasma ammonia levels compared to baseline in patients with propionic acidemia. All supplements were safe, with no adverse events attributed to them nor changes in safety laboratory values (Table 2). Growth remained appropriate during the study. BMI (kg/m2) increased in patients 1 and 3, who started with a BMI lower than average, and decreased in patient 2, who started with an above average BMI (Table 1). Overall, there were no indications that any of the supplements were detrimental to the patients’ health. Our results in this small patient population are slightly different from those reported in patients with the related condition methylmalonic acidemia in which medical foods have been associated with defective growth [26].
Table 2. Safety Laboratory studies in patients with propionic acidemia.
Values are averages ± SD of at least 12 observations. OKG: Ornithine α-ketoglutarate.
| Labs: | Normal | Baseline | Citrate | Glutamine | OKG |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 11–13.3 | 13.1± 2.1 | 12.8±1.4 | 12.6±1.1 | 12.1±1.0 |
| White Blood Cells (k/uL) | 4.5–10.5 | 5.3±1.5 | 5.2±1.8 | 6.0±2.5 | 5.4±1.0 |
| Platelets (k/uL) | 204–405 | 234±70 | 233±70 | 273±90 | 302±94 |
| AST (U/L) | 20–60 | 60.3±24.4 | 59.9±22.4 | 47.9±11.4 | 44.9±6.7 |
| ALT (U/L) | 5–45 | 45.8±29.6 | 54.2±32.4 | 33.5±15.7 | 35.4±11.5 |
| Alk. Phos (U/L) | 145–320 | 189±57 | 207±44 | 191±53 | 175±51 |
| Total Protein (g/dL) | 5.9–7.0 | 6.7±0.7 | 6.6±0.5 | 6.9±0.6 | 6.7±0.7 |
| Albumin (g/dL) | 3.1–4.2 | 3.9±0.4 | 3.8±0.2 | 3.9±0.3 | 4.0±0.3 |
In the first part of the study, none of the supplements significantly raised plasma glutamine or [glutamine+glutamate] concentration (Figure 2A). Ammonia levels also did not change significantly during therapy with any of the 3 supplements and remained slightly elevated. Despite lack of significant changes in glutamine levels, urine organic acids indicated that treatment with citrate, but not the other supplements, significantly increased the excretion of ketoglutarate, succinate and fumarate (Krebs cycle intermediates, Figure 2B), indicating that citrate entered mitochondria and was converted to subsequent intermediates in the Krebs cycle possibly providing successful anaplerotic therapy. For this reason, treatment with citrate was continued over time (30 weeks) to determine its effects on clinical outcomes and biochemical parameters.
Even with a longer period of time on citrate, therapy failed to significantly increase glutamate or glutamine levels (Table 3). There were no changes in the urinary excretion of methylcitric acid, 3-OH-propionic acid, or propionylglycine, metabolites that accumulate in propionic acidemia and remained markedly elevated (Fig.3). Similarly, plasma levels of propionylcarnitine remained markedly increased, indicating that none of the therapies reduced the production of toxic metabolites (Fig. 3). The only changes that were observed in the plasma amino acids were an increase in ornithine in patients receiving ornithine α-ketoglutarate and a reduction in some essential amino acids, possibly reflecting more strict dietary protein restriction (Table 3). With respect to plasma ammonia, the inverse relationship between plasma ammonia and glutamine levels [4] was lost during the trial (Fig. 5), suggesting that more substrate (ketoglutarate) may have been available to hepatocytes to conjugate ammonia escaping the urea cycle in periportal hepatocytes. At the same time, there was a striking direct correlation between ammonia and lysine levels (R2=0.52, p<0.0001) (Fig. 5). Lysine levels increase in many forms of hyperammonemia and in urea cycle defects. Since lysine is an essential amino acid, its increased levels likely reflect decreased degradation. There are two pathways devoted to lysine breakdown: the saccharopine and the pipecolic acid routes that ultimately converge at the level of al α-aminoadipic semialdehyde [27]. In the saccharopine pathway, that is believed to be the main catabolic route in extracerebral tissue [28], L-lysine is converted to saccharopine by α-aminoadipic semialdehyde synthase by condensation with α-ketoglutaric acid [27]. In the pipecolic acid pathway, transamination also requires an acceptor for ammonia that, in most cases, is α-ketoglutarate. In urea cycle defects, ammonia combines with α-ketoglutarate to generate glutamate and then glutamine, leading to α-ketoglutarate depletion and a secondary increase in lysine levels [29]. In propionic acidemia, it would be the primary depletion of α-ketoglutarate that results in a stable increase in lysine levels [30], which further increase when residual α-ketoglutarate is conjugated with ammonia to generate glutamic acid (Fig. 5). If this is the mechanism, normalization of lysine levels would become a further indicator of successful therapy in patients with propionic acidemia. In addition, mild restriction of dietary lysine could spare ketoglutarate for anaplerotic functions.
Most patients with propionic acidemia have developmental delays. We tested the effects of supplements on cognitive and motor delays. Our patients were so severely affected that not all testing could be completed and the small sample size precluded any statistical analysis (Tables 5 and 6).
While there was no effect on cognitive or motor development, the overall health of patients with propionic acidemia might have benefitted from citrate supplementation. In the 2 years prior to the study, patients were hospitalized 15.3 days per year on average (Table 7). During the study, the number remained at 15.8 days per year. In the 2 years post-study, all three patients remained on open label citrate supplements and the average number of days hospitalized per year decreased significantly to 1.8 (p=0.04 as compared to baseline). The significant reduction in the number of hospitalization days during this time suggests that citrate supplements might improve the general health of patients with propionic acidemia, but that the effects of supplements might require time to become evident. The decreased number of hospitalizations could be due to factors other than therapy. In general, patients with propionic acidemia become more clinically stable as they get older. However, the patient who improved the most (patient 2) in this case was the oldest of the group and was already 11 years old when the study started. All patients improved regardless of age. Patients and their families also had extended contacts with the healthcare team consisting of physicians and dietitians, during the study. Improved education about the disease and consistent monitoring could have helped the management of propionic acidemia in the long term. Our clinical impression was that patients seemed to have shorter hospital stays and improved more rapidly while on citrate supplements.
There are several limitations in our study. Our study involved only three patients with propionic acidemia, a rare metabolic disorder, affecting approximately 1:240,000 in the USA [31]. The results of this pilot study results should be extended to a larger population of patients with a multicenter trial enabling enrollment of an adequate number of patients.
Another factor that may have affected the results of the study may be the dose of the supplement since a single dose was used. On a molar basis, OKG provided 2 mmol/kg ornithine and 1 mmol/kg α-ketoglutarate, glutamine was supplemented at 2.7 mmol/kg, and citrate was supplemented at 2.5 mmol/kg. Therefore, considering that ornithine can be converted into ketoglutarate (Fig. 1), the dosage of the supplements was almost equivalent. However, these dosing levels may not have been sufficient to increase glutamine levels. Specifically, the dose of citrate might have increased intermediates of the citric acid cycle, but might have not been sufficiently high to increase glutamate and glutamine levels. In a fish model of propionic acidemia (the medaka fish, Oryzias latipes), supplements of sodium citrate, ornithine α-ketoglutarate, and glutamine resulted in significant improvements of both survival and locomotor activity [32]. For this reason, higher doses of citrate and possibly other supplements should be tested in future patients with dose-escalation.
CONCLUSION
Propionic acidemia is a rare metabolic disorder caused by deficiency of propionyl-CoA carboxylase. Patients with propionic acidemia generally have a poor prognosis due to health complications including pancreatitis or cardiomyopathy. Deficient breakdown of propionyl-CoA may lead to deficient levels of Krebs cycle intermediates and decreased glutamine levels. Current treatment in patients with propionic acidemia includes a low protein diet with an artificial formula that does not contain the amino acids threonine, valine, leucine, and methionine. Patients receive carnitine supplements to replace propionylcarnitine that is lost in urine. Adding an anaplerotic supplement to their treatment regimen would be a simple, effective way to improve the prognosis.
By supplementing citrate in patients with propionic acidemia, we have shown their ability to increase Krebs cycle intermediates through anaplerosis and (for all supplements) to normalize the relationship between ammonia and glutamate levels. This treatment, whose effects seem incremental to those of existing treatments, appears to be safe and effective in the long term in reducing hospitalizations in patients with propionic acidemia. Further testing of citrate therapy at a higher dose on a larger population might prove useful not only in treating patients with propionic acidemia, but also patients with other organic acidemias in which there is depletion of Krebs cycle intermediates.
Acknowledgments
This work was supported in part by NIH grant 1 R21 DK 077415.
The authors thank all the patients and their families for their participation in this complex protocol.
Grant support: National Institutes of Health grant 1 R21 DK 077415
Clinical trial NCT00645879
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification
The authors have no conflicts of interest.
References
- 1.Carrillo-Carrasco N, Venditti C. Propionic Acidemia. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 2.Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142C:104–112. doi: 10.1002/ajmg.c.30090. [DOI] [PubMed] [Google Scholar]
- 3.DF Dionisi-Vici C, Röschinger W, Rhead WJ, Wilcken B. “Classical” Organic Acidurias, Propionic-Methylmalonic- and Isovaleric-aciduria, long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inerit Metab Dis. 2006;29:383–389. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- 4.Filipowicz HR, Ernst SL, Ashurst CL, Pasquali M, Longo N. Metabolic changes associated with hyperammonemia in patients with propionic acidemia. Mol Genet Metab. 2006;88:123–130. doi: 10.1016/j.ymgme.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Ierardi-Curto L, Kaplan P, Saitta S, Mazur A, Berry GT. The glutamine paradox in a neonate with propionic acidaemia and severe hyperammonaemia. J Inherit Metab Dis. 2000;23:85–86. doi: 10.1023/a:1005659132147. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hassnan ZN, Boyadjiev SA, Praphanphoj V, Hamosh A, Braverman NE, Thomas GH, Geraghty MT. The relationship of plasma glutamine to ammonium and of glycine to acid-base balance in propionic acidaemia. J Inherit Metab Dis. 2003;26:89–91. doi: 10.1023/a:1024048118294. [DOI] [PubMed] [Google Scholar]
- 7.Tuchman M, Yudkoff M. Blood levels of ammonia and nitrogen scavenging amino acids in patients with inherited hyperammonemia. Mol Genet Metab. 1999;66:10–15. doi: 10.1006/mgme.1998.2783. [DOI] [PubMed] [Google Scholar]
- 8.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. The Journal of clinical investigation. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mochel F, DeLonlay P, Touati G, Brunengraber H, Kinman RP, Rabier D, Roe CR, Saudubray JM. Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol Genet Metab. 2005;84:305–312. doi: 10.1016/j.ymgme.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Vockley J, Burton B, Berry GT, Longo N, Phillips J, Sanchez-Valle A, Tanpaiboon P, Grunewald S, Murphy E, Humphrey R, Mayhew J, Bowden A, Zhang L, Cataldo J, Marsden DL, Kakkis E. UX007 for the treatment of long chain-fatty acid oxidation disorders: Safety and efficacy in children and adults following 24weeks of treatment. Mol Genet Metab. 2017;120:370–377. doi: 10.1016/j.ymgme.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 12.Gibala MJ, MacLean DA, Graham TE, Saltin B. Anaplerotic processes in human skeletal muscle during brief dynamic exercise. J Physiol. 1997;502(Pt 3):703–713. doi: 10.1111/j.1469-7793.1997.703bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunengraber H, Roe CR. Anaplerotic molecules: Current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- 14.Renner C, Sewell AC, Bervoets K, Forster H, Bohles H. Sodium citrate supplementation in inborn argininosuccinate lyase deficiency: a study in a 5-year-old patient under total parenteral nutrition. Eur J Pediatr. 1995;154:909–914. doi: 10.1007/BF01957504. [DOI] [PubMed] [Google Scholar]
- 15.Iafolla AK, Gale DS, Roe CR. Citrate therapy in argininosuccinate lyase deficiency. J Pediatr. 1990;117:102–105. doi: 10.1016/s0022-3476(05)82456-4. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad A, Kahler SG, Kishnani PS, Artigas-Lopez M, Pappu AS, Steiner R, Millington DS, Van Hove JL. Treatment of pyruvate carboxylase deficiency with high doses of citrate and aspartate. Am J Med Genet. 1999;87:331–338. doi: 10.1002/(sici)1096-8628(19991203)87:4<331::aid-ajmg10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of glutamine and glutamic acid in humans. Am J Physiol. 1993;264:E848–854. doi: 10.1152/ajpendo.1993.264.6.E848. [DOI] [PubMed] [Google Scholar]
- 18.Kalhan SC, Parimi PS, Gruca LL, Hanson RW. Glutamine supplement with parenteral nutrition decreases whole body proteolysis in low birth weight infants. J Pediatr. 2005;146:642–647. doi: 10.1016/j.jpeds.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Aquino VM, Harvey AR, Garvin JH, Godder KT, Nieder ML, Adams RH, Jackson GB, Sandler ES. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant. 2005;36:611–616. doi: 10.1038/sj.bmt.1705084. [DOI] [PubMed] [Google Scholar]
- 20.Cynober L, Coudray-Lucas C, de Bandt JP, Guechot J, Aussel C, Salvucci M, Giboudeau J. Action of ornithine alpha-ketoglutarate, ornithine hydrochloride, and calcium alpha-ketoglutarate on plasma amino acid and hormonal patterns in healthy subjects. J Am Coll Nutr. 1990;9:2–12. doi: 10.1080/07315724.1990.10720343. [DOI] [PubMed] [Google Scholar]
- 21.Cynober LA. The use of alpha-ketoglutarate salts in clinical nutrition and metabolic care. Curr Opin Clin Nutr Metab Care. 1999;2:33–37. doi: 10.1097/00075197-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Moukarzel AA, Goulet O, Salas JS, Marti-Henneberg C, Buchman AL, Cynober L, Rappaport R, Ricour C. Growth retardation in children receiving long-term total parenteral nutrition: effects of ornithine alpha-ketoglutarate. Am J Clin Nutr. 1994;60:408–413. doi: 10.1093/ajcn/60.3.408. [DOI] [PubMed] [Google Scholar]
- 23.Coudray-Lucas C, Le Bever H, Cynober L, De Bandt JP, Carsin H. Ornithine alpha-ketoglutarate improves wound healing in severe burn patients: a prospective randomized double-blind trial versus isonitrogenous controls. Crit Care Med. 2000;28:1772–1776. doi: 10.1097/00003246-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Moller SE. Quantification of physiological amino acids by gradient ion-exchange high-performance liquid chromatography. J Chromatogr. 1993;613:223–230. doi: 10.1016/0378-4347(93)80136-r. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K, West-Dull A, Hine DG, Lynn TB, Lowe T. Gas-chromatographic method of analysis for urinary organic acids. II. Description of the procedure, and its application to diagnosis of patients with organic acidurias. Clin Chem. 1980;26:1847–1853. [PubMed] [Google Scholar]
- 26.Manoli I, Myles JG, Sloan JL, Carrillo-Carrasco N, Morava E, Strauss KA, Morton H, Venditti CP. A critical reappraisal of dietary practices in methylmalonic acidemia raises concerns about the safety of medical foods. Part 2: cobalamin C deficiency. Genet Med. 2015 doi: 10.1038/gim.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houten SM, Te Brinke H, Denis S, Ruiter JP, Knegt AC, de Klerk JB, Augoustides-Savvopoulou P, Haberle J, Baumgartner MR, Coskun T, Zschocke J, Sass JO, Poll-The BT, Wanders RJ, Duran M. Genetic basis of hyperlysinemia. Orphanet journal of rare diseases. 2013;8:57. doi: 10.1186/1750-1172-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallen A, Jamie JF, Cooper AJ. Lysine metabolism in mammalian brain: an update on the importance of recent discoveries. Amino acids. 2013;45:1249–1272. doi: 10.1007/s00726-013-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamoun P, Richard V, Rabier D, Saudubray JM. Plasma lysine concentration and availability of 2-ketoglutarate in liver mitochondria. J Inherit Metab Dis. 2002;25:1–6. doi: 10.1023/a:1015195009330. [DOI] [PubMed] [Google Scholar]
- 30.Scholl-Burgi S, Sass JO, Zschocke J, Karall D. Amino acid metabolism in patients with propionic acidaemia. J Inherit Metab Dis. 2012;35:65–70. doi: 10.1007/s10545-010-9245-9. [DOI] [PubMed] [Google Scholar]
- 31.Therrell BL, Jr, Lloyd-Puryear MA, Camp KM, Mann MY. Inborn errors of metabolism identified via newborn screening: Ten-year incidence data and costs of nutritional interventions for research agenda planning. Mol Genet Metab. 2014;113:14–26. doi: 10.1016/j.ymgme.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginocchio V, DeFelice E, Barrows FT, Sepe RM, Sordino P, Salierno FG, Conte I, Brunetti-Pierri N. A fish model for propionic acidemia: increased survival and improvement of neurological phenotype by anaplerotic diet. J Inherited Metab Dis. 2016;39(Suppl 1):0–022. [Google Scholar]


