Abstract
Somatic stem cells maintain tissue homeostasis by organizing themselves in such a way that they can maintain proliferative output while simultaneously protecting themselves from DNA damage that may lead to oncogenic transformation. There is considerable debate as to how such stem cell compartments are organized. Burgeoning evidence from the small intestine and colon provides support for a two-stem cell model involving actively proliferating, but injury-sensitive stem cell and a rare, injury-resistant pool of quiescent stem cells. Parallel to this evidence, recent studies have revealed considerable plasticity within the intestinal stem cell compartment. We discuss the evidence for plasticity and hierarchy within the intestinal stem cell compartment and how these properties govern tissue regeneration and contribute to oncogenic transformation leading to colorectal cancers.
Stem cell organization in rapid turnover tissues
High-turnover tissues including the blood, skin, testes, and intestinal epithelium lose millions of cells daily due to basal turnover associated with tissue function and environmental exposure. This tremendous turnover highlights the need for exquisite coordination between self-renewal of upstream stem and progenitor cells and downstream production of differentiated effector cells.
It is becoming increasingly clear that the highest turnover tissues (the intestinal epithelium and hematopoietic system) organize their stem cell compartments into a hierarchical structure with a slow cycling, long-term, injury-resistant stem cell residing at the top of the hierarchy giving rise to an actively cycling stem cell that bears the proliferative burden required for tissue function. The benefits of such an organizational structure include the capacity to maintain the proliferative output necessary to keep up with the demands of high-turnover tissues using a relatively small stem cell pool, the ability to efficiently regenerate the tissue after damage, and the maintenance of regenerative capacity throughout the lifetime of the organism through preservation of stem cell function. Recent studies have not only revealed the existence of this hierarchical stem cell organization, but have also demonstrated considerable plasticity within the hierarchy. Here we discuss the existing evidence for both hierarchical organization and plasticity in the downstream progeny of ISCs, along with the rapid and ongoing shift in our understanding of the cellular identify of the intestinal stem cell niche. We discuss the implications for these findings in an effort to create a framework for understanding how the intestinal epithelium responds to injury and oncogenic transformation, and draw parallels to the more mature data informing our understanding of the hematopoietic stem cell compartment.
Active and reserve stem cells of the intestinal epithelium
As a result of constant exposure to pathogens and xenobiotics, intestinal epithelial cells have a short half-life, and have therefore evolved the ability to rapidly regenerate both during basal homeostasis and in response to injury. The ability to regenerate rapidly after injury makes the intestine an excellent model system to study tissue homeostasis, regeneration and tumorigenesis. In the intestinal epithelium, the most highly proliferative tissue in the body, there is mounting evidence supporting the existence of a hierarchically organized stem cell compartment (Figure 1). Actively proliferating and relatively abundant crypt-base columnar (CBCs) stem cells exhibit high expression of the canonical Wnt pathway target gene Lgr5 and were the first genetically marked intestinal stem cell (ISC) population functionally validated to give rise to all cell-types in the intestinal epithelium through lineage tracing experiments [1] (Table S1). The robust contribution of actively cycling Lgr5+ CBCs to intestinal homeostasis has been clearly shown under basal conditions and may be conceptually analogous to the pool of active hematopoietic progenitors recently described in native hematopoiesis (Box 1) [2]. Remarkably, however, genetic ablation of Lgr5+ cells with diphtheria toxin showed that they are dispensable for intestinal homeostasis under basal conditions [3]. Consistent with this, Lgr5+ cells, and particularly Wnthigh Lgr5high CBCs, like all proliferative cells are quantitatively ablated in response to DNA damaging injury, such as high dose gamma-irradiation [4,5] (Figure 2). Interestingly, diphtheria toxin ablation of Lgr5+ CBCs shortly after or concomitant to radiation injury revealed a requirement for these cells for a robust regenerative response, suggesting that post-injury de novo generated Lgr5+ CBCs and/or a small fraction of Lgr5+ cells that survive the radiation injury play an important role in epithelial regeneration [6]. The expendable nature of Lgr5+ CBCs during intestinal homeostasis and the susceptibility of actively cycling CBCs to DNA damage imply the presence of additional epithelial cells capable of compensating for CBC loss.
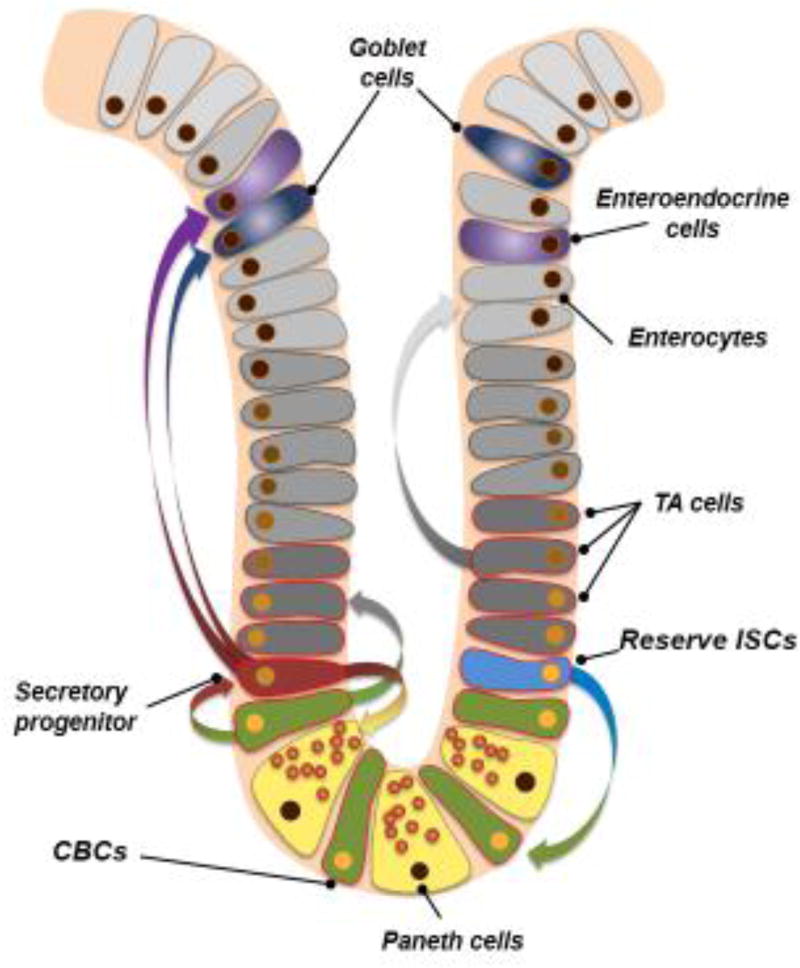
Figure 1.
Intestinal stem cell fate determination under basal conditions. In the resting state, reserve ISCs (blue) periodically divide to give rise to the active crypt base columnar stem cells (CBCs, green). These active CBCs then either produce transit-amplify progeny (T/A cells, dark grey), which go on to divide very rapidly in order to produce large quantities of enterocytes (light grey), or can generate secretory progenitor cells (maroon). These secretory progenitor cells then commit to Paneth, goblet, or enteroendocrine cell lineages (yellow, navy blue, or purple, respectively).
Box.1. Stem cell dynamics in the hematopoietic system.
Hematopoietic stem cells (HSCs) serve as a paradigm for studying adult stem cells. HSCs are defined as cells with the ability to self-renew and the potential to give rise to all hematopoietic lineages [63]. HSCs are a heterogeneous population with subsets of long-term (LT) and short-term (ST) HSCs. LT-HSCs vs ST-HSCs are distinguished by their ability to support hematopoiesis post-transplantation into recipient mice for a lifetime (LT) or a few months (ST), however these definitions are arbitrary and subject to change as the field progresses. ST-HSCs give rise to multipotent progenitor cells (MPPs) that have the potential to give rise to all hematopoietic lineages, but lack long-term self-renewal ability as evidenced by their limited ability to support hematopoiesis post-transplantation. MPPs can further give rise to common myeloid progenitors (CMPs), which produce mega-erythroid progenitors (MEPs) and granulocyte-macrophage progenitors (GMPs). The latter two are the resources of mature megakaryocytes, erythrocytes, granulocytes, and macrophages. On the axis of immune lineages, MPPs can give rise to common lymphoid progenitors (CLPs), and the latter further produce T, B, and NK cells [64,65]. This ‘clonal succession’ model in which a very small number LT-HSCs sitting at the apex of the hematopoietic pedigree generate the entire hematopoietic system at any given time has been dominant in the field for decades. However, this model is based on the transplantation assay, a non-physiological condition that can be considered highly stressful and relies upon a cell’s ability to survive in the circulation and home to the bone marrow. Thus, the transplantation assay may better represent a post-injury regenerative scenario rather than a readout of homeostatic HSC function. Whether basal (or native) hematopoiesis behaviors follow this model has recently been challenged [2].
Recent observations indicate that under basal conditions, LT-HSCs rarely divide over a lifetime [66,67]; and mice can tolerate loss of LT-HSCs for up to 6-months without showing any lineage bias [65]. These observations suggest that LT-HSCs function only as a back-up or reserve subpopulation under basal conditions on shorter-term (6-month) time frames [68,69]. Taking an alternative approach by employing retrotranposition as a means to uniquely barcode individual cells and their lineage, researchers demonstrated that a pool of long-lived progenitors, rather than classically defined LT-HSCs, are the main drivers of basal hematopoiesis during most of adulthood [2]. Our understanding of stem cell activity in the intestinal epithelium is derived largely from in vivo lineage tracing in native (non-transplanted) tissue, however in vitro organoid culture and transplantation assays have recently been pioneered in this tissue. These assays may be more analogous to the transplantation assays used to define the HSC compartment. We should therefore be cognizant of the limitations of each assay, and may find value in comparing results from transplantation versus native stem cell activity in both the blood and intestine.
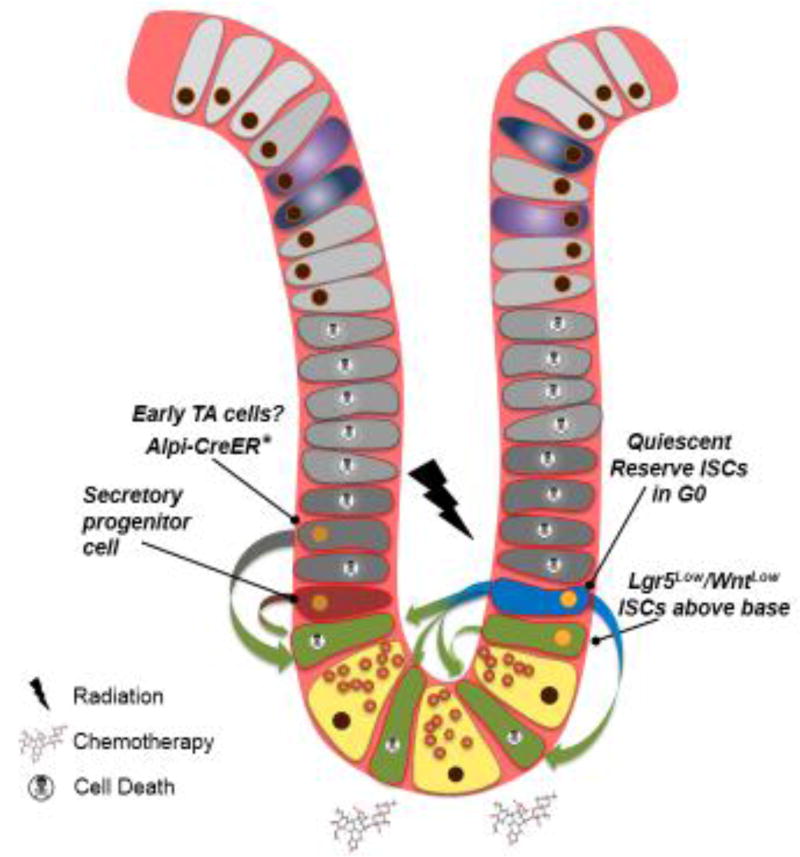
Figure 2.
Intestinal regeneration after injury. Exposure to DNA damaging agents such as high-dose gamma radiation or chemotherapeutics ablates actively cycling cells, including CBCs and transit-amplifying cells. Some cells are able to survive DNA damage, and some of these cells can contribute to the post-injury regenerative process. In response to DNA damage, reserve ISCs enter the cell cycle to replenish the CBC compartment and epithelium. Additionally, some reports indicate that WntLow CBCs above the crypt base as well as cells downstream of the CBC can resist DNA damage-induced cell death and contribute to repopulation, including a rare subpopulation of secretory progenitor cells. Further, in non-physiological injury settings in which CBCs are genetically ablated with diphtheria toxin (asterisk), transit-amplifying cells marked by Alpi-CreER can fall back into the CBC niche and re-establish stem cell identity. The quantitative contribution of secretory progenitors and Alpi-CreER-marked cells to regeneration based on lineage tracing is, however, minimal, and no evidence exists demonstrating its functional importance.
Concomitant to the identification of Lgr5 as a marker of crypt base columnar stem cells, a second population of functionally distinct cells was identified through insertion of a CreER reporter into the endogenous Bmi1 locus [7]. These cells are slower cycling and less frequent than CBCs and, unlike the CBCs, appear indispensable for epithelial maintenance under basal conditions [7]. Since this initial report, numerous additional studies have identified rare cells with shared characteristics of the Bmi1-CreER-marked cells, including those marked by Hopx-CreER [8–10], mTert-CreER [11–13], and likely subpopulations of much larger groups of cells marked by more broadly expressed reporter alleles including Krt19-CreER [14], Lrig1-CreER [15], and Sox9-CreER[16] (Table SI and Figure 1). These rare stem cells are often referred to as ‘reserve ISCs’ based on their slow cycling relative to CBCs [5,7,10,13], their resistance to DNA damage injury [5,8,13,17], as well as their proliferative response and increase in lineage tracing in response to such injury [5,8,13,17] (Figure 2). They have also been denoted as ‘+4’ cells based on their position above the crypt base, although we now appreciate that numerous molecularly and functionally distinct cells reside around this position (Figure 2). They have similarly been referred to as ‘quiescent’ stem cells, although only recently has their residence in the quiescent state outside of the cell cycle (G0) been formally demonstrated [8,17]. Curiously, the endogenous mTert, Hopx, and Bmi1 mRNAs are non-specifically distributed throughout all cells of the crypt base, and thus the presence of the transcripts cannot be taken as evidence of cell identity [10,18,19] (Table S1). Why CreER reporters in all of these instances mark a fairly specific subpopulation of reserve ISCs remains unclear, however it is tempting to speculate that because these CreER insertions disrupt native 3’UTR sequences, the CreER reporters may mark cells in which these loci are actively transcribed, while distribution of endogenous mRNAs may reflect message stabilization and inheritance to daughter cells through activity of the long 3’UTR sequences transcribed from these loci, as has been observed in other stem cell compartments [20]. Interestingly, there are considerable disconnects between cells marked by reporter transgenes, endogenous protein, and mRNA for many intestinal stem cell markers (Table S1), and thus caution must be taken when assigning stem cell identity based on mRNA/protein/reporter expression as most functional demonstrations of stem cell activity rely solely on lineage tracing assays conducted with Cre recombinase.
Single cell expression profiling confirmed an overlap in the molecular identity of the majority of cells marked by Hopx-CreER and Bmi1-CreER, with the Hopx-CreER reserve ISCs being as homogenous a population as that marked by Lgr5-CreER, but also one that is molecularly distinct from CBCs [10]. The population marked by Bmi1-CreER largely overlaps with that marked by Hopx-CreER, although Bmi1-CreER marks a more heterogeneous population, including some presumably differentiated cells within the villi as well as CBCs, with approximately 80% of the population having a signature consistent with an undifferentiated reserve ISC [10]. Indeed, careful analysis of cell position shortly after reporter activation with Bmi1-CreER identifies some cells in the crypt base columnar position, as well as presumptive reserve ISCs above this position and differentiated cells within the villi, consistent with the single cell expression analyses [18,10]. Interestingly, another knockin reporter at the Bmi1 locus, Bmi1-eGFP, marks cells of the endocrine lineage[21], however the degree of overlap, if any, between the Bmi1-eGFP and Bmi1-CreER populations (the latter being used for functional demonstration of stem cell activity) remains unknown. Ultimately, caution should be exercised when interpreting molecular profiles taken from bulk populations, as population heterogeneity may prove misleading.
In contrast to CBCs, the reserve ISCs have little to no detectable readouts of canonical Wnt pathway activity (e.g., expression of Lgr5, Ascl2, Ccnd1, etc.), little expression of genes associated with cellular metabolism and proliferation (e.g., H6PD, Myc, Msi1), and higher expression of genes encoding cell cycle inhibitors (Cdkn1a) [9,10,17,22]. In summary, these reserve ISCs, including those marked by mTert-CreER, share a number of unique functional properties: i) they appear highly resistant to DNA damage (e.g., chemotherapeutics or 12–15Gy of gamma irradiation) [5,8,13,17,23], ii) they reside primarily outside of the cell cycle in G0 [8,17], iii) they appear refractory to stimulation of the canonical Wnt pathway in vivo [5], iv) they routinely give rise to lineages (assessed by crypt-villus lineage tracing) containing all intestinal cell types, including CBCs [3,5,7,10,13,14,17], and v) they are necessary for both epithelial maintenance in the basal state as well as proper epithelial regeneration after injury [7,17] (Table S1).
Importantly, numerous groups have independently confirmed that the WntOFF reserve stem cells give rise to WntHigh CBCs relatively frequently during homeostasis, both through histological lineage tracing techniques and molecular profiling of progeny [3,5,7,10,13,14,17]. An assessment of daughter cell identity from reserve ISCs done by coupling a Hopx-CreER-Lox-Stop-Lox-tdTomato lineage trace with a nuclear H2B-GFP pulse-chase as a measure of cell division suggests that upon division, these reserve stem cells produce CBCs (evidenced by high Lgr5 and Wnt target gene expression) and additional reserve ISCs at similar frequencies, indicating that asymmetric self-renewal/commitment (to CBC identity) divisions may occur [10]. Consistent with this, quantification of clonal exhaustion reveals that CBCs undergo exhaustion much more rapidly in comparison to reserve ISCs, the latter exhibiting no clonal exhaustion after 6 months, while approximately 2/3 of CBC clones undergo extinction over this same period [10]. The culmination of data therefore supports a model in which a long-lived, injury resistant reserve stem cell resides upstream of an active, shorter-lived, Wnt-driven crypt base columnar stem cell (Figures 1 and 3).
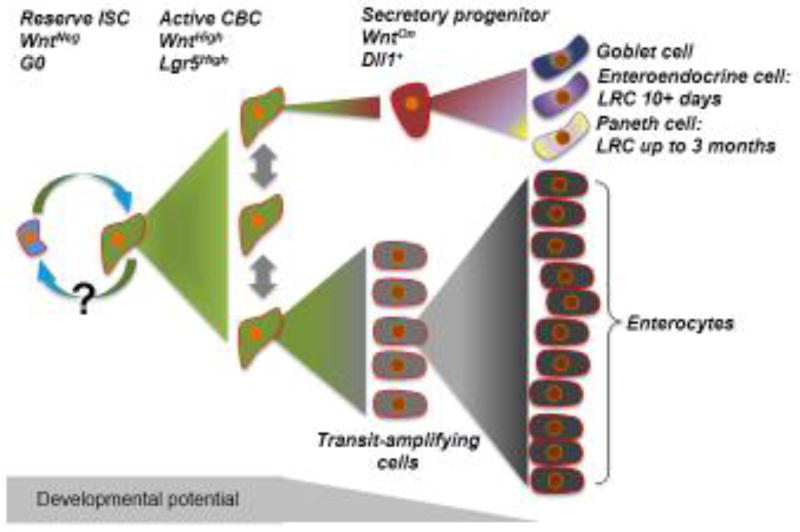
Figure 3.
Model of intestinal stem cell compartment organization. In the hierarchical model, a population of rare stem cells that lack canonical Wnt pathway activity and reside in the quiescent G0 state sits atop the hierarchy. During homeostasis, these cells periodically divide to generate CBCs driven by high Wnt pathway activity and marked by Lgr5 expression. Whether CBCs can re-enter the WntNegative reserve ISC state remains an outstanding question. CBCs divide symmetrically and stochastically give rise to transit-amplifying cells which begin to lose developmental potency as they undergo the massive proliferation required to generate large numbers of short-lived enterocytes. Conversely, CBCs can activate Dll1 expression and commit to the secretory lineage. These secretory progenitor cells retain some developmental potency, even after they exit the cell cycle and retain DNA label while they acquire hallmarks of enteroendocrine and Paneth cell lineages. The secretory progenitor cells can also give rise to goblet cells, a fate decision that is presumably coupled to additional cell divisions as no evidence for goblet cell identity is found in the short-term label retaining cell population. Long-term label retaining cells are terminally differentiated Paneth cells that have lost developmental potency.
Plasticity within the hierarchy
Several recent studies have also identified surprising plasticity in the tissue as well, in which the more committed progeny of CBCs can re-acquire CBC identity, albeit at a low frequency [24] and in the case of non-physiological injury models (such as genetic ablation of specific cell types) [25] (Figure 2). Nonetheless, these studies clearly demonstrate plasticity in cell identity exists within the crypt.
The first evidence of such plasticity arose from studies of label-retaining cells (LRCs). DNA label retention was originally posited to mark cells undergoing asymmetric partitioning of the nascent and parental genomes into distinct daughter cells (originally employing tritiated thymidine as the label). This ‘immortal strand’ hypothesis was posited over 40 years ago to act as a mechanism protecting long-lived stem cells (which, theoretically, retain the parental genome) against replication-induced mutation [26–29]. While this hypothesis is elegant, little evidence supports it, and significant evidence opposes it [30]. Nonetheless, the existence of LRCs within the intestine is clear, but what is less clear is the identity and function of intestinal LRCs. Since the original tritiated thymidine pulse-chase experiments, fusion proteins between fluorophores and histone H2B have become the favored tool for identifying LRCs, as H2B fusions enable prospective identification and isolation of living cells for molecular profiling and functional assays (Table S1). The LRC assay is now used as a means to identify non-dividing cells, with the caveat that truly non-dividing cells are unable to incorporate the label because the label can only be incorporated during S-phase. Thus, LRC labeling is usually performed by pulsing after injury or early during development, when a presumptive dormant stem cell is actively dividing prior to reentering/entering dormancy [29].
The first study to test the potential of LRCs to function as stem cells in the intestine in vivo incorporated the label not after injury or in an immature animal, but during a relatively short pulse in a mature animal [24]. This finding suggests that the LRCs identified by this method represent cells undergoing cell cycle exit downstream of a proliferative cell rather than a dormant stem cell population. Indeed, it is now broadly appreciated that long-term (one month chase or longer) LRCs of the intestine are a homogenous population of terminally differentiated Paneth cells downstream of the CBCs [8,24,29,31]. These long-term LRCs are restricted to the small intestine, consistent with the absence of Paneth cells in the colon. Perhaps more interesting was the population of LRCs found after shorter-term (8–12 days) chase. These cells reside at or near the crypt base, and unlike long-term LRCs, single cell expression profiling reveals this population to be highly heterogeneous, including cells with gene expression signatures consistent with Paneth cells, enteroendocrine cells, as well as very rare cells with reserve ISC molecular identity [8,24]. Indeed, compound reporter mice, where Hopx-CreER marks reserve ISCs and H2B-GFP marks label retaining cells, revealed only a very small fraction of double-positive cells (i.e. label retaining reserve ISCs), and thus these data lead us to conclude that the reserve ISCs and short-term LRCs/secretory progenitor cells are largely mutually exclusive populations [8]. Employing an inducible H2B-Split-Cre system to enable in vivo lineage tracing from LRCs demonstrated extremely rare tracing events, particularly in response to injury (less than 15 clonal tracing events along the entire length of the intestine), consistent with the presence of very rare cells with reserve ISC identity within this short-term LRC population [8,24]. The majority of the short-term LRC population that exhibits hallmarks of Paneth or enteroendocrine cell identity retains the ability to form small intestinal organoids in vitro, albeit at significantly lower efficiencies than purified populations of reserve ISCs [8,24]. When the label retention chase is extended to one month, LRCs are no longer able to form organoids.
The functional and molecular properties of short-term label-retaining secretory progenitor cells are shared with cells marked by a Dll1-CreER allele [32]. These Dll1-CreER-marked cells are the progeny of CBCs, normally generate cells of the secretory lineage, form organoids in vitro in the presence of Wnt3a (which is not required for organoid formation from CBCs or reserve ISCs), and exhibit rare lineage tracing activity in response to mid-dose radiation injury (6Gy gamma-IR). Taken together, these findings support a model where CBCs activate Dll1 as they enter the secretory lineage (in opposition to the transit-amplifying enterocyte lineage, Figure 3) and shortly thereafter exit the cell cycle. These now post-mitotic LRCs retain developmental plasticity for some time, enabling them to re-acquire stem cell activity within a favorable environment (Figures 2 and 3).
The LRCs of the intestine and other non-dividing cells are often referred to as being quiescent [24], however the distinction between true quiescence and differentiation/G1 arrest are unclear, as both can be associated with lack of proliferation. For example, LRCs are referred to as being quiescent, yet the vast majority of these cells are arrested in the G1 phase of cell cycle, typical of a terminally differentiated cell [8]. The definition of quiescence, to be “in a state or period of inactivity or dormancy” implies that i) quiescent cells have a dormant genome (and thus reside in G0 not G1, usually distinguished by global levels of transcription, translation and cellular metabolism) and that ii) quiescent cells can re-enter the cycle and act as a stem/progenitor cell. By this definition, the vast majority of non-dividing cells are not quiescent, and thus drawing this distinction becomes important for creating clear framework for understanding the intestinal stem cell compartment.
Developmental plasticity downstream of the CBC state can also be observed in transit-amplifying (TA) cells committing to the enterocyte lineage (Figure 2). These cells, marked with Alpi-CreER, give rise to full crypt-villus lineage tracing events at low frequency in response to genetic ablation of CBCs using an Lgr5-DTR allele [25]. Similarly, generation of small intestinal organoids followed by Lgr5-DTR CBC ablation results in organoid maintenance from an Alpi-CreER-marked population. While this provides some evidence for plasticity downstream of the CBC state, whether this mechanism functionally contributes to intestinal regeneration in any physiological injury setting is unclear. It might be expected that an injury which kills cycling cells such as DNA damage would ablate both the CBC compartment as well as these Alpi-CreER-marked transit-amplifying cells [4].
Similarly, a recent study employing Bmi1-eGFP as a marker of endocrine lineage cells demonstrated that upon ablation of CBCs, these more committed cells undergo chromatin reorganization reverting to a CBC-like chromatin state, indicating that the functional plasticity can be observed at the level of chromatin organization [21].
Ultimately, the ability of short-term LRCs and transit-amplifying cells displaying hallmarks of lineage commitment downstream of the CBC state to acquire stem cell properties, either forming organoids in vitro or giving rise to clonal lineage tracing events in vivo is a clear demonstration of the plasticity of cellular identity within the intestinal crypt.
Intestinal stem cells and cancer
Colorectal tumors evolve clonally by acquiring a series of mutations that ultimately convert normal epithelial cells to metastatic carcinoma. The initiating mutation, which provides a selective growth advantage to a normal epithelial cell, is thought to be homozygous inactivation of the APC tumor suppressor gene [33,34]. APC is a large protein with diverse cellular functions, including antagonism of canonical Wnt signaling, microtubule nucleation, RNA binding and regulation, among others[35–39]. Acute and broad loss of Apc throughout the epithelium in mouse models results in crypt hyperplasia, a differentiation block, and crypt fission [40]. Subsequent mutations in KRAS, TGF-B, P53, and PI3K pathways occur later and result in clone expansion and progression from small adenoma to malignant invasive carcinoma [40–44].
It is not completely clear whether the heterogeneous population of cells observed in tumors are the progeny of a single undifferentiated cell with multi-lineage differentiation capacity (i.e., a cancer stem cell), or a combination of several cell types with more limited differentiation capacity. Xenotransplantation of a single colon adenocarcinoma cell can reconstitute a tumor containing differentiated cells and cancer stem cells reminiscent of the original tumor, supporting the cancer stem cell hypothesis [45]. However, whether a colorectal cancer stem cell is a transformed descendent of a normal intestinal stem cell, or whether differentiated cells can acquire a cancer stem cell phenotype upon transformation is not known.
The high turnover rate in the intestinal epithelium (with the bulk epithelium being replaced every 4–5 days, and stem cells and some secretory lineage cells surviving for weeks to years) implies that the tumor-initiating cell should have self-renewal capacity or be able to dedifferentiate to a stem cell state. Several studies have shown that loss of Apc/activation of canonical Wnt signaling specifically in intestinal stem cells can lead to adenoma formation. Apc deletion using Lgr5-CreER or Lrig1-CreER and activation of **β-Catenin using Bmi1-CreER results in hyperplasia and adenoma formation [7,15,46]. DCLK1-CreER+ Tuft cells, which have stem cell activity, form adenomas after Apc loss and dextran sulfate sodium-induced inflammation [47]. Adenoma formation in response to ISC-specific modulation of APC/Wnt activity and tumor initiating capacity of ISC-like tumor cells point to ISCs as major contributors to colorectal cancer [48].
The great plasticity of the intestinal epithelium and presence of non-stem cells capable of de-differentiation under certain conditions suggests that there may also be a non-stem cell of origin in colorectal cancer. This is often referred to as ‘top-down’ tumor initiation, in opposition to the ‘bottom-up’ model in which an intestinal stem cell is the tumor cell-of-origin. However, the evidence for adenoma formation from differentiated cells is limited and it normally requires multiple concomitant oncogenic events that are highly unlikely to arise clonally in short-lived differentiated cells during natural tumor ontogeny in humans. Further, models of top-down tumorigenesis also frequently rely on broad oncogene activation (using widely-expressed Cre drivers), rather than clonal activation in single cells as would occur naturally (see, for example [49]).
The fact that most oncogenic mutations in mouse models (both in top-down and bottom-up models) are induced in a population of cells rather than in a single cell (clonally) makes comparison of tumor initiating capacity between cell types and interpretation of tumor formation difficult. For example, genetic ablation of Apc throughout the population of CBCs using Lgr5-CreER results in broad hyperplasia, but whether this hyperplasia can be equated to tumorigenesis is debatable [46]. This is because analogous genetic ablation of Apc clonally (rather than in an entire stem cell population) does not result in such rapid hyperplasia or tumorigenesis. Rather, when Apc is lost in a single stem cell, that cell gains a modest competitive advantage over its Apcwt neighbors, but no hyperplasia or differentiation block are observed [45]. Similar results are seen with clonal activation of Kras. Although mutation of Apc or Kras in a single cell gives that cell a competitive advantage, it does not guarantee the fixation of that clone in the population, and the mutated cell may still be replaced by its wild-type neighbors [45]. This is an example of how presence of a driver mutation does not necessarily lead to tumorigenesis and additional events over long time periods may be required for conversion of a stem cell to a tumor-initiating cell, consistent with the long latency (sometimes decades) of CRC progression, from an aberrant crypt focus to invasive adenocarcinoma. Ultimately, these findings point to the existence of powerful field effects in mouse models of broad oncogene activation, and thus might serve as a cautionary tale for how colorectal cancer is best modeled in animals.
The intestinal stem cell niche
The stem cell niche provides signaling cues that maintain stem cell self-renewal and dictate the balance of stem cell proliferation with differentiation. The importance of Wnt signaling in maintenance of the intestinal epithelium, and specifically CBC function [50,51], raised the question about the cellular identity of the Wnt-secreting niche. Sato et al. originally hypothesized that Paneth cells are the CBC niche because they are intercalated at the crypt base between CBCs, secret Wnt3, EGF, and the Notch ligand Dll4, their co-culture with CBCs enhances organoid formation efficiency from CBCs in vitro, and addition of Wnt3a can substitute co-culturing Paneth cells with CBCs.
Despite these findings, several recent studies have called into question the role of the Paneth cells in supporting CBCs. Paneth cell loss via deletion of Atoh1/Math1 (the master Paneth cell specifying transcription factor) or in other experimental settings such as forced expression of Lin28b has no effect on CBC activity or intestinal homeostasis under basal conditions, nor does it adversely affect regeneration following radiation injury, clearly indicating the presence of other sources of niche factors [52–55]. Moreover, epithelial-specific deletion of Wnt3 or the pan-Wnt processing protein Porcupine (Porcn) using Villin-CreER has no effect on intestinal stem cell activity, suggesting the presence non-epithelial sources of Wnt and other niche factors [56,57].
Turning to potential non-epithelial Wnt sources, deletion of Porcn in myofibroblasts using Myh11-CreER alone or in combination with epithelial deletion using Villin-CreER did not affect intestinal stem cell proliferation or differentiation [58]. In contrast, a recent study showed that rare Foxl1+ cells, which are located just under intestinal epithelium, have mesenchymal characteristics, highly express niche factors such as Wnt and Fgf2, and are distinct from myofibroblasts. Further, their ablation using diphtheria toxin halts proliferation and Wnt signaling activity in the intestinal stem cell compartment leading to tissue failure, in contrast to what is observed upon Paneth cell ablation [59]. This recent study, for the first time, shows the requirement for a single cell type in providing niche signals to CBCs. However, whether the requirement for Foxl1+ cells is due to their production of Wnt ligands or other niche factors remains to be definitively demonstrated.
Concluding remarks
The intestinal epithelium and hematopoietic system represent two of the most proliferative tissues in the body, and thus it might be expected that commonalities exist in our understanding of their stem cell compartments, despite the largely unique approaches taken to study them (e.g., lineage tracing versus transplantation). More recently, tools traditionally used to study the hematopoietic stem cell compartment are being increasingly applied to the intestinal stem cell compartment, including flow cytometry and transplantation assays. One commonality that is becoming increasingly evident is the residence of long-term stem cells in quiescence (G0) outside of the cell cycle. Reserve ISCs have longer clonal lifespans than their active CBC counterparts [10], analogous to the differences observed in LT-HSCs versus ST-HSCs. Similarly, the majority of reserve ISCs (>60%) and LT-HSCs (≈90%) reside in G0, in contrast to active CBCs (<10% in G0)[8,60]. While the molecular underpinnings and functional benefits of maintaining long term HSCs in quiescence has been studied extensively, little is known about this phenomenon in the intestinal stem cell compartment. However, recent data has begun to illustrate common molecular mechanisms controlling quiescence in reserve ISCs and LT-HSCs. For example, activity of PI3K/PTEN signaling was recently shown to play a critical role in governing the activity of reserve ISCs marked by mTERT-CreER. These data indicate that PTEN inhibition drives activation of these reserve ISCs in a model reminiscent of the role of PTEN in HSCs [11,61]. Similarly, studies of the MSI family of RNA binding proteins reveal that MSI activity is both necessary and sufficient for driving proper exit from quiescence and cell cycle entry both in quiescent LT-HSCs and quiescent reserve ISCs marked by Hopx-CreER or Bmi1-CreER [17,62]. Despite this emerging picture of the molecular control of reserve ISC activity, several questions remain unanswered (see outstanding questions). The emerging parallels with HSC biology offer an opportunity to apply our much more mature knowledge of HSC regulation to the nascent field of reserve ISC biology. By looking to the hematopoietic stem cell field, we may be able to better accelerate the development of therapeutic approaches to diseases involving the intestinal stem cells, including radiation enteropathy, inflammatory bowel disorders, and particularly colorectal cancers.
Outstanding Questions.
Do reserve and active ISCs exist in an interchangeable continuum or rigid hierarchy?
Do reserve and active ISCs act differentially as cells-of-origin in colorectal cancer?
Does clonal exhaustion occur in active ISCs in a manner analogous to active hematopoietic progenitors?
Can the transition between reserve ISCs in G0 and active ISCs in cell cycle be therapeutically targeted in degenerative diseases such as inflammatory bowel disease and radiation enteropathy, and in colorectal cancers?
What is the extent of the parallels in the molecular control of reserve ISC dormancy and long-term hematopoietic stem cell quiescence?
Does residence in the quiescent, G0 state outside of the cell cycle protect ISCs against DNA damage?
Supplementary Material
Trends.
Parallels between the long-term hematopoietic stem cell and reserve intestinal stem cell are emerging
At least two robust populations of intestinal stem cells (ISCs) contribute to homeostasis: A WntHigh active ISC and a WntNegative quiescent reserve ISC
Genetic ISC proxy reporter alleles mark heterogeneous populations of cells
Plasticity can be observed in more committed epithelial cells upon return to a vacated crypt base niche
Reserve ISCs exist primarily in G0 and are able to re-enter the cell cycle, while long-term (1 month+) label retaining cells are arrested in G1 and cannot
Glossary
- Label retaining cells
DNA label retention is used to identify non-dividing cells, as DNA label is not exchanged in interphase, and only diluted upon mitosis. This assay has the caveat that in order to incorporate the label initially, a cell must be dividing.
- Tritiated thymidine pulse-chase experiments
A method of label retention. Giving cells a short exposure (pulse) of radiolabeled thymidine such as tritiated thymidine followed by a chase period in the absence of labeled nucleotide is a way to assess rate of cell division. Highly proliferative cells rapidly lose the label due to its dilution during DNA replication. Proliferative cells that undergo differentiation, senescence, or enter G0 immediately after incorporation of the label retain the label for the longer periods of time.
- Lineage tracing
Irreversibly activating a label (usually a fluorescent protein) in a stem and progenitor cell results in all of its progeny being labeled, thus allowing for assessment of the differentiation and/or self-renewal capacity of the initially labeled cell, depending on the time between labeling and tissue harvest.
- Quiescence
Quiescence is a period of resting or dormancy. In a cell, this can imply that a cell is not dividing, however in modern stem cell biology quiescence is used to describe a cell residing outside of the cell cycle (G0), where it is metabolically inactive, often characterized by low rates of global transcription, translation, and respiration.
- Stem cell niche
Stem cell niche is an external microenvironment that provides stem cells with signaling molecules that regulates their fate (self-renewal, quiescence, cycling, differentiation, etc).
- DNA damaging injury
Cancer treatments such as radiotherapy and chemotherapy are efficiently kill dividing cells by inducing extensive DNA damage that exceeds the cell’s capacity for repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao S, et al. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J. 2015;34:624–640. doi: 10.15252/embj.201490700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan KS, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. U. S. A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metcalfe C, et al. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, et al. Mouse Label-Retaining Cells Are Molecularly and Functionally Distinct From Reserve Intestinal Stem Cells. Gastroenterology. 2016;151:298–310.e7. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda N, et al. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, et al. Single-cell analysis of proxy reporter allele-marked epithelial cells establishes intestinal stem cell hierarchy. Stem Cell Rep. 2014;3:876–891. doi: 10.1016/j.stemcr.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richmond CA, et al. Dormant Intestinal Stem Cells Are Regulated by PTEN and Nutritional Status. Cell Rep. 2015;13:2403–2411. doi: 10.1016/j.celrep.2015.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breault DT, et al. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10420–10425. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery RK, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asfaha S, et al. Krt19(+)/Lgr5(−) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell. 2015;16:627–638. doi: 10.1016/j.stem.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell AE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roche KC, et al. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149:1553–1563.e10. doi: 10.1053/j.gastro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousefi M, et al. Msi RNA-binding proteins control reserve intestinal stem cell quiescence. J. Cell Biol. 2016;215:401–413. doi: 10.1083/jcb.201604119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz J, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent “+4” cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itzkovitz S, et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol. 2011;14:106–114. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crist CG, et al. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11:118–126. doi: 10.1016/j.stem.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Jadhav U, et al. Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory Precursors into Lgr5+ Intestinal Stem Cells. Cell Stem Cell. 2017 doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, et al. Heterogeneity in readouts of canonical wnt pathway activity within intestinal crypts. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2016;245:822–833. doi: 10.1002/dvdy.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinkum KL, et al. Fasting protects mice from lethal DNA damage by promoting small intestinal epithelial stem cell survival. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E7148–7154. doi: 10.1073/pnas.1509249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buczacki SJA, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 25.Tetteh PW, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Potten CS, et al. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-x. [DOI] [PubMed] [Google Scholar]
- 27.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 28.Potten CS, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differ. Res. Biol. Divers. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 29.Potten CS, et al. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 30.Kiel MJ, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth S, et al. Paneth cells in intestinal homeostasis and tissue injury. PloS One. 2012;7:e38965. doi: 10.1371/journal.pone.0038965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Es JH, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fearnhead NS, et al. The ABC of APC. Hum. Mol. Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 34.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 35.Mili S, et al. Genome-wide screen reveals APC-associated RNAs enriched in cell protrusions. Nature. 2008;453:115–119. doi: 10.1038/nature06888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preitner N, et al. APC is an RNA-binding protein, and its interactome provides a link to neural development and microtubule assembly. Cell. 2014;158:368–382. doi: 10.1016/j.cell.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith KJ, et al. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- 38.Munemitsu S, et al. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- 39.Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 40.Sansom OJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogelstein B, et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 43.Markowitz S, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 44.Samuels Y, Waldman T. Oncogenic mutations of PIK3CA in human cancers. Curr. Top. Microbiol. Immunol. 2010;347:21–41. doi: 10.1007/82_2010_68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermeulen L, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 47.Westphalen CB, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Invest. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merlos-Suárez A, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Schwitalla S, et al. Intestinal Tumorigenesis Initiated by Dedifferentiation and Acquisition of Stem-Cell-like Properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Kuhnert F, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. U. S. A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 52.Durand A, et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc. Natl. Acad. Sci. U. S. A. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim T-H, et al. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3932–3937. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sailaja BS, et al. The regulatory niche of intestinal stem cells. J. Physiol. 2016;594:4827–4836. doi: 10.1113/JP271931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madison BB, et al. LIN28B promotes growth and tumorigenesis of the intestinal epithelium via Let-7. Genes Dev. 2013;27:2233–2245. doi: 10.1101/gad.224659.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farin HF, et al. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 57.Kabiri Z, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Dev. Camb. Engl. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 58.San Roman AK, et al. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Rep. 2014;2:127–134. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aoki R, et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pietras EM, et al. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 62.Kharas MG, et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat. Med. 2010;16:903–908. doi: 10.1038/nm.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weissman IL, et al. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 64.Kostetskii I, et al. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 2005;96:346–354. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- 65.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 66.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 67.Bernitz JM, et al. Hematopoietic Stem Cells Count and Remember Self-Renewal Divisions. Cell. 2016;167:1296–1309.e10. doi: 10.1016/j.cell.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haug JS, et al. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell. 2008;2:367–379. doi: 10.1016/j.stem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.