Abstract
We have previously demonstrated that anti-Aβ DNA vaccine (AV-1959D) based on our proprietary MultiTEP platform technology is extremely immunogenic in mice, rabbits, and monkeys. Importantly, MultiTEP platform enables development of vaccines targeting pathological molecules involved in various neurodegenerative disorders. Taking advantage of the universality of MultiTEP platform, we developed DNA vaccines targeting three B cell epitopes (aa85–99, aa109–126, aa126–140) of human alpha-Synuclein (hα-Syn) separately, or all three epitopes simultaneously. All four DNA vaccines (i) generate high titers of anti-hα-Syn antibodies; (ii) induce robust MultiTEP-specific Th cell responses without activation of potentially detrimental autoreactive anti-hα-Syn Th cells. Generated antibodies recognize misfolded hα-Syn produced by neuroblastoma cells, hα-Syn in the brain tissues of transgenic mouse strains and in the brain tissues of Dementia with Lewy Bodies (DLB) cases. Based on these results, the most promising vaccine targeting three B cell epitopes of hα-Syn simultaneously (PV-1950D) has been chosen for ongoing pre-clinical assessment in mouse models of hα-Syn with the aim to translate it to the human clinical trials.
Keywords: DNA Vaccines, MultiTEP platform, Anti-α-Synuclein Antibodies, B-cell mapping, T-cell mapping, Immunogenicity, Therapeutic potency
1. Introduction
Intracellular aggregates of alpha-Synuclein (α-Syn) accumulate as Lewy bodies (LB) and Lewy neurites (LN) in patients with Dementia with Lewy bodies (DLB) and Parkinson’s disease (PD). However, synucleinopathy is not restricted to pure DLB and PD. Up to 50% of AD cases exhibit Lewy bodies, and the presence of LB pathology in AD is associated with a more aggressive disease course and accelerated cognitive dysfunction (McGeer and McGeer, 2008). Overlap of clinical and neuropathological features of AD and PD is observed in DLB (Kotzbauer et al., 2012; Obi et al., 2008). Likewise, co-occurrence of tau and α-Syn inclusions could be observed in Parkinson’s disease with dementia (PDD), DLB, Lewy body variant of AD (LBVAD) and Guam-Parkinson-ALS dementia complex (Badiola et al., 2011; Forman et al., 2002; Giasson et al., 2003a; Marui et al., 2000). Furthermore, there are multiple reports demonstrating that amyloidogenic proteins can interact in vivo to promote an aggregation and accumulation of each other (Gallardo et al., 2008; Giasson et al., 2003b; Mandal et al., 2006; Tsigelny et al., 2008). Today medications may help control some symptoms of PD/DLB, but very few disease-modifying treatments have been developed yet (Fahn, 2005; Fahn et al., 2004). The era of vaccination against neurodegenerative disorders was initiated almost 16 years ago when a group of researchers from Elan Pharmaceuticals reported on effective clearance of extracellular amyloid pathology after immunizations of AD transgenic (Tg) mice with fibrillar beta-amyloid (Aβ42) peptide formulated in adjuvant (Schenk et al., 1999). Several years later Masliah et al. (Masliah et al., 2005) and Asuni et al. (Asuni et al., 2007) demonstrated that vaccination strategies could be viable not only for targeting extracellular, but also intracellular pathological human α-Syn (hα-Syn) and tau aggregates, respectively. More specifically, vaccination of hα-Syn transgenic mice mimicking certain aspects of PD/DLB, with recombinant hα-Syn protein induced therapeutically potent antibodies capable of reducing intracellular toxic hα-Syn aggregates. Notably, these antibodies generated by full-length protein recognized four epitopes spanning amino acids (aa) 85–99, 109–123, 112–126, and 126–138 of hα-Syn (Masliah et al., 2005). Later the same group of scientists demonstrated that administration of monoclonal antibodies (mAb) 9A4 specific to aa118–126 of hα-Syn reduced neurological deficits and improved behavior in the same mouse model of PD/DLB (line D) (Masliah et al., 2011) while two other mAbs (1H7 & 5D12) mitigated neurodegeneration in the second mouse model (Thy1-α-Syn) mimicking PD (line 61) (Games et al., 2014). Later it was shown that short peptides mimicking aa sequence 110–130 of hα-Syn attached to Keyhole Limpet Hemocyanin (KLH) carrier (e.g. AFF 1 vaccine) also induced therapeutically potent antibodies in these two mouse models of PD/DLB, and the authors showed improvements in both motor function and memory in hα-Syn Tg mice (Mandler et al., 2014). Finally, active immunization with AFF 1 vaccine formulated in alhydrogel reduced hα-Syn accumulation, demyelination in the neocortex, striatum and corpus callosum, as well as neurodegeneration in mouse models of another synucleinopathy, multiple system atrophy (MBP-α-Syn Tg) (Games et al., 2014; Mandler et al., 2015; Mandler et al., 2014; Masliah et al., 2005; Masliah et al., 2011). Thus, anti-hα-Syn immunotherapy reduced the accumulation of pathological α-Syn in axons and synapses and alleviated motor and learning deficits in various models of α-synucleinopathies.
By providing genes encoding a specific antigen, DNA vaccination represents a unique alternative method of immunization and has properties that may be advantageous in developing a range of vaccines against a variety of pathogens and human diseases (Abdulhaqq and Weiner, 2008; Agadjanyan et al., 1998; Davtyan et al., 2014b; Ghochikyan, 2009; Kim et al., 1998; Li et al., 2012; Ugen et al., 2006). Previously, we demonstrated that an anti-Aβ DNA vaccine based on the MultiTEP platform technology (AV-1959D) generated very strong cellular and humoral immune responses in mice, rabbits, and monkeys without induction of potentially harmful autoreactive T cell responses (Davtyan et al., 2014a; Davtyan et al., 2014b; Evans et al., 2014; Ghochikyan et al., 2013). Importantly, at least in monkeys, this vaccine did not induce antibodies specific to MultiTEP (Davtyan et al., 2014a), suggesting that this vaccine platform may be beneficial in human clinical trials. To take advantage of this immunogenic and universal vaccine platform technology we decided to generate MultiTEP-based DNA vaccines targeting three different B cell antigenic determinants of hα-Syn, spanning aa85–99 (PV-1947D), aa109–126 (PV-1948D), aa126–140 (PV-1949D) separately, or simultaneously (PV-1950D). Here we present data demonstrating that none of the DNA epitope vaccines induced potentially harmful autoreactive Th cell responses, while all of them generated strong cellular responses to the vaccine platform. Importantly, anti-MultiTEP specific Th cells have activated B cells producing high titers of antibodies that recognized pathological forms of hα-Syn.
2. Material and methods
2.1. Mice
Female, 6–8 weeks old C57BL/6 mice (H2b haplotype) were obtained from Jackson Laboratory. All animals were housed in a temperature and light-cycle controlled facility, and their care was under the guidelines of NIH and an approved IACUC protocol at UC Irvine.
2.2. Plasmids and peptides
The generation of plasmid encoding the MultiTEP platform composed of a string of twelve foreign Th epitopes was described previously (Ghochikyan et al., 2013). In this study, four different minigenes encoding three copies of B cell epitopes aa85–99, aa109–126, aa126–140, or all three epitopes together [3×(aa126–140)+3×(aa109–126)+3×(aa85–99)], have been synthesized by GenScript Company and ligated with a gene encoding MultiTEP. The resulting genes were cloned into the pVAX1 vector (Invitrogen) designed to be consistent with current Food and Drug Administration (FDA) guidelines. Additionally, we have generated a plasmid encoding full-length hα-Syn fused with MultiTEP platform (phα-Syn-MultiTEP). Immunization-grade plasmids were purified by Aldevron, and the correct sequences of the generated plasmids were confirmed by nucleotide sequence analysis. Expression and secretion of the proteins encoded by generated DNA constructs were analyzed in the lysates and supernatants from transiently transfected Chinese hamster ovary (CHO) cells by western blotting (WB) as described previously (Movsesyan et al., 2008a; Movsesyan et al., 2008b), except that immunoblots were stained with anti-hα-Syn85–99, anti-hα-Syn109–126 and anti-hα-Syn126–140 antibodies generated at the Institute for Molecular Medicine (IMM). Peptides spanning hα-Syn, aa85–99 (α-Syn85–99), aa109–126 (α-Syn109–126), aa126–140 (α-Syn126–140), as well as 20mer overlapping peptides aa1–20 (α-Syn1–20), aa16–35 (α-Syn16–35), aa31–50 (α-Syn31–50), aa46–65 (α-Syn46–65), aa61–80 (α-Syn61–80), aa76–95 (α-Syn76–95), aa91–110 (α-Syn91–110), aa106–125 (α-Syn106–125), aa121–140 (α-Syn121–140) and irrelevant peptide were synthesized by GenScript.
2.3. Immunizations
Mice were injected into both tibialis anterior muscles with 20µg per leg of the appropriate plasmid vaccine in 30µl PBS. Immediately after the DNA administration electrical pulses were applied using the AgilPulse™ device from BTX Harvard Apparatus, as previously described (Davtyan et al., 2014b). Briefly, the needle electrode made of two parallel rows of four 5-mm needles 0.3mm in diameter (1.5 × 4-mm gap) was inserted in such a way that the i.m. injection site was located between the two needle rows. The EP pulses were applied (“high amplitude, short duration” (450/0.05) two pulses and “low amplitude, long duration” (110/10) eight pulses) using the AgilPulse™ device from BTX Harvard Apparatus (Holliston, MA). The mice were immunized with indicated DNA vaccines on days 0, 14, 28 and 42. The sera were collected on 12th day after the third immunization (Day 40) and were used both for analyzing of the humoral immune responses and purification of polyclonal antibodies. On the 7th day after the fourth immunization (Day 49) mice were terminated, spleens were harvested, and splenocytes were used for detection of cellular immune responses. For the B and T cell epitope mapping studies, two groups of mice were immunized with either phα-Syn-MultiTEP plasmid followed by EP, or with the full-length hα-Syn protein (rPeptide) formulated with AdvaxCpG adjuvant. Mice were vaccinated 4 times biweekly. Blood was collected 10 days after the 3rd immunization and sera were used for B cell epitope mapping. On the 7th day after the last immunization mice were terminated and T cell epitope mapping was performed on splenocytes (see below). In the second set of T cell epitope mapping experiment mice were immunized twice with full-length hα-Syn protein (rPeptide) and terminated 7 days after the second immunization.
2.4. Detection of IFN-γ-producing T cells and mapping of T cell epitopes
The analysis of cellular immune responses was performed in splenocyte cultures from individual animals by detection of T cells producing IFN-γ cytokine by ELISPOT (BD Biosciences, San Jose, CA, USA) as previously described (Cribbs et al., 2003; Davtyan et al., 2014a; Davtyan et al., 2013; Petrushina et al., 2007). For T cell epitope mapping the splenocyte cultures collected from phα-Syn-MultiTEP- and full-length hα-Syn protein immunized mice were re-stimulated with 10µg/ml of each indicated peptide spanning hα-Syn or irrelevant peptide for 20 hours in vitro. Splenocyte cultures from mice immunized with different DNA epitope vaccines were re-stimulated in vitro with a cocktail of 12 peptides representing the Th epitopes in MultiTEP platform (Davtyan et al., 2014a) (2µg/ml of each peptide), hα-Syn protein or peptides(s) representing self B cell epitope(s) at 10µg/ml for 20 hours. The numbers of SFC per 106 splenocytes were then counted.
2.5. Detection of the titer of anti-hα-Syn antibody and mapping of B cell epitopes
B cell epitopes were mapped by ELISA, as previously described (Ghochikyan et al., 2014). Binding of sera from phα-Syn-MultiTEP and hα-Syn protein immunized mice (at dilutions 1:1000 and 1:5000, respectively) to 20-mer overlapping peptides spanning hα-Syn was analyzed. The titers of total anti-hα-Syn antibodies, as well as antibodies specific to hα-Syn85–99, hα-Syn109–126 and hα-Syn126–140 were detected by ELISA as described previously (Ghochikyan et al., 2014). Briefly, plates were coated with 1µg/ml of hα-Syn protein or the appropriate peptide. Sera from individual mice were diluted (from 1:1,000 to 1:2,560,000) and endpoint titers were calculated as the reciprocal of the highest sera dilution that gave a reading twice above the background levels of binding of non-immunized sera at the same dilution (cutoff). HRP-conjugated anti-IgG1, IgG2ab, IgG2b and IgM specific antibodies (Bethyl Laboratories, Inc.) were used to characterize the isotype profiles of anti-hα-Syn antibodies in individual sera at dilution 1:1000.
2.6. Purification of anti-hα-Syn antibodies
Anti-hα-Syn antibodies from sera of mice immunized with PV-1947D, PV-1948D and PV-1949D epitope vaccines were purified by an affinity column (SulfoLink, ThermoFisher Sci.) using an immobilized α-Syn85–99-C, α-Syn109–126-C and α-Syn126–140-C peptides (GenScript), respectively, as we previously described(Mamikonyan et al., 2007). Purified antibodies were analyzed via 10% Bis-Tris gel (ThermoFisher Sci.), and the concentrations were determined using a BCA protein assay kit (ThermoFisher Sci.).
2.7. Human α-Syn overexpressing cell line and Western blotting
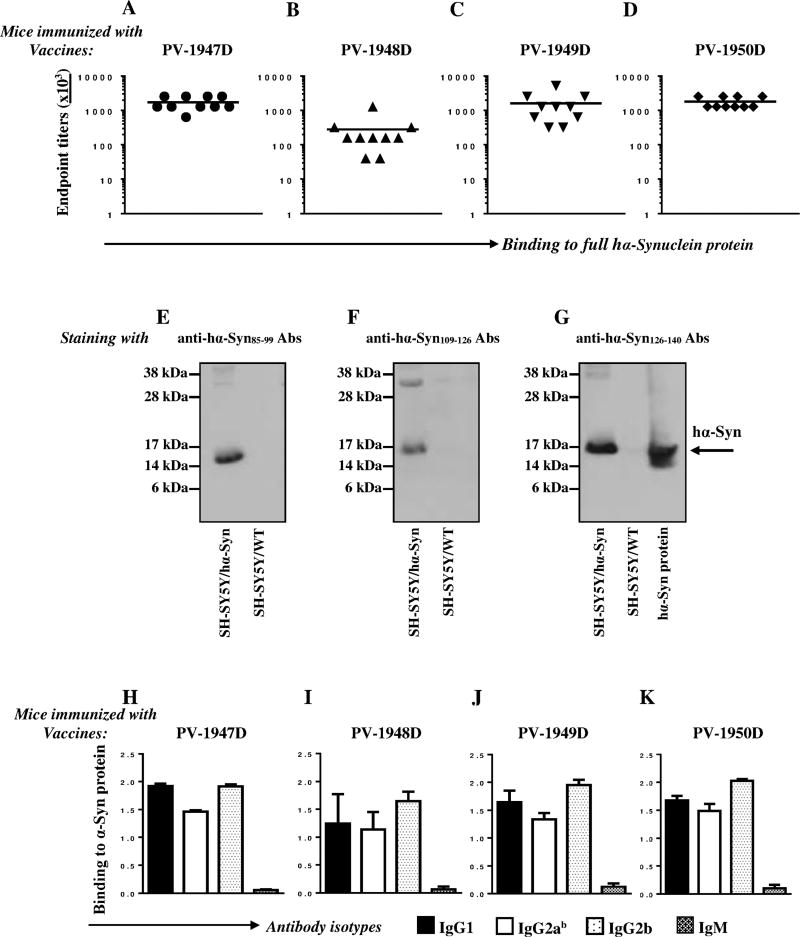
SH-SY5Y neuroblastoma cells stably overexpressing human α-Synuclein (SH-SY5Y/hα-Syn) were generated by AMAXA nucleofection with a plasmid expressing wild type hα-Syn and puromycin resistance under control of a CAG promoter. Control (SH-SY5Y/WT) cells were concurrently produced by nucleofection with an empty vector. Stable cells were selected by treatment with puromycin for 6 weeks and hα-Syn expression confirmed by WB (data not shown). All SH-SY5Y cells were grown in DMEM supplemented with 10% FBS, in a 5% CO2 atmosphere. Cell lysates were subjected to electrophoresis on NuPAGE 10% Bis-Tris gel in MES buffer under reducing conditions and electro-transferred onto a nitrocellulose membrane (GE Healthcare). Membranes were stained with purified anti-hα-Syn85–99, anti-hα-Syn109–126 and anti-hα-Syn126–140 antibodies at concentration 1µg/ml and goat anti-mouse IgG-HRP (Santa Cruz Biotechnology). As a positive control, 0.5µg of full-length hα-Syn was loaded onto the gel.
2.8. Mouse brain extraction and Western blotting
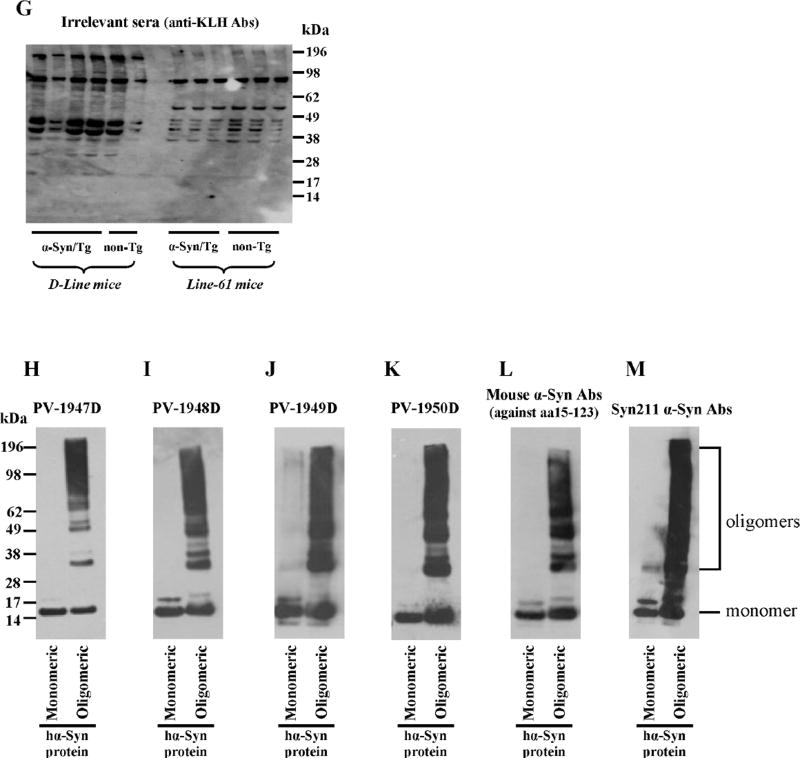
Brain homogenates from hα-Syn tg (line D) mice were prepared using T-PER (Tissue Protein Extraction Reagent) buffer (ThermoFisher Sci.) with added protease inhibitor cocktail (Sigma). Brain homogenates from hα-Syn tg (line 61) mice were prepared using PDGF buffer (1mM HEPES, 5mM Benzamidine, 2mM 2-Mercaptoethanol, 3mM EDTA, 0.5mM Magnesium sulfate, 0.05% Sodium azide, pH=8.8) with added phosphatase and protease inhibitors (Calbiochem). Lysates were centrifuged (14,000×g for 1 hr at 4°C), supernatants were collected, and protein concentrations were determined by BCA protein assay kit (ThermoFisher Sci). Striatal tissue lysate from a α-Syn knockout mouse (Jackson Laboratories #016123) was prepared along with experimental samples and it is present on every α-Synuclein Western blot as a negative control. Samples were subjected to electrophoresis on NuPAGE 4–12% Bis-Tris gel in MES buffer under reducing conditions and electro-transferred onto a nitrocellulose membrane (GE Healthcare). α-Syn was visualized by incubating with sera from mice immunized with PV-1947D, PV-1948D, PV-1949D or PV-1950D followed by HRP-conjugated anti-mouse IgG (Santa Cruz Biotechnology). Sera were used after normalization of antibody endpoint titers measured by ELISA. Mouse anti-α-Syn antibody against aa15–123 (1:1000; BD Biosciences) and Syn211 anti-α-Syn antibody (1:1000; EMD Millipore) served as positive controls. Sera from mice immunized with irrelevant antigen were used as a negative control.
2.9. Human brain extraction and Western blotting
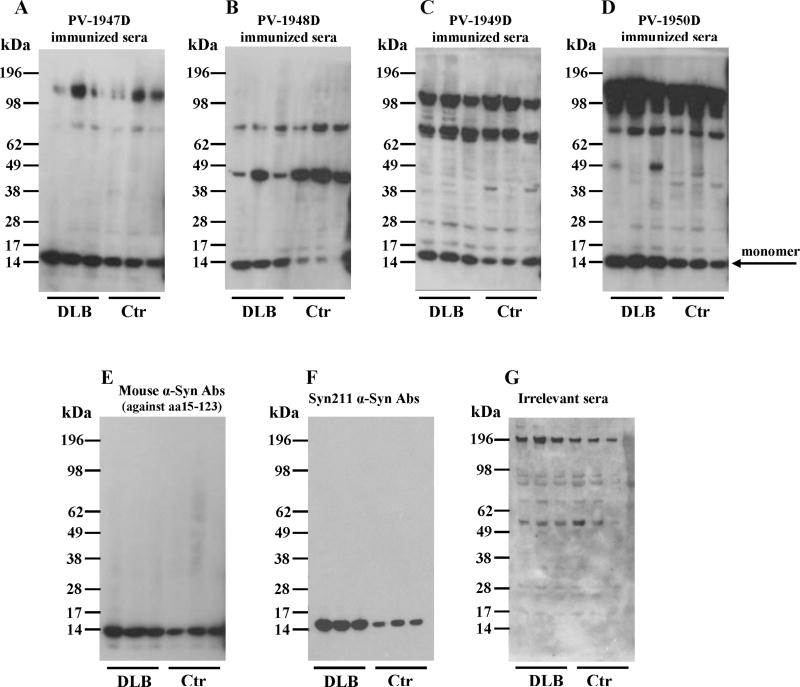
Brain homogenates from DLB cases and control subjects were prepared using PDGF buffer with added phosphatase and protease inhibitors (Calbiochem). Samples were analyzed using WB performed as described above.
2.10. Preparation and analysis of α-Synuclein oligomers
Oligomers were prepared as described by Roberts et al.(Roberts et al., 2015). Briefly, oligomers were produced by incubating recombinant hα-Syn (rPeptide) at 1mg/ml (~70µM) with 30:1 M excess of 4-hydroxy-2-nonenal (HNE; EMD Millipore) at 37°C for 18h. After the incubation, unbound aldehyde was removed with an Amicon 3kDA cut-off ultra-centrifugal unit (EMD Millipore). WB analysis was carried out as described above. 0.25µg hα-Syn monomers and oligomers were used in each well. Additionally, α-Syn was also visualized with monoclonal mouse anti-α-Syn antibody (aa15–123; 1:1000; BD Biosciences) and Syn211 anti-α-Syn antibody (1:1000; EMD Millipore).
2.11. Immunohistochemistry
The brain samples were obtained from the Brain Bank and Tissue Repository (MIND, UCI) as formalin-fixed blocks of anterior cingulate cortex of control normal brain and the brain of a human subject with established DLB pathology. The brains were processed for immunohistochemistry (IHC) by previously published methods (Ghochikyan et al., 2014; Petrushina et al., 2007) employing proteinase K pretreatment, and Lewy bodies (LBs) and Lewy neurites (LNs) were detected using sera from mice immunized with PV-1947D, PV-1948D, PV-1949D, and PV-1950D. The images were captured via Digital Whole Slide Scanning System Aperio AT2 (HistoWiz, Brooklin, NY), and the photomicroghraphs of immunostained images at 40X magnification are presented in this paper.
2.12. Statistical Analysis
Statistical parameters (mean, standard deviation (SD), significant difference, etc.) were calculated using the Prism 6 software (GraphPad Software, Inc.). Statistically significant differences were examined using analysis of variance (one-way ANOVA) and Tukey's multiple comparisons post-test (a P value of less than 0.05 was considered significant).
3. Results and Discussion
3.1. Mapping of B and T cell epitopes of hα-Syn after DNA- and protein-based immunizations and construction of vaccine plasmids
Previous epitope mapping studies showed that hα-Syn/Tg mice responded to immunization with full-length hα-Syn recombinant protein formulated in the adjuvant CFA/IFA with production of antibodies binding to the middle and C-terminal regions of this protein (aa85–99, aa109–123, aa112–126, and aa126–138) (Masliah et al., 2005). These epitope-mapping data became instrumental for future immunotherapeutic studies demonstrating that mAb specific to aa118–126 (Games et al., 2014) of hα-Syn and antibodies against hα-Syn mimotope of aa110–130 (Mandler et al., 2015) effectively reduce pathology and improve behavior in three transgenic mouse models of α-synucleinopathies.
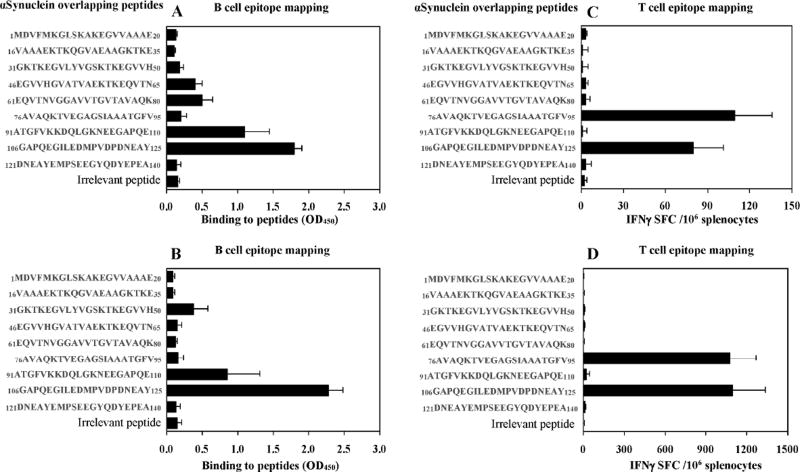
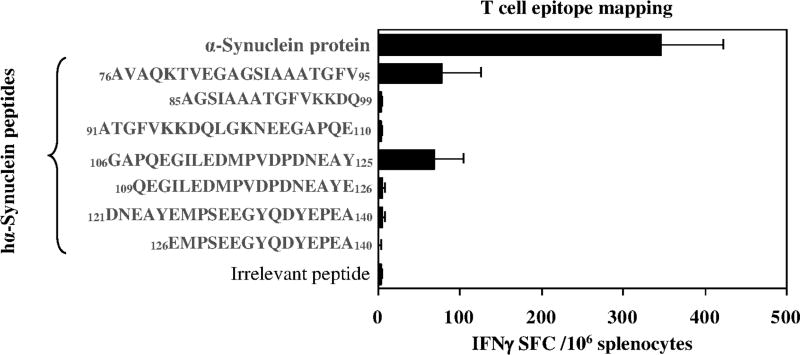
Here we compared the B cell antigenic determinant of hα-Syn synthesized in eukaryotic mouse cells versus that produced in prokaryotic bacterial cells (e.g., DNA vaccine versus recombinant protein vaccine). Accordingly, we immunized mice of H2b immune haplotype with plasmid encoding full-length of hα-Syn gene or recombinant hα-Syn protein formulated in AdvaxCpG adjuvant and then mapped the B cell epitopes using immune sera from vaccinated mice with a series of overlapping peptides covering the entire sequence of hα-Syn protein (Fig. 1A, B). Both DNA and protein-based full-length hα-Syn generated antibodies that bind strongly to the peptides spanning aa91–110 and aa106–125, but not to aa126–138, as was shown earlier by Masliah et al. (Masliah et al., 2005). Injection of a plasmid encoding hα-Syn also induced antibodies that weakly bound to aa46–65 and aa61–80 peptides, while administration of protein-based vaccine generated antibodies that bound weakly to peptide spanning aa31–40 of hα-Syn. Of note, no natural anti-α-syn antibody had been detected in non-immunized mice. Prior to using these B cell epitopes for generation of MultiTEP platform-based vaccines, we tested an ability of each of the overlapping peptides to stimulate in vitro autoreactive Th cells in mice immunized with full-length hα-Syn. These experiments demonstrated that regardless of the nature of the vaccine, DNA vs. recombinant protein, two peptides spanning aa76–95 and aa106–125 of hα-Syn activated Th cells in immunized mice (Fig. 1C, D). Therefore, at least in mice of H2b immune haplotype, one peptide (span aa106–125) of hα-Syn possessed B and Th cell antigenic determinants. Previously, we showed that slightly smaller peptides, aa85–99 and aa109–126 of hα-Syn did not possess Th cell epitopes (Ghochikyan et al., 2014), therefore it was suggested that aa76–85 and aa106–108 of hα-Syn may be important for activation of Th cells. To test this possibility, we performed a separate study and tested Th cell responses in mice immunized and boosted once with full-length hα-Syn protein formulated with adjuvant. The data showed that small peptides spanning aa85–99 and aa109–125 do not activate autoreactive Th cells (Fig. 2). Collectively, these results suggested that B cell epitopes spanning aa85–99 and aa109–126 at the C-terminus of hα-Syn are candidates for prototype PD/DLB vaccine development.
Fig. 1.
Mapping of B cell and T cell epitopes of hα-Syn in C57BL/6 mice immunized with plasmid encoding full length hα-Syn protein followed by electroporation (A, C) or full length hα-Syn recombinant protein formulated with AdvaxCpG adjuvant (B, D). (A, B) B cell epitopes were mapped by analyzing the binding of immune sera (1:1000 for DNA immunized sera and 1:5000 for protein immunized sera) to overlapping hα-Syn peptides or irrelevant peptide by ELISA. (C, D) Th cell responses were detected in splenocytes of immunized mice in vitro re-stimulated with the same overlapping peptides.
Fig. 2.
Peptides spanning aa85–99 and aa109–126 do not possess a T helper cell epitope. Th cell responses specific to indicated peptides were detected in mice immunized and boosted once with recombinant hα-Syn protein.
At the same time, data presented in Fig. 1 did not confirm the existence of B cell antigenic determinants in the hα-Syn region spanning aa126–140 as was shown after immunization of hα-Syn/Tg mice (line D) with recombinant hα-Syn protein formulated with CFA/IFA adjuvant (Masliah et al., 2005). Such differences could be explained by different strains of mice (wild-type mice of H2b immune haplotype vs. hα-Syn/Tg mice of H2dxb immune haplotype), as well as by different adjuvants used for formulation of recombinant protein (AdvaxCpG vs. CFA/IFA). Furthermore, we have previously demonstrated that the B cell epitope aa126–140 attached to one Th epitope (P30) and a carrier peptide is immunogenic. In fact, it induced higher titers of anti-hα-Syn antibodies than B cell epitopes aa85–99 and aa109–125 attached to P30 and the same carrier peptide (Games et al., 2014; Ghochikyan et al., 2014; Mandler et al., 2015; Masliah et al., 2005; Masliah et al., 2011). Therefore, based on these data and results published earlier (Games et al., 2014; Ghochikyan et al., 2014; Mandler et al., 2015; Masliah et al., 2005; Masliah et al., 2011), we decided to use B cell epitopes spanning aa85–99 and aa109–126 of hα-Syn, as well as the epitope aa126–140 for the generation of MultiTEP-based DNA vaccines.
3.2. Generation of MultiTEP platform-based DNA vaccines and analysis of their immunogenicity
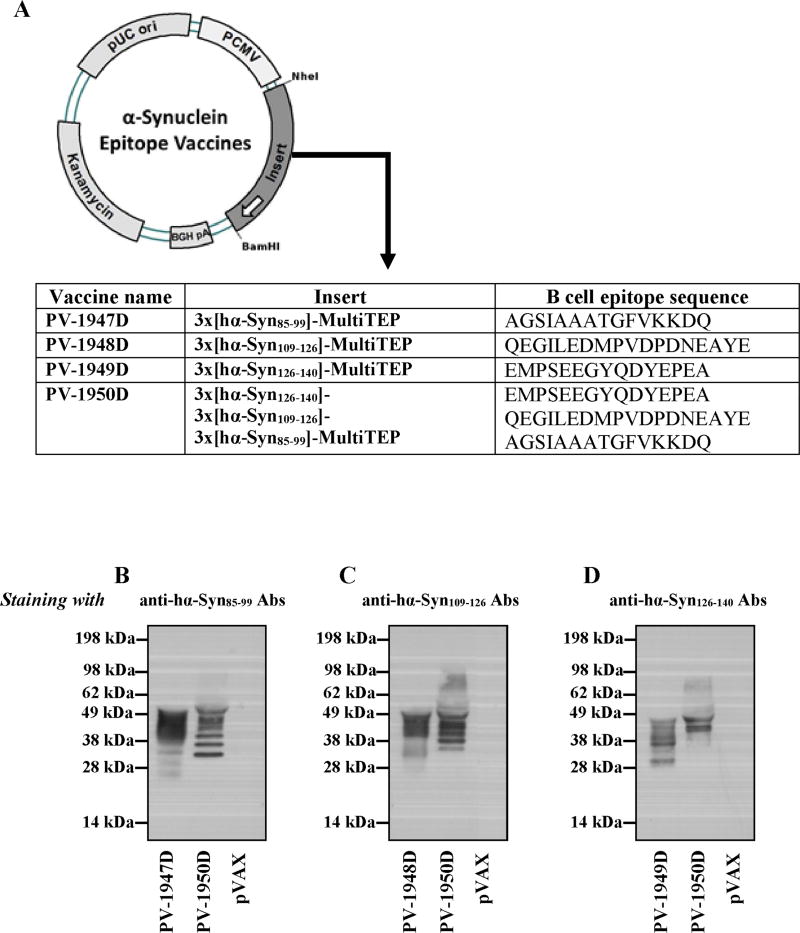
Four plasmids were constructed containing the MultiTEP platform fused with three copies of B cell epitopes, aa85–99 (PV-1947D), aa109–126 (PV-1948D), aa126–140 (PV-1949D) separately or consecutively, but in reverse order [aa126–140 + aa109–126 + aa85–99 (PV-1950D)] (Fig. 3A). Expression of the plasmid construct was confirmed by analyzing the supernatants of transiently transfected CHO cells (Fig. 3B–D) using previously developed antibodies specific to the epitopes (Ghochikyan et al., 2014). We detected several discrete bands on immunoblotting with anti-α-Syn antibodies indicating that α-Syn-MultiTEP proteins are proteolytically processed in CHO cells at varying sites within the MultiTEP sequence. Although it would be interesting to know precisely where these cleavages occur, such experiments are beyond the scope of the current study.
Fig. 3.
Schematic representation of DNA hα-Syn vaccine constructs and expression in transfected CHO cells. (A) Strategy for cloning genes encoding several hα-Syn B cell epitope/s fused with MultiTEP into the pVAX1 vector and schematic representation of PV-1947D, PV-1948D, PV-1949D, and PV-1950D constructs. (B–D) The expression of PV-1947D (B), PV-1948D (C), PV-1949D (D), and PV-1950D (B–D) and secretion of protein was demonstrated by WB of conditioned media of transiently transfected CHO cells. Proteins were visualized by staining with anti-hα-Syn85–99 Abs (A), anti-α-hSyn109–126 Abs (B), and anti-α-hSyn126–140 Abs (C) followed by HRP conjugated anti-mouse IgG.
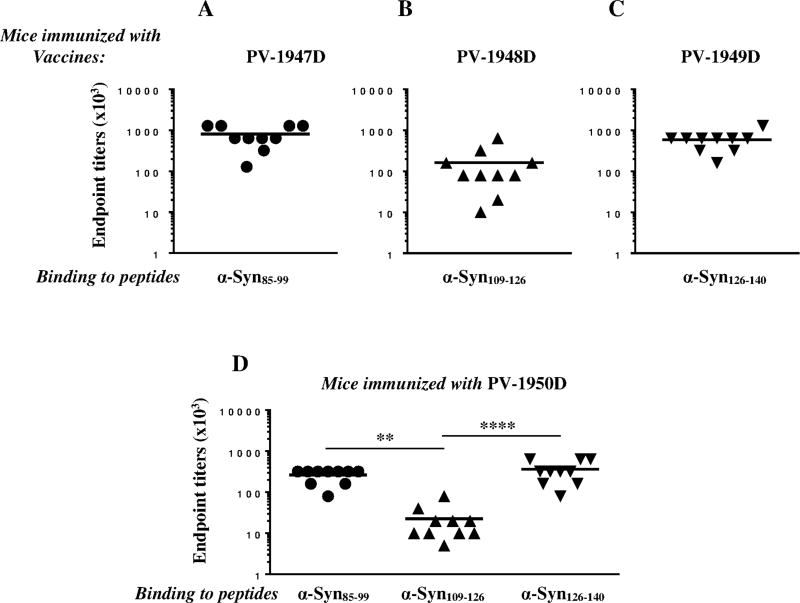
To analyze the immunogenicity of these constructs we immunized mice with PV-1947D, PV-1948D, PV-1949D and PV-1950D vaccines and tested the cellular and humoral immune responses. All four DNA vaccines induced strong Th cell responses specific to the MultiTEP platform, but not self-peptides (aa85–99, aa109–126, aa126–140) or recombinant hα-Syn (Fig. 4A). Therefore, the data suggest that at least in mice of H2b immune haplotype, PV-1947D, PV-1948D, PV-1949D and PV-1950D vaccines do not stimulate potentially harmful autoreactive Th cells specific to B cell epitopes used for construction of the plasmids (Fig. 4). As expected, these MultiTEP-specific Th cells supported production of high titers of antibodies specific to the hα-Syn B cell epitopes chosen for generation of constructs (Fig. 5). Notably, two vaccines, PV-1947D and PV-1949D (Fig. 5A, C) induced significantly higher titers of antibodies specific to their corresponding peptides (p<0.001 and p<0.05, respectively) than PV-1948D vaccine (Fig. 5B). Similar results were obtained after immunization with PV-1950D targeting all three hα-Syn B cell epitopes where significantly higher titers of antibodies specific to aa85–99 and aa126–140 epitopes (p<0.01 and p<0.001, respectively), compared to aa109–126 epitope were detected (Fig. 5D).
Fig. 4.
Immunization with PV-1947D (3hα-Syn85–99-MultiTEP; A), PV-1948D (3hα-Syn109–126-MultiTEP; B), PV-1949D (3hα-Syn126–140-MultiTEP; C), and PV-1950D (3hα-Syn126–140-3hα-Syn109–126-3hα-Syn85–99-MultiTEP; D) vaccines did not induce potentially detrimental autoreactive Th cells, but induced strong Th cell responses specific to the MultiTEP platform. Numbers of IFN-γ producing T-cells were calculated by ELISPOT in splenocyte cultures obtained from immunized animals. Bars represent average ± SD (n=4 per group).
Fig. 5.
DNA vaccines PV-1947D (3hα-Syn85–99-MultiTEP; A), PV-1948D (3hα-Syn109–126-MultiTEP; B), PV-1949D (3hα-Syn126–140-MultiTEP; C), and PV-1950D (3hα-Syn126–140-3hα-Syn109–126-3hα-Syn85–99-MultiTEP; D) induced high titers of antibodies specific to appropriate peptide/s. Endpoint titers of antibodies specific to hα-Syn85–99, hα-Syn109–126, hα-Syn126–140 peptides were detected in sera collected after the third immunization with indicated vaccines by ELISA. Lines indicate the mean values of antibody titers (**P<0.01, ****P<0.0001).
In order to investigate the therapeutic potential of the antibodies induced by the DNA vaccines, we examined the binding of antibodies generated against the various B cell epitopes to hα-Syn recombinant protein. These analyses demonstrated that vaccinated mice produced very high titers of antibodies recognizing recombinant hα-Syn (Fig. 6A–D), and the titers of antibodies generated with PV-1947D and PV-1949D vaccines were significantly higher (p<0.01 and p<0.05, respectively) compared with PV-1948D vaccine (Fig. 6A, C vs. B). These differences in binding to full-length hα-Syn recombinant protein could be explained by stronger immunogenicity of B cell epitopes used for generation of PV-1947D and PV-1949D vs. PV-1948D (Fig. 5A–D), or better accessibility of aa85–99 and aa126–140 epitopes vs. aa109–126 in the vaccines to B cell receptors. Of note, the vaccine composed of three B cell epitopes of hα-Syn, PV-1950D induced significantly higher antibody titers specific to aa109–126 than PV-1948D (p<0.05) (Fig. 6A–D). These data demonstrate that all three B cell epitopes, aa85–99, aa109–126 and aa126–140 are exposed on recombinant hα-Syn protein. Next, we tested accessibility of these epitopes in the protein produced by a human SH-SY5Y neuroblastoma cell line that was modified to stably overexpress hα-Syn. Western blot data presented in Fig. 6E–G show that in reducing conditions purified antibodies specific to all three B cell antigenic determinants bind very well to hα-Syn in lysates of hα-Syn-SH-SY5Y cells. Thus, the Th cells activated by the MultiTEP platform promoted production of antibodies against all three B cell antigenic determinants, aa85–99, aa109–126 and aa126–140 of hα-Syn that are exposed on recombinant hα-Syn protein from E. coli and hα-Syn synthesized in a human neuroblastoma cell line.
Fig. 6.
DNA vaccines induced IgG1, IgG2ab and IgG2b antibodies specific to recombinant hα-Syn and hα-Syn synthesized by SH-SY5Y/hα-Syn cells. (A–D) Endpoint titers of antibodies specific to recombinant hα-Syn protein were detected in sera collected after the third immunization with PV-1947D (p3hα-Syn85–99-MultiTEP; A), PV-1948D (p3hα-Syn109–126-MultiTEP; B), PV-1949D (p3hα-Syn126–140-MultiTEP; C), PV-1950D (p3hα-Syn126–140-3hα-Syn109–126-3hα-Syn85–99-MultiTEP; D). (E–G) Staining of SH-SY5Y/α-Syn and SH-SY5Y/WT control cell lysates with purified anti-hα-Syn antibodies: anti-hα-Syn85–99 (E), anti-hα-Syn109–126 (F), and anti-hα-Syn126–140 (G). Full recombinant hα-Synuclein protein was loaded as a positive control. (H–K) Isotypes of antibodies generated by DNA vaccines. Sera from individual mice were diluted 1:1000.
More than a decade ago Bard et al. showed that the isotype of the anti-Aβ antibody is important for in vitro clearance of pathology (Bard et al., 2003). On the other hand, it was shown that full-length and F(ab')2 fragments of the same antibody clear pathology in APP/Tg mice equally well (Bacskai et al., 2002). Recently, this finding was confirmed when Lee et al. demonstrated that the effector function of an anti-tau antibody Fc fragment was not required for the reduction of pathology in vivo (Lee et al., 2016). Importantly, the authors of this elegant study showed that the antibody with an effector function not only promotes uptake of tau by microglia, but also triggers secretion of pro-inflammatory cytokines in these cells that could have harmful effects on neurons. One advantage of AgilPulse™ mediated delivery of our DNA vaccines for AD is that we can choose different routes of immunization, intradermal (i.d.) or intramuscular (i.m.). Previously we have reported that injections of AV-1959D into the skin of mice induced predominantly Th2-type of humoral responses, while i.m. injections of the same vaccine in the same strain of mice induced predominantly a Th1 type of humoral immune responses (Davtyan et al., 2014b). Accordingly, we measured IgG1, IgG2ab, IgG2b and IgM responses in mice vaccinated with DNA vaccines (Fig. 6H–K). As expected, i.m immunizations with PV-1947D, PV-1948D, PV-1949D, and PV-1950D induced equal levels of antibodies of all three IgG isotypes, with predominant Th1 type of humoral immune response. Since anti-hα-Syn antibody isotypes may be important for their efficacy, in our future studies we are planning to test different routes of vaccination (i.e., i.m. vs. i.d.) in mouse models of PD and DLB.
3.3. DNA vaccine-induced antibody was capable of binding to native hα-Syn
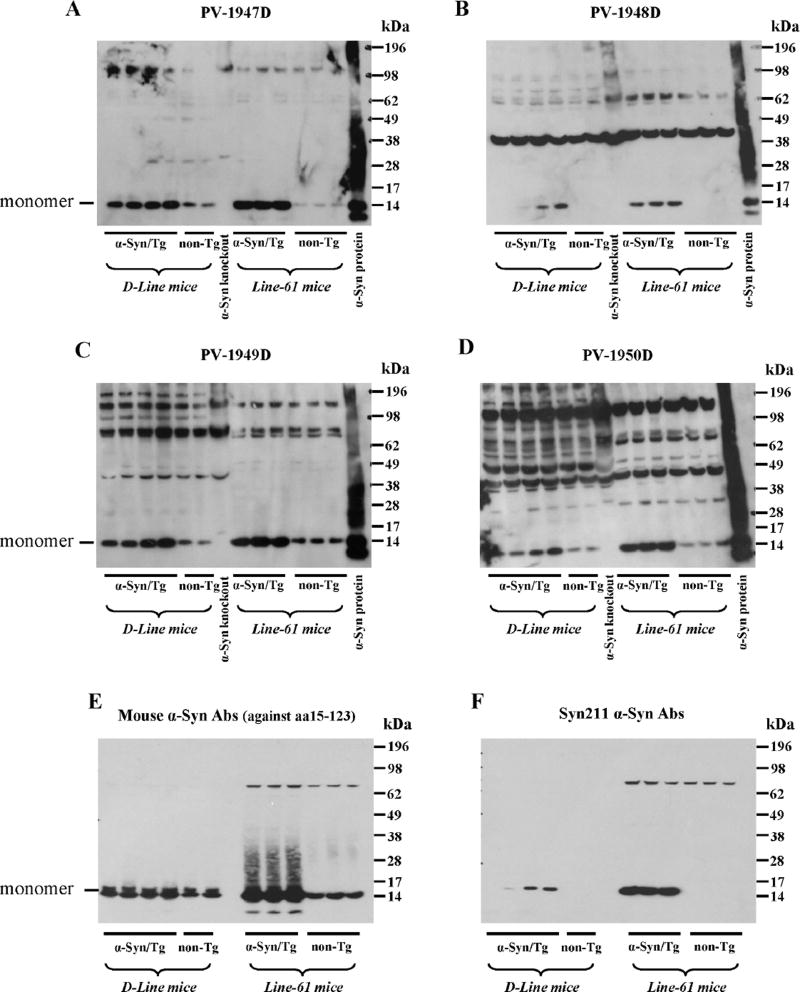
Next, we evaluated the specificity of antibodies generated with all four vaccines to hα-Syn expressed in the brains of two α-Syn/Tg mouse strains (line D and line 61) and their non-transgenic littermates (non-Tg). Line D expressing hα-syn under the regulatory control of the platelet-derived growth factor-β (PDGFβ) promoter (Masliah et al., 2000) displayed abnormal accumulation of detergent-insoluble hα-syn and developed hα-syn-immunoreactive inclusion-like structures in cortical and subcortical regions of the brain. Line 61 mice expressing hα-syn under the mThy1 promoter showed higher level of expression than line D. In these mice hα-syn accumulates in cell bodies of pyramidal neurons in the neocortex and limbic system (Rockenstein et al., 2002). Of note, we used two different methods for preparation of the soluble fractions of brain homogenates (see Method section) and used brain extract of α-Syn knockout mice as a negative control (Fig. 7A–G). Brain homogenates were analyzed using immune sera from vaccinated mice (Fig. 7A–D), two commercial monoclonal antibodies, Syn211 (recognizing only hα-Syn) and anti-Syn15–123 (recognizing both hα-Syn and mouse α-Syn) (Fig. 7E, F), as well as immune sera from mice vaccinated with irrelevant antigen (Fig.7G). This data demonstrated that antibodies generated by PV-1947D, PV-1948D, and PV-1949D bound to monomeric hα-Syn isolated from the brains of both strains of Tg mice (Fig. 7). However, antibodies generated by PV-1948D (aa109–126), as well as Syn211 mAb bound only to hα-Syn (Fig. 7B, F), while anti-aa85–99 and anti-aa126–140 antibodies induced by PV-1947D and PV-1949D along with anti-Syn15–123 mAb were capable of binding to both hα-Syn and mouse α-Syn (Fig. 7A, C, E). As expected from these data, the PV-1950D vaccine induced antibodies that also recognized both hα-Syn and mouse α-Syn (Fig. 7D). Of note, binding of antibodies to mouse α-Syn in brain homogenates of non-Tg littermates is significantly weaker than binding to hα-Syn in brain homogenates of both strains of Tg mice (Fig. 7). Previously it was reported that oligomeric forms of hα-Syn in the soluble fraction of brain homogenates of line D and line 61 mice (Games et al., 2014; Mandler et al., 2014; Masliah et al., 2005), as well as MBP-α-Syn Tg mice (Games et al., 2014; Mandler et al., 2015; Mandler et al., 2014; Masliah et al., 2005; Masliah et al., 2011) could be detected by WB. However, our WB analysis of brain extracts from both line D, line 61 and non-Tg littermates suggested that antibodies generated by all four vaccines recognized monomeric forms of hα-Syn, while high molecular weight bands are likely non-specific (Fig. 7). In fact, similar bands have been detected after analyzing the same homogenates with irrelevant immune sera (Fig. 7G), but not with commercial mAb that recognized only one non-specific high MW band in Line 61 Tg mice (Fig. 7E,F). To confirm these data, we tested the binding of these antibodies to the soluble fraction of brain homogenates of α-Syn knockout mice. As we expected, the same high molecular weight bands were visualized in lanes loaded with brain extracts of α-Syn knockout mice in all immunoblots (Fig. 7A–D). We further analyzed insoluble fractions of brain homogenates from hα-Syn/Tg and non-Tg mice. Similarly, to soluble fractions, all high-molecular weight bands detected by WB appeared to be non-specific and were detected in non-Tg animals (data not shown). Of note, at least one epitope used for generation of antibodies is the same that was described in (Games et al., 2014), allowing to suggest that the difference between our data and results reported previously is likely related to the varying methods of detection of oligomers in brain homogenates. Therefore, we analyzed the reactivity of immune sera generated by PV-1947D, PV-1948D, PV-1949D, and PV-1950D to the monomeric and aggregated forms of recombinant hα-Syn generated by cross-linking with 4-hydroxy-2-nonenal (Roberts et al., 2015). Data demonstrated that all four vaccines induced antibodies that specifically recognized not only monomers, but also all types of hα-Syn oligomers generated in vitro (Fig. 7H–K). Interestingly, two control commercial anti-α-Syn antibodies (anti-Syn15–123 and Syn211) also recognized hα-Syn monomers and oligomers (Fig. 7L, M). Next, we tested the reactivity of antibodies to the pathological hα-Syn molecules in brains homogenates from DLB and control non-DLB cases. Our data showed that all immune sera recognized monomeric hα-Syn in the soluble fraction of brain homogenates (Fig. 8A–D). Notably, antibodies generated by PV-1947D and PV-1950D vaccine recognized hα-Syn stronger compared to PV-1948D and PV-1949D vaccines and it was slightly different from the results obtained with hα-Syn in brain homogenates of Tg mice (Fig. 7). Binding of immune sera from vaccinated mice to homogenates from postmortem DLB cases was compared to that with commercial mAb (Fig. 8E, F) and immune sera from mice vaccinated with irrelevant antigen (Fig. 8G). Data (Fig. 8) suggested that the method used for preparation of homogenates did not allow obtaining oligomers visualized in Western blot. Analyses of insoluble fractions of DLB and control brain homogenates also showed strong binding to hα-Syn monomers, whereas binding to high molecular weight bands was found to be non-specific as processing of blots with irrelevant serum produces the same high molecular weight bands (data not shown).
Fig. 7.
DNA vaccines induced antibodies that recognize hα-Syn monomers in brain homogenates from hα-Syn/tg mice (line D and line 61) and both monomers and oligomers generated in vitro from recombinant protein. Brain homogenates from non-Tg mice and α-Syn KO mice were used as controls. Staining was done with sera collected from mice immunized with different DNA vaccines: PV-1947D (p3hα-Syn85–99-MultiTEP; A), PV-1948D (p3hα-Syn109–126-MultiTEP; B), PV-1949D (p3hα-Syn126–140-MultiTEP; C), PV-1950D (p3hα-Syn126–140-3hα-Syn109–126-3hα-Syn85–99-MultiTEP; D). Of note, PV-1948D immunized sera recognized only hα-Syn, while all other DNA vaccines induced antibodies that also recognized mouse α-Syn (A–D). Mouse anti-α-Syn antibody against aa15–123; (E) and Syn211 anti-α-Syn antibody (F) were used as positive controls, and irrelevant immune sera served as a negative control (G). Oligomers and monomer were prepared from recombinant protein and immunostained with the same immune sera (H–K) or commercial mouse α-Syn antibody (L) and Syn211 anti-α-Syn antibody (M).
Fig. 8.
DNA vaccines induced antibodies that recognize hα-Syn in DLB brain homogenates. Staining was done with sera collected from mice immunized with different DNA vaccines: PV-1947D (p3hα-Syn85–99-MultiTEP; A), PV-1948D (p3hα-Syn109–126-MultiTEP; B), PV-1949D (p3hα-Syn126–140-MultiTEP; C), PV-1950D (p3hα-Syn126–140-3hα-Syn109–126-3hα-Syn85–99-MultiTEP; D). Mouse anti-α-Syn antibody against aa15–123 (E) and Syn211 anti-α-Syn antibody (F) were used as positive controls, and irrelevant sera was used as a negative control (G). DLB –Dementia with Lewy Bodies brain extract; Ctr – Non-DLB brain extract.
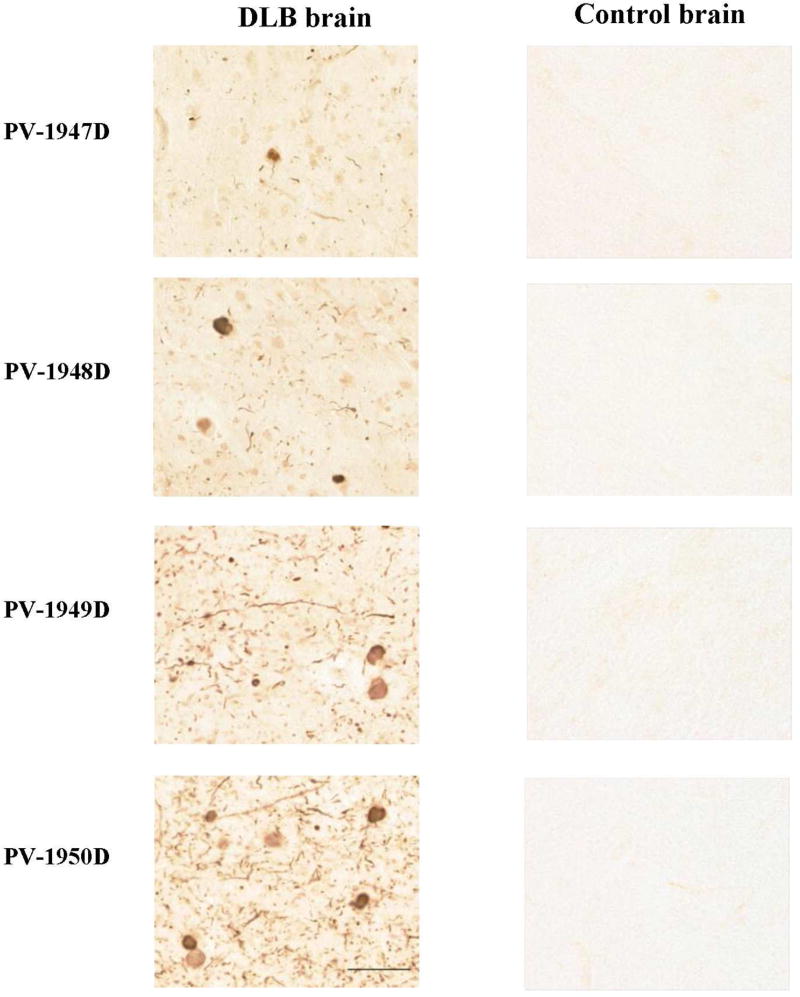
Therefore, in this study we could not detect α-Syn oligomers in brain extracts not only from two hα-Syn Tg mice strains, but also in three DLB cases via standard WB methods. A potential explanation of this phenomenon could be the instability of α-Syn oligomers leading to their disruption during sample preparation or electrophoresis. Such instability would indeed be expected, considering the evidence of functional polymerization of α-Syn, which would likely involve a functional de-polymerization as well (Burre et al., 2014; Kim et al., 2013; Wang et al., 2014). Therefore, α-Syn oligomers would be expected to be unstable and easily dissociable, to compensate for their high formation rate (Lashuel et al., 2013). In light of this, we decided to detect binding of antibodies generated by all four vaccines to pathological forms of hα-Syn (LBs, LNs) using the 40 um-thick sections of the anterior cingulate cortex of a DLB case compared to the similar region of a control non-DLB brain, in regard to a total Synuclein pathology of LBs and affected neuropil containing LNs. We observed a wide range of variation in positive specific staining for hα-Syn pathology. The strongest immunoreactivity with the most densely clustered LBs and pronounced LNs was observed with antibodies generated against PV-1950D, followed by PV-1949D, PV-1948D and PV-1947D, respectively (Fig. 9). The relative differences in immunoreactivity detected by IHC could be explained by better accessibility of hα-Syn126–140 versus hα-Syn85–99 and both versus hα-Syn109–126, since in these studies we have used equal concentrations of antibodies based on ELISA data. Importantly, none of the antibodies recognized Synuclein-related pathology in the control brain sections (Fig. 9). Although all vaccines induced Abs that clearly recognize monomeric recombinant and cellular hα-Syn, we did not detect normal hα-Syn in control brains since tissues were pretreated with protinase K that may completely break down normal synuclein, while sparing aggregated synuclein. These data suggest that the PV-1950D vaccine targeting all three B cell epitopes of the hα-Syn simultaneously may represent a feasible immunotherapeutic strategy, and we are currently testing the immunogenicity and efficacy of this DNA vaccine and its recombinant protein version (PV-1950R) in line D hα-Syn Tg mice.
Fig. 9.
Antibodies induced by DNA vaccines and full-length hα-Syn protein recognize LBs and LNs in the anterior cingulate gyrus of DLB case, but not in the similar region of a control brain, showing the range of variation in the positive specific staining for total Synuclein pathology. Most pronounced and densely clustered LBs and LNs were observed in DLB brain sections stained with antibodies generated through vaccination with PV-1950D (p3hα-Syn126–140-3hα-Syn109–126-3hα-Syn85–99-MultiTEP), followed by PV-1949D (p3hα-Syn126–140-MultiTEP), PV-1948D (p3hα-Syn109–126-MultiTEP) and PV-1947D (p3hα-Syn85–99-MultiTEP), respectively. Photomicrographs depict immunoreactive profiles at 40x original magnification, scale bar = 50 um.
4. Conclusions
We have used a DNA vaccination strategy for the development of vaccines that target various individual B cell epitopes of hα-Syn and several epitopes simultaneously. Taking advantage of our proprietary universal vaccine platform, MultiTEP, we generated four different vaccines and demonstrated their immunogenicity in wild-type mice. We have also demonstrated that our anti-hα-Syn vaccines did not induce potentially harmful Th cell responses, generating instead the therapeutically relevant concentrations of hα-Syn antibodies. Furthermore, testing of the therapeutic efficacy of these novel DNA epitope vaccines in Tg mouse models of α-synucleinopathies will facilitate translation of the most effective DNA vaccine to human clinical trials.
In fact, the only anti-hα-syn active vaccine that is currently in clinical trial is PD01 (Affiris) composed of B cell epitope mimicking hα-Syn110–130 linked to KLH and formulated in Alum [(Mandler et al., 2015; Mandler et al., 2014) and NCT01568099]. All three other similarly designed anti-Aβ vaccines from Affiris (AD01, 02, 03) have failed in clinical trials likely because they did not induce sufficient anti-Aβ antibody responses in vaccinated people, although they did generate undesirable strong antibody responses to the KLH. Our vaccination strategy based on the MultiTEP platform technology provides a unique opportunity to generate high antibody responses in the great majority of vaccinated subjects by activating broad repertoire of both naive and memory (previously generated in the human population in response to infection or vaccination by TT, HBV and Flu) Th cells that could be especially beneficial in the elderly population with immunosenescence. In this manuscript, we are reporting for the first time on immunogenicity of all four DNA vaccines based on this universal platform. Future efficacy studies will demonstrate which DNA vaccine described herein is most suitable for translation to clinical trials.
Highlights.
Four novel MultiTEP platform based anti-hα-Syn DNA vaccines are developed
All four vaccines generate high titers of anti-hα-Syn antibodies in mice
Generated antibodies recognize pathological hα-Syn in the brain tissues of DLB cases
Acknowledgments
We would like to thank Mr. Tommy Saing for helping with homogenization of DLB brains. This work was supported by fundings from NIH (AG-20241, NS-50895) and Alzheimer’s Association (IIRG-12-239626 and NIRG-13-281227). Additional support for DLB case tissues was provided by University of California, Irvine Alzheimer’s Disease Research Center Grant P50 AG16573.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
MGA is a co-founder of Neuroimmune that licensed MultiTEP vaccine platform technology from the Institute for Molecular Medicine.
The remaining authors have no financial or commercial conflict of interests.
Authors’ contribution
HD, KZ, DHC, MGA and AG conceived the study and participated in its design. HD, KZ, IP, KK, JP and NRSG performed the experiments. MBJ generated the SH-SY5Y/hα-Syn cells. MBJ, EM and DHC helped with interpretation of data. HD, MGA and AG wrote the paper. All authors read and approved the final manuscript.
References
- Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol Res. 2008;42(1–3):219–232. doi: 10.1007/s12026-008-8076-3. [DOI] [PubMed] [Google Scholar]
- Agadjanyan MG, Wang B, Nyland SB, Weiner DB, Ugen KE. DNA plasmid based vaccination against the oncogenic human T cell leukemia virus type 1. Current Topics in Microbiology and Immunology. 1998;226(3):175–192. doi: 10.1007/978-3-642-80475-5_11. [DOI] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22(18):7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiola N, de Oliveira RM, Herrera F, Guardia-Laguarta C, Goncalves SA, Pera M, Suarez-Calvet M, Clarimon J, Outeiro TF, Lleo A. Tau enhances alpha-synuclein aggregation and toxicity in cellular models of synucleinopathy. PLoS One. 2011;6(10):e26609. doi: 10.1371/journal.pone.0026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100(4):2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Sudhof TC. alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A. 2014;111(40):E4274–4283. doi: 10.1073/pnas.1416598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Tran M, Vasilevko V, Petrushina I, Sadzikava N, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol. 2003;15(4):505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Cribbs DH, Agadjanyan MG. The MultiTEP platform-based Alzheimer's disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimers Dement. 2014a;10(3):271–283. doi: 10.1016/j.jalz.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Poghosyan A, Marleau AM, Movsesyan N, Kiyatkin A, Rasool S, Larsen AK, Madsen PJ, Wegener KM, Ditlevsen DK, Cribbs DH, Pedersen LO, Agadjanyan MG. Immunogenicity, Efficacy, Safety, and Mechanism of Action of Epitope Vaccine (Lu AF20513) for Alzheimer's Disease: Prelude to a Clinical Trial. J Neurosci. 2013;33(11):4923–4934. doi: 10.1523/JNEUROSCI.4672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Hovakimyan A, Zagorski K, Davtyan A, Petrushina I, Agdashian D, Murthy V, Cribbs DH, Agadjanyan MG, Ghochikyan A. BTX AgilePulse(TM) system is an effective electroporation device for intramuscular and intradermal delivery of DNA vaccine. Curr Gene Ther. 2014b;14(3):190–199. doi: 10.2174/1566523214666140522121427. [DOI] [PubMed] [Google Scholar]
- Evans CF, Davtyan H, Petrushina I, Hovakimyan A, Davtyan A, Hannaman D, Cribbs DH, Agadjanyan MG, Ghochikyan A. Epitope-based DNA vaccine for Alzheimer's disease: Translational study in macaques. Alzheimers Dement. 2014;10(3):284–295. doi: 10.1016/j.jalz.2013.04.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351(24):2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM, Trojanowski JQ. Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam. Am J Pathol. 2002;160(5):1725–1731. doi: 10.1016/s0002-9440(10)61119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo G, Schluter OM, Sudhof TC. A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci. 2008;11(3):301–308. doi: 10.1038/nn2058. [DOI] [PubMed] [Google Scholar]
- Games D, Valera E, Spencer B, Rockenstein E, Mante M, Adame A, Patrick C, Ubhi K, Nuber S, Sacayon P, Zago W, Seubert P, Barbour R, Schenk D, Masliah E. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson's disease-like models. J Neurosci. 2014;34(28):9441–9454. doi: 10.1523/JNEUROSCI.5314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A. Rationale for peptide and DNA based epitope vaccines for Alzheimer's disease immunotherapy. CNS Neurol Disord Drug Targets. 2009;8(2):128–143. doi: 10.2174/187152709787847298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, Davtyan H, Petrushina I, Hovakimyan A, Movsesyan N, Davtyan A, Kiyatkin A, Cribbs DH, Agadjanyan MG. Refinement of a DNA based Alzheimer's disease epitope vaccine in rabbits. Hum Vaccin Immunother. 2013;9(5):1002–1010. doi: 10.4161/hv.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, Petrushina I, Davtyan H, Hovakimyan A, Saing T, Davtyan A, Cribbs DH, Agadjanyan MG. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human alpha-synuclein: feasibility study. Neurosci Lett. 2014;560:86–91. doi: 10.1016/j.neulet.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003a;300(5619):636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Lee VM, Trojanowski JQ. Interactions of amyloidogenic proteins. Neuromolecular Med. 2003b;4(1–2):49–58. doi: 10.1385/NMM:4:1-2:49. [DOI] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Nottingham LK, Wilson DM, Bagarazzi ML, Tsai A, Morrison LD, Javadian A, Chalian AA, Agadjanyan MG, Weiner DB. Engineering DNA vaccines via co-delivery of co-stimulatory molecule genes. Vaccine. 1998;16(19):1828–1835. doi: 10.1016/s0264-410x(98)00177-7. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, Tabbal SD, Perlmutter JS. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol. 2012;69(10):1326–1331. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Le Pichon CE, Adolfsson O, Gafner V, Pihlgren M, Lin H, Solanoy H, Brendza R, Ngu H, Foreman O, Chan R, Ernst JA, DiCara D, Hotzel I, Srinivasan K, Hansen DV, Atwal J, Lu Y, Bumbaca D, Pfeifer A, Watts RJ, Muhs A, Scearce-Levie K, Ayalon G. Antibody-Mediated Targeting of Tau In Vivo Does Not Require Effector Function and Microglial Engagement. Cell Rep. 2016;16(6):1690–1700. doi: 10.1016/j.celrep.2016.06.099. [DOI] [PubMed] [Google Scholar]
- Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012;162(2–3):171–182. doi: 10.1016/j.jbiotec.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamikonyan G, Necula M, Mkrtichyan M, Ghochikyan A, Petrushina I, Movsesyan N, Mina E, Kiyatkin A, Glabe C, Cribbs DH, Agadjanyan MG. Anti-Abeta 1–11 antibody binds to different beta-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils, but not the most toxic oligomers. J Biol Chem. 2007;282:22376–22386. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R. Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer's and Parkinson's in dementia with Lewy body disease. Neurochem Res. 2006;31(9):1153–1162. doi: 10.1007/s11064-006-9140-9. [DOI] [PubMed] [Google Scholar]
- Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, Adame A, Schmidhuber S, Santic R, Schneeberger A, Schmidt W, Mattner F, Masliah E. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener. 2015;10:10. doi: 10.1186/s13024-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler M, Valera E, Rockenstein E, Weninger H, Patrick C, Adame A, Santic R, Meindl S, Vigl B, Smrzka O, Schneeberger A, Mattner F, Masliah E. Next-generation active immunization approach for synucleinopathies: implications for Parkinson's disease clinical trials. Acta Neuropathol. 2014;127(6):861–879. doi: 10.1007/s00401-014-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui W, Iseki E, Ueda K, Kosaka K. Occurrence of human alpha-synuclein immunoreactive neurons with neurofibrillary tangle formation in the limbic areas of patients with Alzheimer's disease. J Neurol Sci. 2000;174(2):81–84. doi: 10.1016/s0022-510x(99)00327-5. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46(6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6(4):e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The alpha-synuclein burden hypothesis of Parkinson disease and its relationship to Alzheimer disease. Exp Neurol. 2008;212(2):235–238. doi: 10.1016/j.expneurol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG. Reducing AD-like pathology in 3×Tg-AD mouse model by DNA epitope vaccine- a novel immunotherapeutic strategy. PLos ONE. 2008a;3(5):e21–24. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan N, Mkrtichyan M, Petrushina I, Ross TM, Cribbs DH, Agadjanyan MG, Ghochikyan A. DNA epitope vaccine containing complement component C3d enhances anti-amyloid-beta antibody production and polarizes the immune response towards a Th2 phenotype. J Neuroimmunol. 2008b;205(1–2):57–63. doi: 10.1016/j.jneuroim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210(2):409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, Patel A, Head E, Cribbs DH, Agadjanyan MG. Alzheimer's Disease Peptide Epitope Vaccine Reduces Insoluble But Not Soluble/Oligomeric A{beta} Species in Amyloid Precursor Protein Transgenic Mice. J Neurosci. 2007;27(46):12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson's disease brain. Brain. 2015;138(Pt 6):1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68(5):568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse [see comments] Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX, Masliah E. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer's and Parkinson's diseases. PLoS One. 2008;3(9):e3135. doi: 10.1371/journal.pone.0003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugen KE, Kutzler MA, Marrero B, Westover J, Coppola D, Weiner DB, Heller R. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 2006;13(10):969–974. doi: 10.1038/sj.cgt.7700973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. alpha-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr Biol. 2014;24(19):2319–2326. doi: 10.1016/j.cub.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]