Abstract
Heat shock protein beta-1 (HSPB1), is a ubiquitously expressed, multifunctional protein chaperone. Mutations in HSPB1 result in the development of a late-onset, distal hereditary motor neuropathy type II (dHMN) and axonal Charcot-Marie Tooth disease with sensory involvement (CMT2F). The functional consequences of HSPB1 mutations associated with hereditary neuropathy are unknown. HSPB1 also displays neuroprotective properties in many neuronal disease models, including the motor neuron disease amyotrophic lateral sclerosis (ALS). HSPB1 is upregulated in SOD1-ALS animal models during disease progression, predominately in glial cells. Glial cells are known to contribute to motor neuron loss in ALS through a non-cell autonomous mechanism. In this study, we examined the non-cell autonomous role of wild type and mutant HSPB1 in an astrocyte-motor neuron co-culture model system of ALS. Astrocyte-specific overexpression of wild type HSPB1 was sufficient to attenuate SOD1(G93A) astrocyte-mediated toxicity in motor neurons, whereas, overexpression of mutHSPB1 failed to ameliorate motor neuron toxicity. Expression of a phosphomimetic HSPB1 mutant in SOD1(G93A) astrocytes also reduced toxicity to motor neurons, suggesting that phosphorylation may contribute to HSPB1 mediated-neuroprotection. These data provide evidence that astrocytic HSPB1 expression may play a central role in motor neuron health and maintenance.
Keywords: Motor Neuron Disease, Neuroprotection
INTRODUCTION
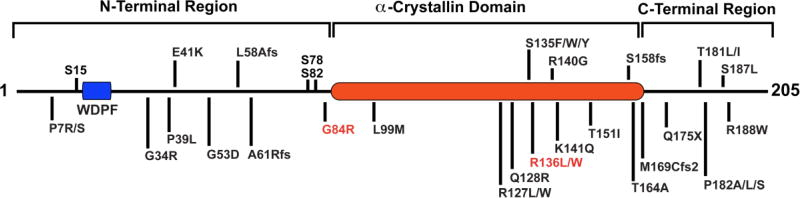
Heat shock protein beta-1 (HSPB1/Hsp27) is a member of the conserved molecular chaperone family of small heat shock proteins (sHSPs). This ubiquitously expressed, 27kDa protein contains the hallmark α-crystallin domain of sHSPs, along with flexible N- and C-terminal regions. HSPB1 is a multifunctional protein with roles in protein aggregation (1–3), apoptosis (4–8), cytoskeletal maintenance (9–11), and gene transcription (12–14). Mutations in HSPB1 result in late-onset, distal hereditary motor neuropathy type II (dHMN) and axonal Charcot-Marie Tooth disease (CMT2F) (15–21). These neuropathies are characterized by length-dependent axonal degeneration of peripheral nerve axons, resulting in sensory loss, muscle wasting, and weakness (18, 22). To date, 31 mutations have been identified in patients located throughout the protein (Figure 1). The specific functional consequences of mutations on cellular mechanisms are unknown. Overexpression of neuropathy-associated HSPB1 mutations, mutHSPB1, disrupts neurofilament assembly (23), and causes motor neuron toxicity and degeneration (24). In vitro studies have suggested that mutations in HSPB1 alter its chaperone activity, however this does not hold true for all currently identified mutations (25, 26).
Figure 1. Domain structure of human HSPB1.
Domain structure of human HSPB1 showing dHMN and CMT2F associated point mutations. S15, S78 and S82 are the major sites of phosphorylation. Mutations in red were studied.
To study mutant HPSB1 in vivo, we and others have developed mouse models of HSPB1-associated CMT2F. Overexpression of mutHSPB1 in neurons results in the development of a very mild phenotype, with only electrophysiological deficits (27), or a more severe phenotype with behavioral and electrophysiological deficits (28, 29). Peripheral nerves from the latter model displayed decreased abundance of acetylated α-tubulin, and DRG neurons cultured from these mice exhibited axonal degeneration and impaired axonal transport (28). Remarkably, treatment of these mice with small molecules inhibiting histone deactylase 6 increased acetylated α-tubulin levels, corrected the axonal transport deficits and improved the CMT2F phenotype in mutant HSPB1 mice (28). It is not clear how these overexpression models reflect pathology under physiologic conditions, and it is notable that mouse models expressing mutHSPB1 at endogenous levels in all cell types failed to develop a neuropathy phenotype (30). While missense mutations in HSPB1 result in hereditary sensory-motor neuropathy affecting primarily the lower motor neuron, wild type HSPB1, HSPB1(WT), has neuroprotective properties in an additional disease of the motor neuron, amyotrophic lateral sclerosis (ALS) (31–36).
ALS is an adult-onset, progressive motor neuron disease that is characterized by degeneration of upper and lower motor neurons in the motor cortex, brain stem and spinal cord (37). 10% of ALS cases follow a dominant inheritance pattern, and are classified as familial ALS (fALS), with the other 90% being termed sporadic ALS (sALS). Approximately 20% of fALS cases are a result of mutations in the gene encoding Cu/Zn superoxide dismutase 1 (SOD1) (38). The exact mechanism of SOD1-mediated toxicity remains unclear, however most evidence suggests that mutations confer a toxic gain of function due partly to the propensity of mutant SOD1 to misfold and aggregate with itself and other proteins. In vitro studies have demonstrated that HSPB1 and HSPB5, another small heat shock protein, can reduce the rate of mutant SOD1 aggregation in a concentration dependent manner (36). Overexpression of HSPB1 in SOD1-astrocyte cell cultures subjected to oxidative stress resulted in a 2-fold increase in cell viability, and reduced mutant SOD1 aggregation (31). Additionally, viral delivery of HSPB1 and HSP70 to ND7 and dorsal root ganglion cells expressing mutant SOD1 reduces cell death in response to serum deprivation and a variety of other lethal stimuli (34). To test if HSPB1 is neuroprotective in vivo, SOD1(G93A)-ALS mice have been crossed with mice overexpressing HSPB1 in two laboratories. These studies yielded different results. Krishnan et al. found no neuroprotective effect in SOD1(G93A)-HSPB1 double transgenic mice (33), while Sharp et al. found that overexpression of HSPB1 delayed disease progression and increased motor unit survival (35). Recent human data points to a possible neuroprotective role; two HSPB1 variants of uncertain significance were identified in individuals with apparent sALS, further indicating a potential link between ALS and HSPB1 malfunction (39).
Additional supporting evidence that HSPB1 is neuroprotective in SOD1-ALS involves the endogenous levels of Hsp25 (murine HSPB1) that are reliably upregulated in transgenic mouse models of SOD1(G93A)-ALS (33, 40, 41). This is also true in human post-mortem spinal cord from ALS patients (40). These HSPB1 positive cells were predominately glial in nature. Moreover, astrocytic inclusion bodies in ALS patient spinal cords co-stain for SOD1 and HSPB1 (42), and inclusion bodies isolated from various mutant SOD1 mouse models contain heat shock proteins (HSPs), including HSPB1. In this study, we utilized an in vitro, astrocyte-motor neuron co-culture system (43–46) to study the consequences of overexpression of wild type or mutHSPB1 in SOD1(G93A) astrocytes. We find that overexpression of wild type HSPB1 in SOD1(G93A) astrocytes improves survival of co-cultured motor neurons, and that motor neuropathy-linked mutations in HSPB1 attenuate this neuroprotection. Further, we show that expression of mutHSPB1 in wild type astrocytes has no effect on motor neuron survival. Finally, our data suggests that HSPB1 phosphorylation may be important for its non-cell autonomous neuroprotection.
RESULTS
Murine HSPB1 is upregulated in mouse neural progenitor cell-derived SOD1(G93A) astrocytes
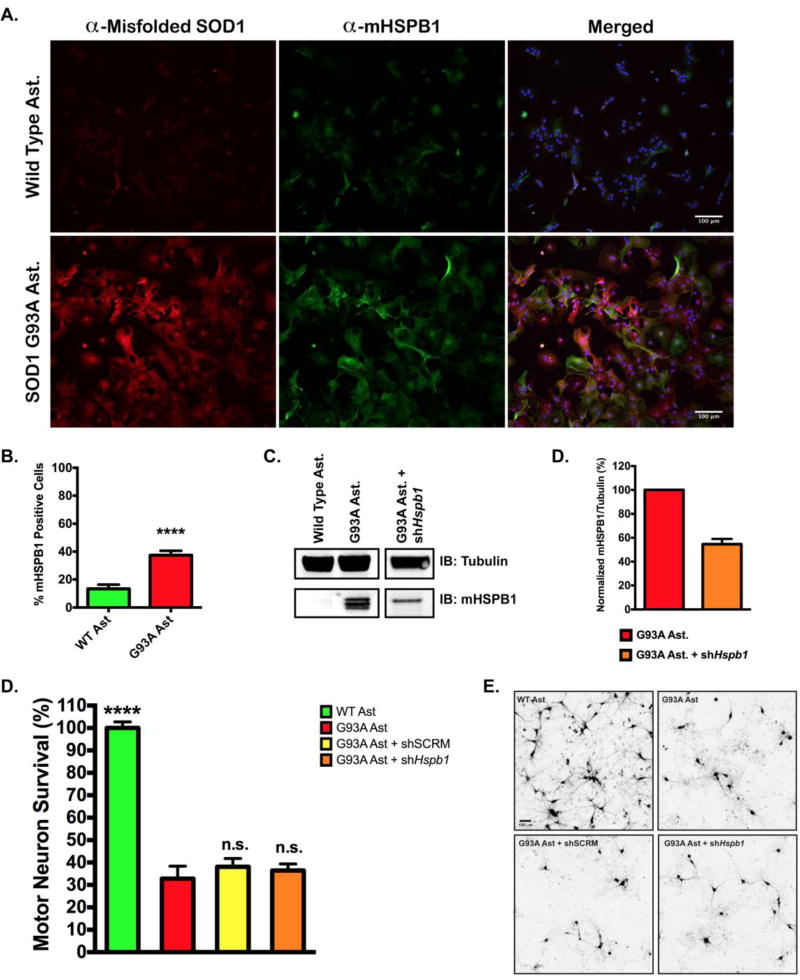
To determine whether endogenous Hsp25 (murine HSPB1, herein referred to as mHSPB1) protein expression is increased in neural progenitor cell (NPC) derived astrocytes, we prepared total cellular lysates from both wild type and SOD1(G93A) astrocytes. mHSPB1 protein levels were measured by semi-quantitative western blot using an antibody specific to murine HSPB1 (Figure 2C). Endogenous mHSPB1 levels were enriched in lysates from SOD1(G93A) astrocytes compared to wild type astrocytes. Immunofluorescent staining of mHSPB1 similarly showed upregulation in SOD1(G93A) astrocytes. 13.25 ± 3.05% of wild type astrocytes were positive for mHSPB1, whereas 37.40 ± 3.18% of SOD1(G93A) astrocytes were positive for mHSPB1 (Figure 2A, 2B). Co-staining, with antibodies specific to misfolded SOD1 (47–51), revealed that SOD1 and mHSPB1 are visualized in the cytoplasm, and that 41.03 ± 3.25% of mHSPB1 positive astrocytes were also positive for misfolded SOD1.
Figure 2. Knockdown of murine HSPB1 in SOD1(G93A) astrocytes has no effect on motor neuron survival.
A. Immunofluorescent staining of murine HSPB1 (mHSPB1) and misfolded SOD1 in wild type and SOD1(G93A) mouse astrocytes. Nuclei were visualized with DAPI. B. Percentage of wild type or SOD1(G93A) astrocytes stained positive for mHSPB1. Cells were counted in 5 random fields of view and expressed as the number of mHSPB1 positive cells over total cells. C. Representative immunoblot of mHSPB1 protein levels in wild type and SOD1(G93A) mouse astrocytes (left panel) and SOD1(G93A) astrocytes infected with lentiviral shRNA against Hspb1. D. Quantification of mHSPB1 immuno-staining in SOD1(G93A) astrocytes and SOD1(G93A) astrocytes transduced with shHspb1. mHSPB1 signal intensity was normalized to mHSPB1 signal intensity from SOD1(G93A) astrocyte lysates. Error bars denote s.e.m. E. MN survival at day 6 of MN co-culture assay with wild type, SOD1(G93A), SOD1(G93A) + sh Hspb1 RNA or SOD1(G93A) + shSCRM RNA astrocytes. MN survival was normalized to counts from MNs cultured with wild type astrocytes (N = 3 for all groups, each n was run in triplicate). Error bars denote s.e.m. ****P<0.0001, ns, non-significant. F. Representative images of HB9-GFP expressing MNs (shown in black) after 6 days in co-culture with wild type astrocytes, SOD1(G93A) astrocytes, and SOD1(G93A) astrocytes transduced with shSCRM or shHspb1.
Our group, and others, have shown that SOD1(G93A) astrocytes exhibit non-cell autonomous toxicity to motor neurons (MNs) in vitro (43, 45, 52). To determine if mHSPB1 expression in astrocytes affects MN survival, we used lentiviral-mediated knockdown to reduce mHSPB1 protein levels by approximately 50% in SOD1(G93A) astrocytes (Figure 2C, 2D). These astrocytes were then co-cultured with wild type MNs (Figure 2E, 2F). There was no observable difference in survival at 6 days between MNs cultured with shHspb1 astrocytes, 36.44 ± 5.86%, or a scrambled control shRNA, 38.07 ± 3.75%, versus non-transduced SOD1(G93A) astrocytes, 32.81 ± 5.57%, suggesting that reduced levels of mHSPB1 protein do not affect SOD1(G93A) astrocyte-mediated MN toxicity.
Overexpression of HSPB1(WT) in SOD1(G93A) astrocytes improves motor neuron survival
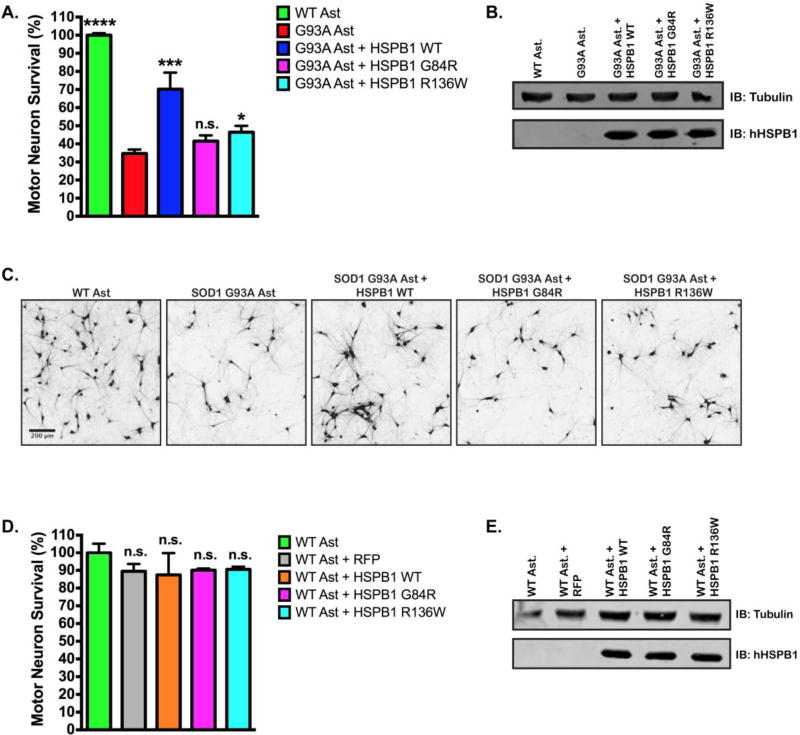
Next, we determined whether human HSPB1(WT) overexpression in SOD1(G93A) astrocytes could improve MN survival in our in vitro co-culture model. Using lentiviral transduction, we overexpressed a FLAG-tagged HSPB1(WT) construct in SOD1(G93A) astrocytes, which resulted in a 100% increase in MN survival compared to non-transduced SOD1(G93A) astrocytes, 70.18 ± 9.19% vs. 34.66 ± 2.21% respectively (P<0.0005) (Figure 3A, Figure S1A). Previous, in vitro studies found that HSPB1 expression can influence neurite maintenance and growth (53, 54). Consistent with previous reports, we also found that motor neurons cultured on SOD1(G93A) astrocytes exhibit reduced soma size and neurite length compared to motor neurons cultured on wild type astrocytes (Figure S1B and S1C). Interestingly, the soma size of MNs cultured on SOD1(G93A) astrocytes overexpressing HSPB1(WT) did not differ from astrocytes bearing the SOD1(G93A) mutation alone, but neurite length was increased by 75% when MNs were cultured in contact with SOD1(G93A) astrocytes overexpressing HSPB1(WT) (Figure S1B and S1C).
Figure 3. Mutations in HSPB1 attenuate non-cell autonomous motor neuron protection.
A. MN survival at day 6 of MN co-culture assay with wild type, SOD1(G93A), SOD1(G93A) + HSPB1(WT), SOD1(G93A) + HSPB1(G84R), SOD1(G93A) + HSPB1(R136W) astrocytes. MN survival was normalized to counts from MNs cultured with wild type astrocytes. (n = 3 for all groups, each n was run in triplicate.) B. Representative immunoblot confirming the expression of human HSPB1 constructs in SOD1(G93A) astrocytes. Western blots were probed with an antibody specific to human HSPB1 C. Representative images of HB9-GFP expressing MNs (shown in black) after 6 days in co-culture with wild type astrocytes, SOD1(G93A) astrocytes, and SOD1(G93A) astrocytes transduced with HSPB1(WT), HSPB1(G84R) or HSPB1(R136W). D. MN survival at day 6 of MN co-culture assay with wild type, WT+RFP, WT + HSPB1 (WT), WT + HSPB1(G84R), WT + HSPB1(R136W) astrocytes. MN survival was normalized to counts from MNs cultured with wild type astrocytes. (n = 3 for all groups, each n was run in triplicate.) E. Representative immunoblot confirming the expression of HSPB1 constructs in wild type astrocytes using an antibody specific to human HSPB1. Error bars denote s.e.m. ***P<0.0005, *P<0.01, n.s., non-significant.
Previous studies have demonstrated that overexpression of SOD1(G93A) in primary cortical astrocyte cultures was toxic to motor neurons, but did not affect the viability of the astrocytes (55, 56). To confirm this in our experiments, we compared the viability of wild type astrocytes to non-transduced SOD1 (G93A) astrocytes and SOD1 (G93A) astrocytes transduced with HSPB1 (WT) (Figure S2). We observed no difference in the viability between cell lines, with 97.13 ± 0.80% viable wild type astrocytes and 96.74 ± 0.98% viable SOD1 (G93A) astrocytes at day 9 in culture. Similarly, transduction of SOD1 (G93A) astrocytes with HSPB1 (WT) had no effect on astrocyte viability compared to a mock transduction, 92.22 ± 1.57% vs. 92.31 ± 0.68% respectively, suggesting that the observed increase in motor neuron survival in our co-culture assay is not from an alteration in astrocyte viability.
Since mutations in HSPB1 result in motor neuropathy, we examined the consequences of mutHSPB1 overexpression on motor neuron survival in the SOD1(G93A) co-culture system. We selected two mutations to study, a glycine to arginine mutation near the phosphorylation sites in the N-terminal region, HSPB1(G84R) and an arginine to tryptophan mutation in the α-crystallin domain of the protein, HSPB1(R136W) (Figure 1, mutations in red) and overexpressed FLAG-tagged versions of these isoforms in astrocytes via lentiviral transduction. These mutations were selected for their divergent effects on HSPB1 chaperone activity, with HSPB1(G84R) exhibiting reduced chaperone activity (57) and HSPB1(R136W) exhibiting increased chaperone activity in vitro (26). In contrast to the overexpression of wild type HSPB1 in SOD1(G93A) astrocytes, overexpression of HSPB1(G84R) failed to alter MN survival compared to non-transduced astrocytes, 41.50 ± 3.19% vs. 34.66 ± 2.21%, (P>0.1), while overexpression of HSPB1(R136W) resulted in a mild increase in MN survival, 46.38 ± 3.56%, vs. 34.66 ± 2.21%, (P<0.01) (Figure 3A, 3B, 3C), indicating that both mutations interfere with the protective effect of HSPB1 in our co-culture system.
To determine whether the expression of the mutant HSPB1 isoforms in astrocytes had a negative effect on motor neurons, we next overexpressed mutHSPB1 in wild type astrocytes and recorded MN survival. Wild type astrocyte lines overexpressing RFP, HSPB1(WT), HSPB1(G84R) or HSPB1(R136W) were generated and co-cultured with wild type MNs. Expression of RFP has previously been shown to have no effect on MN survival (58), and was used as a control for toxicity associated with infecting astrocytes with a viral vector. Similar to RFP expressing astrocytes, overexpression of HSPB1(WT) or mutHSPB1 constructs resulted in an ~10% loss of MN survival compared to non-transduced astrocytes (Figure 3C, D). These results indicated that specific overexpression of mutHSPB1 in wild type astrocytes had little effect on motor neuron survival in co-culture.
Overexpression of HSPB1 does not alter SOD1 localization or protein levels in astrocytes
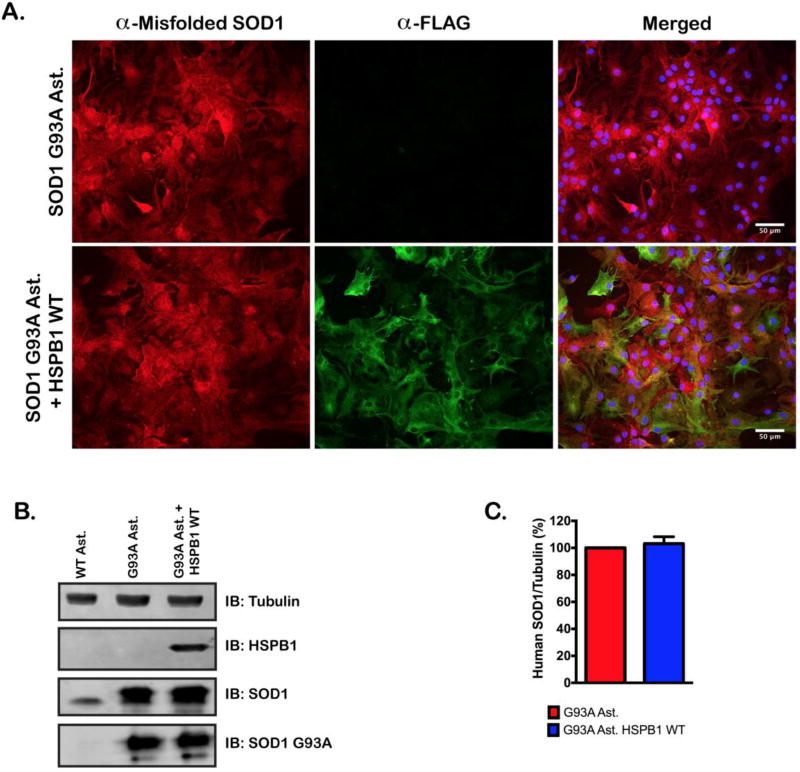
We addressed the possibility that HSPB1 increases MN survival through altering SOD1 localization and expression. We examined the immunohistochemical staining pattern for HSPB1 and misfolded SOD1 in both SOD1(G93A) and SOD1(G93A) + HSPB1(WT) astrocyte lines. Staining for misfolded SOD1 was indistinguishable between the two cell lines (Figure 4A). To examine SOD1 protein levels in a more quantitative fashion, we compared SOD1 and misfolded SOD1 protein expression in total cellular lysates from our wild type, SOD1(G93A) and SOD1(G93A) + HSPB1(WT) astrocytes by western blot. There was no difference in the amount of misfolded SOD1, or SOD1 protein in astrocytes overexpressing HSPB1(WT) (Figure 4B, 4C), suggesting that overexpression of HSPB1 has no effect on total SOD1 protein levels. Similar results were obtained for astrocytes overexpressing neuropathy-associated HSPB1 mutants (Data not shown).
Figure 4. Overexpression of HSPB1(WT) does not reduce SOD1(G93A) expression.
A. Immunofluorescent staining of FLAG and, misfolded SOD1 in non-transduced SOD1(G93A) astrocytes and, HSPB1(WT) transduced SOD1(G93A) astrocytes. Nuclei were visualized with DAPI. B. Representative immunoblot of hHSPB1, SOD1 and misfolded SOD1 protein levels in wild type, SOD1(G93A) and SOD1(G93A) + HSPB1(WT) cell lines. HSPB1 and misfolded SOD1 were stained with antibodies specific to human isoforms of the respective proteins. SOD1 was stained using an antibody that detects both human and murine SOD1. C. Quantification of misfolded human SOD1 staining in SOD1(G93A) and SOD1(G93A) + HSPB1(WT) cell lines. Band intensities were quantified and normalized to a tubulin loading control. Data represents the average of 3 independent western blots. Error bars denote (s.e.m.) n.s., not significant.
Phosphorylation of HSPB1 is likely required for non-cell autonomous MN neuroprotection
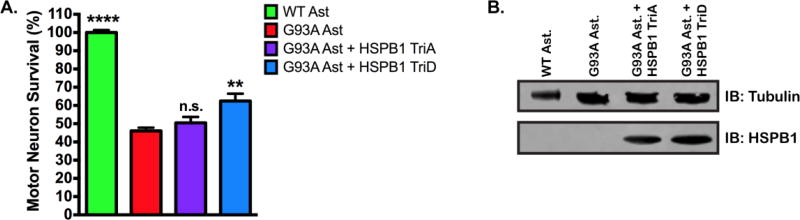
HSPB1 is a phosphoprotein, with 3 phosphorylation sites located in the N-terminal region (Figure 1). Previous studies have shown that phosphorylation of HSPB1 is critical to its neuroprotective properties (53, 59, 60). To determine whether phosphorylation was required for neuroprotection in our assay, SOD1(G93A) astrocytes were transduced with one of two mutHSPB1 constructs, in which the 3 major phosphorylation sites had been mutated to alanine or aspartic acid respectively, to mimic a non-phosphorylated HSPB1, HSPB1(TriA), or a constitutively phosphorylated protein, HSPB1(TriD). When astrocytes expressing HSPB1(TriA) are co-cultured with MNs (Figure 5A), we observed no difference in MN survival as compared to non-transduced astrocytes, 50.48 ± 3.25% and 46.02 ± 1.84% respectively. In contrast, overexpression of HSPB1(TriD) in astrocytes resulted in increased MN survival compared to non-transduced SOD1(G93A) astrocytes (Figure 5A), 63.01 ± 4.73% and 46.01 ± 1.84% respectively (P<0.001). Expression of HSPB1 transgenes was confirmed by western blot (Figure 5B). These data support the hypothesis that phosphorylation of HSPB1 may contribute to non-cell autonomous neuroprotection.
Figure 5. Phosphomimetic HSPB1 expression in SOD1(G93A) astrocytes protects MNs from astrocyte-mediated toxicity.
A. MN survival at day 6 of MN co-culture assay with wild type, SOD1(G93A), SOD1(G93A) + HSPB1(TriA) or SOD1(G93A) + HSPB1(TriD) astrocytes. MN survival was normalized to counts from MNs cultured with wild type astrocytes. (n = 3 for all groups, each n was run in triplicate.) Error bars denote s.e.m. **P<0.001, ****P<0.0001, n.s., non-significant. B. Representative immunoblot confirming the expression of HSPB1(TriA) and HSPB1(TriD) in SOD1(G93A) astrocytes using an antibody specific to human HSPB1.
DISCUSSION
The molecular consequences of mutations in HSPB1 that result in the development of axonal neuropathies is interesting because of what it teaches us about the unique role that HSPB1 plays in the maintenance of motor neurons. Previously characterized mouse models suggest a cell autonomous mechanism(s); neuronal expression of HSPB1 variants is sufficient to cause axonal neuropathy phenotypes similar to those observed in dHMNII and CMT2 patients (27, 28). Boughy et al. recently characterized two new transgenic mouse models of HSPB1 neuropathy that used the CreloxP system to express HSPB1 transgenes at physiological levels specifically in neurons (30). These mice failed to develop a phenotype, and one interpretation of this result and others, is that glial cell types may be involved in the development of axonal neuropathies.
Multiple studies have shed light on the roles of astrocytes, microglia and oliogodendrocytes in the progression of neurodegenerative disorders. Our results demonstrate that expression of HSPB1(WT) in SOD1(G93A) astrocytes improves wild type motor neuron survival in vitro and that expression of two different HSPB1 variants, HSPB1(G84R) and HSPB1(R136W), attenuate this effect. Furthermore, wild type astrocytes expressing mutant HSPB1 were not found to be toxic to motor neurons. These data support the idea that neuropathy-associated mutations in HSPB1 may result in a loss of a supportive function in glial cells. We propose that this loss of non-cell autonomous function is paired with a toxic cell autonomous function in motor neurons that results in the axonal motor neuropathy.
While the neuroprotective effect of HSPB1 in ALS has been well studied, there is conflicting literature regarding the mechanistic basis for such protection. The function of HSPB1 is regulated by posttranslational modifications and structural oligomerization. Chaperone activity of HSPB1 is associated with large, unphosphorylated oligomeric complexes (61). Phosphorylation of HSPB1 results in a shift from larger oligomeric species to smaller multimers, which can directly regulate cellular apoptotic pathways to promote cell survival (60–64). These smaller multimers also regulate a host of other cellular processes, including inflammatory response, mRNA decay and cytoskeleton stabilization (10, 11,14, 65–67). Our data implies that the smaller HSPB1 species are predominantly responsible for the observed neuroprotection in our SOD1-ALS model. We report that overexpression of HSPB1(WT) or HSPB1(TriD), a phosphomimetic mutant, was able to improve motor neuron survival, while a non-phosphorylatable HSPB1 mutant, HSPB1(TriA), did not. Interestingly, the HSPB1(TriD) construct only demonstrated partial neuroprotection compared to the HSPB1(WT) construct. One reason that HSPB1(TriD) may have partial neuroprotection, is that this mutant form disrupts the ability to fluctuate between phosphorylated, partially phosphorylated and dephosphorylated states, which may reduce the full range of HSPB1-client protein interactions necessary for robust neuroprotection.
These findings agree with a recent study that showed expression of HSPB1(WT) and HSPB1(TriD), but not HSPB1(TriA) was able to reduce cell death from oxygen and glucose deprivation in cortical neurons (60). They also found that transgenic mouse lines expressing HSPB1(WT) or HSPB1(TriD) had decreased infarct volume and improved neurofunctional recovery after neuronal ischemia compared to HSPB1(TriA) mice and non-transgenic controls (60). Furthermore, the authors demonstrate that HSPB1(WT) and HSPB1(TriD) confer this neuroprotection by directly inhibiting apoptosis signal-regulating kinase 1 (ASK1) mediated apoptosis (60, 62). Characterization of these mice showed HSPB1 expression was primarily in neurons, but some glial cells were positive for the transgene (62). Thus, mounting evidence, including our data, suggests that HSPB1 neuroprotection is less linked to protein chaperone activity, and more expressed through interaction with client proteins via as yet undefined signal pathways.
An additional possibility is that HSPB1 may improve motor neuron survival in a non-cell autonomous manner by reducing glutamate excitotoxicity, a mechanism known to contribute to motor neuron death in ALS. Astrocytes expressing mutant SOD1 display reduced expression of the glutamate transporter EAAT2, and that reduced expression of EAAT2 can induce motor neuron toxicity (68–70). Recent evidence has shown that EAAT2 is cleaved by caspase-3 in SOD1 (G93A) astrocytes and animal models (71, 72). This cleavage results in a loss of functional EAAT2 and a nuclear accumulation of a truncated C-terminal fragment in astrocytes. Studies have shown that overexpression of this C-terminal fragment in non-transgenic astrocytes can induce motor neuron toxicity in a co-culture model system (73). HSPB1 could prevent astrocyte toxicity through its interaction with procaspase-3 (74), and/or by targeting the toxic C-terminal fragment for degradation via the ubiquitin-proteasome pathway (14). Future work will study these interactions in astrocytes and clarify the molecular pathway, however, owing to the multifunctional nature of HSPB1 it will be challenging to identify a single pathway that accounts for neuroprotective properties of HSPB1.
Therapies that target the induction of heat shock proteins (HSPs) have been developed as potential treatments for ALS patients. Resveratrol, Celastrol and Arimoclomol are 3 compounds that have been reported to delay disease onset and extend survival in SOD1(G93A) transgenic mice (75–77). Each of these compounds indirectly activate heat shock factor 1, the master regulator of HSP gene transcription, and in vitro studies demonstrate that they can increase HSPB1 expression (75, 78, 79). Each of these treatments hold promise, yet the effect these compounds have on HSP regulation in non-neuronal cells has been poorly characterized. More attention may need to be paid to this aspect of HSP induction therapies given our results. It is likely that HSPB1 inducing therapies would be used as part of a combinatorial strategy to treat motor neuron disease. To maximize the potential benefits of HSP neuroprotection, we recommend that future studies examine the role of HSPs in glial cells in addition to neuronal cell types.
In conclusion, HSPB1(WT) expression in SOD1(G93A) astrocytes improves motor neuron survival in vitro through the regulation of an unidentified signal pathway, and likely not via protein chaperone activity. Overexpression of neuropathy-associated HSPB1 mutants mitigate this protective effect and fail to induce non-cell autonomous motor neuron toxicity. These data suggest that neuropathy-associated mutations in HSPB1 may result in loss of a neuroprotective function in non-neuronal cells contributing to the pathogenesis of axonal neuropathy, and highlight the important role that HSPB1 plays in motor neuron health and maintenance.
MATERIALS AND METHODS
Mice
All procedures were performed in accordance with NIH Guidelines and approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children’s Hospital. Wild type, B6SJL, and B6SJLTg SOD1(G93A) mice were obtained from Jackson Laboratories (Bar Harbor, ME).
ES Motor Neuron Differentiation
Mouse embryonic stem cells expressing GFP under the MN-specific promoter HB9 (HBG3 cells; kind gift from Tom Jessell, Columbia University, New York) were cultured on primary mouse embryonic fibroblasts (Millipore). For differentiation into MNs, cells were lifted with trypsin and re-suspended in DFK10 culture medium consisting of knockout DMEM/F12, 10% knockout serum replacement, 1% N2, 0.5% L-glutamine, 0.5% glucose (30% in water), and 0.0016% 2-mercaptoethanol. The cells were plated on non-adherent Petri dishes to allow formation of embryoid bodies. After 1 day of recovery, 2 µM retinoic acid (Sigma) and 2 µM purmorphamine (Calbiochem) were added freshly every day with new medium. After 5 days of differentiation, the embryoid bodies were dissociated and sorted for GFP on a BD FACSVantage/DiVa sorter.
Mouse NPC Isolation and Differentiation into Astrocytes
NPCs were isolated according to methods previously described (80, 81). Briefly, spinal cords were enzymatically dissociated in the same way as described for astrocytes. The cell suspension obtained was then mixed with an equal volume of isotonic Percoll (GE Healthcare) and was centrifuged at 20,000 RCF for 30 minutes at room temperature. Cells from the low-buoyancy fraction (5–10 ml above the red blood cell layer) were harvested, washed thoroughly with D-PBS/PSF (Invitrogen) and plated in 60 mm uncoated plates. Cells were grown in growth medium (DMEM/F12, Invitrogen) with 1% N2 supplement (Invitrogen), 20 ng/ml of fibroblast growth factor-2 (FGF-2, Peprotech) and 20 ng/ml of endothelial growth factor (EGF, Peprotech). Cells were first grown as neurospheres and then were placed on a polyornithinelaminin (P/L)-coated plates, in which they grow as monolayer cultures. NPC cultures were found to be devoid of astrocytes, microglia, and oligodendrocytes contaminants. Once cultures were established, NPCs from wild type and SOD1(G93A) mice were used to generate astrocytes by withdrawing growth factors and supplementing the medium with 10% FBS. The media was changed every 2 days thereafter. Astrocytes were allowed to mature for 7 days prior to being used in the experiments described above. Highly enriched astrocyte cultures were obtained with no detectable levels of microglia, and oligodendrocytes.
Lentiviral Transduction of Mouse Astrocytes
The wild type HSPB1 viral vector was generated by digestion of M13-HSPB1 plasmid (GeneCopoeia) with BstB1 and NotI, and ligated into the pcDNA4/TO vector (Invitrogen), in frame with an N-terminal FLAG sequence. The FLAG-HSPB1 cDNA was then PCR amplified to create a 5` BamH1 site and 3` Xho1 site. This construct was then ligated into a pCSC-SP-PW (Addgene) backbone. Site directed mutagenesis (Agilent) was used to generate HSPB1(G84R), HSPB1(R136W), HSPB1(TriA) and HSPB1(TriD) vectors (primer sequences available upon request). Viral particles were produced as previously described (82) and delivered to astrocytes with a MOI20.
Co-Culture of Motor Neurons and Astrocytes
Murine NPC-derived astrocytes were plated in 96-well plates coated with human fibronectin (2.5 µg/mL; Millipore) at a density of 3.5×10^4 cells per well. One day later, FACS-sorted GFP-positive MNs were re-suspended in MN media consisting of DMEM/F12, 5% horse serum, 2% N2, 2% B27 plus GDNF (Invitrogen; 10 ng/mL), BDNF (Invitrogen; 10 ng/mL), and CNTF (Invitrogen; 10 ng/mL) and added to the astrocytes at a density of 1.0×10^4 per well. Each plate was scanned every day for 6 days with the fully automated IN CELL 6000 confocal plate reader to capture GFP-positive cells. The IN CELL developer and analyzer software were used to create whole-well pictures and to automatically count MNs.
Astrocyte Viability Assays
Murine NPC-derived astrocytes were plated in 10-cm2 plates coated with human fibronectin (2.5 ug/mL; Millipore) at a density of 6×10^6 cells per dish. Media was changed every 2 days. After 4 days, cells were washed 2× with PBS and incubated with Accutase (Gibco) to lift cells from the culture dish. Accutase was neutralized with culture media, and cells were centrifuged for 5 minutes at 500 RCF. The resulting cell pellets were re-suspended in 1 mL of 1× PBS and viability was determined using an NC-100 Nucleo-counter (Chemometec), according to the manufacturer’s protocol.
Western Blotting
Astrocytes were lysed in a buffer containing 20mM Tris (pH 7.4), 150 mM NaCl, 2mM EDTA, 1mM DTT, 10% Glycerol, 1% Triton X-100, 2 mM sodium pyrophosphate, 25 mM à-glycerophosphate, 1 mM Na3VO4, 10 mM NaF, 10 µg/ml leupeptin, 10 µg/ml aprotinin, and 5 mM PMSF on ice for 5 minutes. Lysates were centrifuged (16,000 RCF) at 4°C for 10 minutes. The resulting supernatant was then boiled at 95°C for 5 minutes in 2× Laemmli Buffer (20% Glycerol, 2% β-Mercaptoethanol (v/v), 100 mM Tris-HCl pH 6.8, 2% SDS (v/v)). 10 µg of each sample was separated by SDS-PAGE and transferred to a PVDF (Bio-Rad) membrane. Immunoblots were blocked in 5% nonfat dry milk in Tris-buffered saline (TBS) for 1 hour at room temperature. The blots were then incubated with primary antibodies against human HSPB1, mHSPB1, SOD1, misfolded SOD1 or Tubulin for 1 hour at room temperature. The antibody against misfolded SOD1 has been previously described to be specific to human SOD1 under denaturing conditions and specific for mutant SOD1 isoforms under non-denaturing conditions (47–51). Blots were then washed 3 times with TBS-T, and incubated with secondary antibody for 1 hour at room temperature, and then scanned using a Licor Odyssey Classic. Primary antibodies used for these experiments were: Anti-HSPB1 (Ab Cam, ab2790 [1:5,000]), Anti-Hsp25/27 (Millipore MAB3842 [1:1,000]), Anti-SOD1 (Santa Cruz sc-8637 [1:250]), Anti-Misfolded SOD1 (MediMabs, MM-0070 [1:1,000]), and Anti-Tubulin (Ab Cam, ab7291 [1:10,000]). Secondary antibodies used were: Anti-Mouse 800CW (Licor, 925–32210 [1:10,000]) All antibodies were diluted in blocking buffer + 0.1% Tween-20.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 15 min and washed 3 times with Phosphate-buffered saline (PBS) before the blocking solution consisting of PBS with 10% goat serum, 3% BSA, and 0.1% Triton X-100 was applied for 1 hour. Incubation with primary antibody was performed for 1 hour at room temperature. Cells were then washed 3 times in PBS + 0.1% Triton X-100 then incubated with secondary antibody and DAPI for 1 hour at room temperature. Coverslips were then washed 3 times with PBS + 0.1% Triton X-100. Coverslips were mounted to glass slides using Fluoromount-G (SouthernBiotech). Antibodies used for these experiments: Anti-GFAP (Ab Cam, ab4676 [1:250]), Anti-Hsp25 (ENZO, ADI-SPA-801 [1:1000]), Anti-Misfolded SOD1 (MediMabs, MM-0070 [1:250]), Anti-Mouse AlexaFluor 488 (Invitrogen, A-11001 [1:1,000]), Anti-Rabbit AlexaFluor 488 (Invitrogen, A-11034 [1:1,000]) and Anti-Chicken AlexaFluor 594 (Invitrogen, A-11042 [1:1,000]). All antibodies were diluted in blocking solution.
Statistics
Statistical analysis was performed by one-way ANOVA unpaired t-test for mean differences between the average of wild type control lines versus SOD1(G93A) derived lines and/or HSPB1 mutant lines (GraphPad Prizm Software). All experiments were performed at least in triplicate.
Supplementary Material
Highlights.
Overexpression of HSPB1 in astrocytes attenuates astrocyte-mediated motor neuron toxicity
Phosphorylation contributes to HSPB1-mediated non-cell autonomous neuroprotection
Axonal neuropathy-associated HSPB1 mutations result in loss of astrocyte-mediated motor neuron neuroprotection
Acknowledgments
We would like to acknowledge the OSU Neuroscience Imaging Core for the use of their Andor Revolution WD Spinning Disk Confocal Microscope, and Dr. Paula Monsma for all of her guidance and expertise. This project was funded by NINDS K08NS067282 (to S.J.K.), the Julie Bonasera ALS Fund and the Fred F. and Herman M. Dreier ALS Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landry J, Chretien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldbaum O, Riedel M, Stahnke T, Richter-Landsberg C. The small heat shock protein HSP25 protects astrocytes against stress induced by proteasomal inhibition. Glia. 2009;57(14):1566–77. doi: 10.1002/glia.20870. [DOI] [PubMed] [Google Scholar]
- 3.Bryantsev AL, Kurchashova SY, Golyshev SA, Polyakov VY, Wunderink HF, Kanon B, et al. Regulation of stress-induced intracellular sorting and chaperone function of Hsp27 (HspB1) in mammalian cells. Biochem J. 2007;407(3):407–17. doi: 10.1042/BJ20070195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278(30):27828–35. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 5.Preville X, Salvemini F, Giraud S, Chaufour S, Paul C, Stepien G, et al. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Experimental Cell Research. 1999;247(1):61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- 6.Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, et al. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19(16):1975–81. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- 7.Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20(20):7602–12. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2(9):645–52. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 9.Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56(2):273–9. [PubMed] [Google Scholar]
- 10.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15(1):505–16. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pivovarova AV, Chebotareva NA, Chernik IS, Gusev NB, Levitsky DI. Small heat shock protein Hsp27 prevents heat-induced aggregation of F-actin by forming soluble complexes with denatured actin. FEBS J. 2007;274(22):5937–48. doi: 10.1111/j.1742-4658.2007.06117.x. [DOI] [PubMed] [Google Scholar]
- 12.Alford KA, Glennie S, Turrell BR, Rawlinson L, Saklatvala J, Dean JL. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta-activated kinase-1 (TAK1)-mediated signaling. J Biol Chem. 2007;282(9):6232–41. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 13.Cuesta R, Laroia G, Schneider RJ. Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev. 2000;14(12):1460–70. [PMC free article] [PubMed] [Google Scholar]
- 14.Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, et al. HSP27 Is a Ubiquitin-Binding Protein Involved in I- B Proteasomal Degradation. Molecular and Cellular Biology. 2003;23(16):5790–802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung KW, Kim SB, Cho SY, Hwang SJ, Park SW, Kang SH, et al. Distal hereditary motor neuropathy in Korean patients with a small heat shock protein 27 mutation. Exp Mol Med. 2008;40(3):304–12. doi: 10.3858/emm.2008.40.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36(6):602–6. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- 17.Houlden H, Laura M, Wavrant-De Vrieze F, Blake J, Wood N, Reilly MM. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71(21):1660–8. doi: 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda Y, Abe A, Ishida C, Takahashi K, Hayasaka K, Yamada M. A clinical phenotype of distal hereditary motor neuronopathy type II with a novel HSPB1 mutation. J Neurol Sci. 2009;277(1–2):9–12. doi: 10.1016/j.jns.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 19.James PA, Rankin J, Talbot K. Asymmetrical late onset motor neuropathy associated with a novel mutation in the small heat shock protein HSPB1 (HSP27) J Neurol Neurosurg Psychiatry. 2008;79(4):461–3. doi: 10.1136/jnnp.2007.125179. [DOI] [PubMed] [Google Scholar]
- 20.Luigetti M, Fabrizi GM, Madia F, Ferrarini M, Conte A, Del Grande A, et al. A novel HSPB1 mutation in an Italian patient with CMT2/dHMN phenotype. J Neurol Sci. 2010;298(1–2):114–7. doi: 10.1016/j.jns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Rossor AM, Morrow JM, Polke JM, Murphy SM, Houlden H, Inc R, et al. Pilot phenotype and natural history study of hereditary neuropathies caused by mutations in the HSPB1 gene. Neuromuscul Disord. 2016 doi: 10.1016/j.nmd.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuchner S, Vance JM. Mechanisms of disease: a molecular genetic update on hereditary axonal neuropathies. Nat Clin Pract Neurol. 2006;2(1):45–53. doi: 10.1038/ncpneuro0071. [DOI] [PubMed] [Google Scholar]
- 23.Ackerley S, James PA, Kalli A, French S, Davies KE, Talbot K. A mutation in the small heat-shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet. 2006;15(2):347–54. doi: 10.1093/hmg/ddi452. [DOI] [PubMed] [Google Scholar]
- 24.Zhai J, Lin H, Julien JP, Schlaepfer WW. Disruption of neurofilament network with aggregation of light neurofilament protein: a common pathway leading to motor neuron degeneration due to Charcot-Marie-Tooth disease-linked mutations in NFL and HSPB1. Hum Mol Genet. 2007;16(24):3103–16. doi: 10.1093/hmg/ddm272. [DOI] [PubMed] [Google Scholar]
- 25.Almeida-Souza L, Asselbergh B, d'Ydewalle C, Moonens K, Goethals S, de Winter V, et al. Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot-Marie-Tooth neuropathy. J Neurosci. 2011;31(43):15320–8. doi: 10.1523/JNEUROSCI.3266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida-Souza L, Goethals S, de Winter V, Dierick I, Gallardo R, Van Durme J, et al. Increased monomerization of mutant HSPB1 leads to protein hyperactivity in Charcot-Marie-Tooth neuropathy. J Biol Chem. 2010;285(17):12778–86. doi: 10.1074/jbc.M109.082644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava AK, Renusch SR, Naiman NE, Gu S, Sneh A, Arnold WD, et al. Mutant HSPB1 overexpression in neurons is sufficient to cause age-related motor neuronopathy in mice. Neurobiol Dis. 2012;47(2):163–73. doi: 10.1016/j.nbd.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.d'Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, et al. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat Med. 2011;17(8):968–74. doi: 10.1038/nm.2396. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Jung SC, Joo J, Choi YR, Moon HW, Kwak G, et al. Overexpression of mutant HSP27 causes axonal neuropathy in mice. J Biomed Sci. 2015;22:43. doi: 10.1186/s12929-015-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouhy D, Geuens T, De Winter V, Almeida-Souza L, Katona I, Weis J, et al. Characterization of New Transgenic Mouse Models for Two Charcot-Marie-Tooth-Causing HspB1 Mutations using the Rosa26 Locus. J Neuromuscul Dis. 2016;3(2):183–200. doi: 10.3233/JND-150144. [DOI] [PubMed] [Google Scholar]
- 31.Jin An Jea. Transduced HSP27 Protein Protects Neuronal Cell Death by Enhancing FALS-associated SOD1 Mutant Activity. 2008 doi: 10.5483/bmbrep.2009.42.3.136. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan J, Lemmens R, Robberecht W, Van Den Bosch L. Role of heat shock response and Hsp27 in mutant SOD1-dependent cell death. Exp Neurol. 2006;200(2):301–10. doi: 10.1016/j.expneurol.2006.02.135. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan J, Vannuvel K, Andries M, Waelkens E, Robberecht W, Van Den Bosch L. Overexpression of Hsp27 does not influence disease in the mutant SOD1(G93A) mouse model of amyotrophic lateral sclerosis. J Neurochem. 2008;106(5):2170–83. doi: 10.1111/j.1471-4159.2008.05545.x. [DOI] [PubMed] [Google Scholar]
- 34.Patel YJ, Payne Smith MD, de Belleroche J, Latchman DS. Hsp27 and Hsp70 administered in combination have a potent protective effect against FALS-associated SOD1-mutant-induced cell death in mammalian neuronal cells. Brain Res Mol Brain Res. 2005;134(2):256–74. doi: 10.1016/j.molbrainres.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Sharp PS, Akbar MT, Bouri S, Senda A, Joshi K, Chen HJ, et al. Protective effects of heat shock protein 27 in a model of ALS occur in the early stages of disease progression. Neurobiol Dis. 2008;30(1):42–55. doi: 10.1016/j.nbd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Yerbury JJ, Gower D, Vanags L, Roberts K, Lee JA, Ecroyd H. The small heat shock proteins alphaB-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones. 2013;18(2):251–7. doi: 10.1007/s12192-012-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsuno M, Tanaka F, Sobue G. Perspectives on molecular targeted therapies and clinical trials for neurodegenerative diseases. J Neurol Neurosurg Psychiatry. 2012;83(3):329–35. doi: 10.1136/jnnp-2011-301307. [DOI] [PubMed] [Google Scholar]
- 38.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 39.Capponi S, Geuens T, Geroldi A, Origone P, Verdiani S, Cichero E, et al. Molecular Chaperones in the Pathogenesis of Amyotrophic Lateral Sclerosis: The Role of HSPB1. Hum Mutat. 2016;37(11):1202–8. doi: 10.1002/humu.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vleminckx V, Van Damme P, Goffin K, Delye H, Van Den Bosch L, Robberecht W. Upregulation of HSP27 in a transgenic model of ALS. J Neuropathol Exp Neurol. 2002;61(11):968–74. doi: 10.1093/jnen/61.11.968. [DOI] [PubMed] [Google Scholar]
- 41.Maatkamp A, Vlug A, Haasdijk E, Troost D, French PJ, Jaarsma D. Decrease of Hsp25 protein expression precedes degeneration of motoneurons in ALS-SOD1 mice. Eur J Neurosci. 2004;20(1):14–28. doi: 10.1111/j.1460-9568.2004.03430.x. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, Hayashi H, Nakashima K, Nanba E, Kato M, Hirano A, et al. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am J Pathol. 1997;151(2):611–20. [PMC free article] [PubMed] [Google Scholar]
- 43.Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16(6):1056–64. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, et al. Astrocytes From Familial and Sporadic ALS Patients are Toxic to Motor Neurons. Nature Biotechnology. 2011;29(9) doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. PNAS. 2014;111(2) doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papadeas ST, Kraig SE, O'Banion C, Lepore AC, Maragakis NJ. Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. PNAS. 2011;108(43) doi: 10.1073/pnas.1103141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gros-Louis F, Soucy G, Lariviere R, Julien JP. Intracerebroventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J Neurochem. 2010;113(5):1188–99. doi: 10.1111/j.1471-4159.2010.06683.x. [DOI] [PubMed] [Google Scholar]
- 48.Israelson A, Ditsworth D, Sun S, Song S, Liang J, Hruska-Plochan M, et al. Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1. Neuron. 2015;86(1):218–32. doi: 10.1016/j.neuron.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nizzardo M, Simone C, Rizzo F, Ulzi G, Ramirez A, Rizzuti M, et al. Morpholino-mediated SOD1 reduction ameliorates an amyotrophic lateral sclerosis disease phenotype. Sci Rep. 2016;6:21301. doi: 10.1038/srep21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel P, Julien JP, Kriz J. Early-stage treatment with Withaferin A reduces levels of misfolded superoxide dismutase 1 and extends lifespan in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12(1):217–33. doi: 10.1007/s13311-014-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leyton-Jaimes MF, Benaim C, Abu-Hamad S, Kahn J, Guetta A, Bucala R, et al. Endogenous macrophage migration inhibitory factor reduces the accumulation and toxicity of misfolded SOD1 in a mouse model of ALS. Proc Natl Acad Sci U S A. 2016;113(36):10198–203. doi: 10.1073/pnas.1604600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fritz E, Izaurieta P, Weiss A, Mir FR, Rojas P, Gonzalez D, et al. Mutant SOD1-expressing astrocytes release toxic factors that trigger motoneuron death by inducing hyperexcitability. J Neurophysiol. 2013;109(11):2803–14. doi: 10.1152/jn.00500.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams KL, Mearow KM. Phosphorylation status of heat shock protein 27 influences neurite growth in adult dorsal root ganglion sensory neurons in vitro. J Neurosci Res. 2011;89(8):1160–72. doi: 10.1002/jnr.22634. [DOI] [PubMed] [Google Scholar]
- 54.Williams KL, Rahimtula M, Mearow KM. Heat shock protein 27 is involved in neurite extension and branching of dorsal root ganglion neurons in vitro. J Neurosci Res. 2006;84(4):716–23. doi: 10.1002/jnr.20983. [DOI] [PubMed] [Google Scholar]
- 55.Tortarolo M, Crossthwaite AJ, Conforti L, Spencer JP, Williams RJ, Bendotti C, et al. Expression of SOD1 G93A or wild-type SOD1 in primary cultures of astrocytes down-regulates the glutamate transporter GLT-1: lack of involvement of oxidative stress. J Neurochem. 2004;88(2):481–93. doi: 10.1046/j.1471-4159.2003.02208.x. [DOI] [PubMed] [Google Scholar]
- 56.Benkler C, Ben-Zur T, Barhum Y, Offen D. Altered astrocytic response to activation in SOD1(G93A) mice and its implications on amyotrophic lateral sclerosis pathogenesis. Glia. 2013;61(3):312–26. doi: 10.1002/glia.22428. [DOI] [PubMed] [Google Scholar]
- 57.Nefedova VV, Sudnitsyna MV, Strelkov SV, Gusev NB. Structure and properties of G84R and L99M mutants of human small heat shock protein HspB1 correlating with motor neuropathy. Arch Biochem Biophys. 2013;538(1):16–24. doi: 10.1016/j.abb.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 58.Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, et al. Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81(5):1009–23. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36(1):45–56. doi: 10.1016/s0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- 60.Stetler RA, Gao Y, Zhang L, Weng Z, Zhang F, Hu X, et al. Phosphorylation of HSP27 by protein kinase D is essential for mediating neuroprotection against ischemic neuronal injury. J Neurosci. 2012;32(8):2667–82. doi: 10.1523/JNEUROSCI.5169-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274(27):18947–56. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 62.Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28(49):13038–55. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J, Kang D, Sun BK, Kim JH, Song JJ. TRAIL/MEKK4/p38/HSP27/Akt survival network is biphasically modulated by the Src/CIN85/c-Cbl complex. Cell Signal. 2013;25(1):372–9. doi: 10.1016/j.cellsig.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Shen L, Qi Z, Zhu Y, Song X, Xuan C, Ben P, et al. Phosphorylated heat shock protein 27 promotes lipid clearance in hepatic cells through interacting with STAT3 and activating autophagy. Cell Signal. 2016;28(8):1086–98. doi: 10.1016/j.cellsig.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Hong S, Feng Z, Xin Y, Wang Q, Fu J, et al. Regulation of lipopolysaccharide-induced inflammatory response by heat shock protein 27 in THP-1 cells. Cell Immunol. 2010;264(2):127–34. doi: 10.1016/j.cellimm.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Knapinska AM, Gratacos FM, Krause CD, Hernandez K, Jensen AG, Bradley JJ, et al. Chaperone Hsp27 modulates AUF1 proteolysis and AU-rich element-mediated mRNA degradation. Mol Cell Biol. 2011;31(7):1419–31. doi: 10.1128/MCB.00907-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinsimer KS, Gratacos FM, Knapinska AM, Lu J, Krause CD, Wierzbowski AV, et al. Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay. Mol Cell Biol. 2008;28(17):5223–37. doi: 10.1128/MCB.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18(2):327–38. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 69.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99(3):1604–9. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20(3):589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- 71.Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281(20):14076–84. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- 72.Gibb SL, Boston-Howes W, Lavina ZS, Gustincich S, Brown RH, Jr, Pasinelli P, et al. A caspase-3-cleaved fragment of the glial glutamate transporter EAAT2 is sumoylated and targeted to promyelocytic leukemia nuclear bodies in mutant SOD1-linked amyotrophic lateral sclerosis. J Biol Chem. 2007;282(44):32480–90. doi: 10.1074/jbc.M704314200. [DOI] [PubMed] [Google Scholar]
- 73.Foran E, Bogush A, Goffredo M, Roncaglia P, Gustincich S, Pasinelli P, et al. Motor neuron impairment mediated by a sumoylated fragment of the glial glutamate transporter EAAT2. Glia. 2011;59(11):1719–31. doi: 10.1002/glia.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibert B, Eckel B, Fasquelle L, Moulin M, Bouhallier F, Gonin V, et al. Knock down of heat shock protein 27 (HspB1) induces degradation of several putative client proteins. PLoS One. 2012;7(1):e29719. doi: 10.1371/journal.pone.0029719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han S, Choi JR, Soon Shin K, Kang SJ. Resveratrol upregulated heat shock proteins and extended the survival of G93A-SOD1 mice. Brain Res. 2012;1483:112–7. doi: 10.1016/j.brainres.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 76.Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10(4):402–5. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 77.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2(5):246–54. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 78.Deane CA, Brown IR. Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol. Cell Stress Chaperones. 2016;21(5):837–48. doi: 10.1007/s12192-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, Greensmith L. Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. J Neurochem. 2008;107(2):339–50. doi: 10.1111/j.1471-4159.2008.05595.x. [DOI] [PubMed] [Google Scholar]
- 80.Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, et al. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell. 2012;11(3):542–52. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31(3):560–73. doi: 10.1016/j.mcn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 82.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1(1):241–5. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.