Abstract
Adenosine Deaminases acting on RNA (ADARs) are proteins that catalyze widespread A-to-I editing within RNA sequences. We recently reported that ADAR2 edits and stabilizes nuclear-retained Cat2 transcribed nuclear RNA (Ctn RNA). Here, we report that ADAR1 coordinates with ADAR2 to regulate editing and stability of Ctn RNA. We observe an RNA-dependent interaction between ADAR1 and ADAR2. Furthermore, ADAR1 negatively regulates interaction of Ctn RNA with RNA-destabilizing proteins. We also show that breast cancer (BC) cells display elevated ADAR1 but not ADAR2 levels, compared to non-tumorigenic cells. Additionally, BC patients with elevated levels of ADAR1 show low survival. Our findings provide insights into overlapping substrate preferences of ADARs and potential involvement of ADAR1 in breast cancer.
Keywords: ADAR, Ctn RNA, RNA editing, mRNA stability, breast cancer, HuR
Introduction
RNA-editing proteins are an important class of proteins that regulate key steps in post-transcriptional RNA processing. Adenosine deaminases acting on RNA (ADARs) are double-stranded RNA-editing enzymes that catalyze the hydrolytic deamination of adenosine to inosine, a process referred to as Adenosine-to-Inosine editing (A-to-I editing) [1, 2]. Since the cellular machinery recognizes inosine as guanosine, A-to-I editing results in the alteration of RNA sequence information. While three types of ADAR enzymes, namely ADAR1, ADAR2, ADAR3, have been identified in mammals to-date, only ADAR1 and ADAR2 exhibit editing enzymatic activity [2, 3].
Several studies have shown that A-to-I editing events occur widely within the transcriptome, predominantly in non-coding regions such as introns or untranslated regions (UTRs) of mRNAs and long noncoding RNAs [4–7]. It is estimated that ~85% of pre-mRNAs may be edited within their 3’UTRs or introns [8]. In these non-coding regions, Alu repetitive sequences of short interspersed element (SINE) origin form intra-molecular long RNA duplexes with nearby inverted Alu sequences (IRAlus), and are recognized by ADARs for A-to-I editing [4, 9, 10]. While these studies provide important insights into the global A-to-I editing events in the transcriptome, the individual contributions of ADAR1 and ADAR2 are largely unknown. Such studies would help understand the physiological and pathological effects of the unique and overlapping interactions of ADARs with their substrates.
At the molecular level, A-to-I editing can affect several processes related to gene expression and regulation, such as pre-mRNA splicing, RNA stability, RNA localization, miRNA function, and translation [11–20]. Both Adar1 and Adar2 are essential in mammals; loss of Adar1 is lethal by embryonic day 12.5 and Adar2 knockout mice die within a few weeks after birth [21, 22]. Several physiological roles have been attributed to ADARs. For example, ADAR1 is a major regulator of type-I interferon response [23–25]. Mutations in Adar1 cause the Aicardi-Goutières syndrome (AGS) that results from the chronic activation of the type-I interferon response [26]. Studies have also shown that ADAR1 is a causative gene of the autosomal dominant disorder Dyschromatosis Symmetrica Hereditaria (DSH) [27]. Defective ADAR2 activity is associated with the selective death of motor neurons in a neurodegenerative disorder, amyotrophic lateral sclerosis (ALS) [28]. A-to-I editing is involved in other physiological processes such as human embryogenesis, atherosclerosis, B-cell lineage development and liver inflammation and fibrosis [29–32]. Additionally, A-to-I editing is de-regulated in several cancers such as hepatocarcinoma, glioblastoma, breast cancer, prostate cancer and chronic myeloid leukemia [33–39]. However, a detailed understanding of the role of ADARs in these physiological and cancer-related functions is limited.
We have previously reported a novel function for ADAR2 in A-to-I editing and promoting the stability of nuclear-retained Ctn RNA by antagonizing the interaction between Ctn RNA and the decay-promoting RNA-binding proteins ELAVL1/HuR and PARN [poly(A)-specific ribonuclease] deadenylase [40]. In the present study, we show that like ADAR2, ADAR1 also edits and stabilizes Ctn RNA, suggesting that Ctn RNA requires both ADAR1 and ADAR2 for its stability. Using biochemical- and meta-analysis from transcriptome data, we identified a significant number of RNA substrates that potentially utilize the interplay of both ADAR1 and ADAR2 for their cellular functions. Our data underscore the presence of RNA-mediated dynamic interactions between ADAR1 and ADAR2. In addition to learning about the mechanistic interplay of both ADAR proteins, we also investigated their involvement in disease. Recent studies have reported that A-to-I editing is deregulated in several cancers, including breast cancer [33–39]. These studies indicate the potential for ADARs to serve as prognostic markers and their involvement in regulating breast cancer progression. To extend these observations, we measured ADAR1 and ADAR2 mRNA and protein levels in various breast cancer subtypes by utilizing publicly available cancer genome transcriptome data or breast cancer isogenic model cell lines. Our results suggest that ADAR1 but not ADAR2 is elevated in all breast cancer subtypes compared to non-tumorigenic cells, suggesting its potential prognostic value and involvement in breast cancer progression. Collectively, the present study provides insights into the relative contribution of ADAR1 and ADAR2 in normal physiology and disease.
Materials and Methods
RNA Editing
To measure A-to-I editing levels, FwR and IR2 repeat sequences were amplified using repeat-specific primers by RT-PCR and products of the expected size were sequenced. In the electropherograms, editing sites appeared as mixed A and G peaks. Editing levels were determined by measuring peak heights using Bioedit software and were calculated as a ratio of G-peak height to the A+G-peak height (G/G+A).
To determine changes in RNA editing from RNA-seq data, the known list of A-to-I RNA editing sites was downloaded from Rigorously Annotated Database of A-to-I RNA Editing (version 2), which contained 8823 sites in total [41]. We counted the editing events in these sites using SAMtools mpileup [42] with parameter -q 10 (skipping the alignments with mapping quality less than 10), and only kept the editing sites with at least two supporting reads [40]. This analysis had been performed in our previous study [40]. In the current study, to quantitatively represent changes in editing in WT-MEFs and Adar2-KO MEFs, editing in Adar2-KO was divided by editing in WT-MEFs (Table S1).
RNA stability Assay
To measure RNA stability, 5 µg/ml Actinomycin D (Sigma-aldrich, USA) was added to cells treated with control or Adar1 siRNA for 48 h and incubated for different time durations with Actinomycin D as indicated. At each time point, total RNA was harvested using Trizol and used for reverse transcription and quantitative PCR (RT-qPCR) analysis. RNA decay rate was quantified by fitting an exponential curve to the data points (y = a * e^−bt), whereby y is the (relative) amount of RNA and t is time. The half-life was then calculated using: t(1/2) = ln2 / b [43]. The half-life was calculated from each experiment and the average half-life along with standard deviations shown in the graph.
Ribonucleoprotein Immunoprecipitation (RIP)
RIP was performed using an established protocol [44]. Transformed WT-MEFs and Adar2-KO MEFs (1×107) were transfected with FLAG-Adar1 for 48hrs. These cells were used for RNA immunoprecipitation utilizing reversible chemical crosslinking of RNA-protein interactions by formaldehyde followed by immunoprecipitation using Anti-FLAG antibody (F1804, Sigma, USA). Following IP, extracts were reverse cross-linked and total RNA was extracted using Trizol LS (Invitrogen, USA). Extracted RNA was treated with RNase-free DNase I (Sigma, USA), and RT was conducted using random-hexamer primers as per the manufacturer’s instructions (Applied Biosystems, USA). qPCR analysis was performed using gene-specific primers.
ADAR Co-Immunoprecipitation
Cells were lysed in lysis buffer (50mM Tris-HCl pH7.4, 150mM NaCl, 2mM EDTA, 0.5% NP-40, 1mM PMSF and protease inhibitors) for 30 minutes at 4°C. The lysate was pre-cleared using Gammabind Sepharose protein-G beads for 30 minutes at 4°C prior to incubation with anti-GFP (Roche) antibody overnight. Protein complexes were pulled down using GammaBind Sepharose (GE Healthcare, USA) beads for 1.5 h followed by three 10 min washes with lysis buffer. Loading dye was added to the beads followed by boiling for 5 min and used for western blot analysis. For EtBr treatment, the immunoprecipitation was performed with 50ug/ml of EtBr (Sigma, USA) in the lysate and the beads were washed in the presence of 50ug/ml EtBr in the buffer. For RNAse A treatment, immunoprecipitation was performed as described above. After pulling down the complexes, the beads were incubated in lysis buffer containing 0.1ug/ul RNase A for 30 min at room temperature followed by two washes of 5 min each with lysis buffer before boiling the samples for western blot analysis. Anti-GFP (Roche life sciences) and Anti-FLAG (F1804, Sigma, USA) antibodies were used for IP and to detect proteins by Western Blotting.
Extended materials and methods can be found in the supporting data.
Results
Both ADAR1 and ADAR2 edit the same sites within the 3’UTR of Ctn RNA
Ctn RNA harbors three inverted repeat elements (IR1, IR2, and IR3) of SINE origin in its 3’UTR, that are inverted with respect to the forward repeat (FwR) (Fig. 1A, C). Recently, we have shown that ADAR2 edits several of the adenosines within the FwR and IR2 [40]. We aimed to determine if ADAR1 too contributes to A-to-I editing of Ctn RNA. To this end, we determined the editing profile of endogenous Ctn RNA in Adar1-KO (knock-out) MEFs (mouse embryonic fibroblasts) (Fig. S1). RT-PCR products of FwR and IR2 were directly sequenced and analyzed for A-to-G mismatches (observed as A and G dual peaks in the electropherogram) at the predicted sites (Fig S1). Similar to our results in Adar2-KO MEFs, we observed a dramatic reduction in editing within the FwR and IR2 of Ctn RNA in Adar1-KO MEFs compared to wild-type (WT) MEFs (Fig. 1A–D). As expected, compared to WT-MEFs, Adar1/2-double KO MEFs (DKO) showed complete loss of editing (Fig 1B and D). These results indicate that both ADAR1 and ADAR2 edit the FwR and IR2 in the 3’UTR of Ctn RNA.
Figure 1. ADAR1 edits Ctn RNA.
Schematic of transcript organization of Ctn RNA, indicating edited adenosines (red) in (A) the forward repeat (FwR) and (C) inverted repeat 2 (IR2). Numbers below the sequence indicates the position of the edited adenosine. Relative % of unedited versus edited adenosines at each site in (B) FwR and (D) IR2 of Ctn RNA in WT-MEFs, Adar1-KO and DKO (double knockout, Adar1/Adar2-KO MEFs). Error bars in B & D represent mean ± SD of three independent experiments (biological replicates).
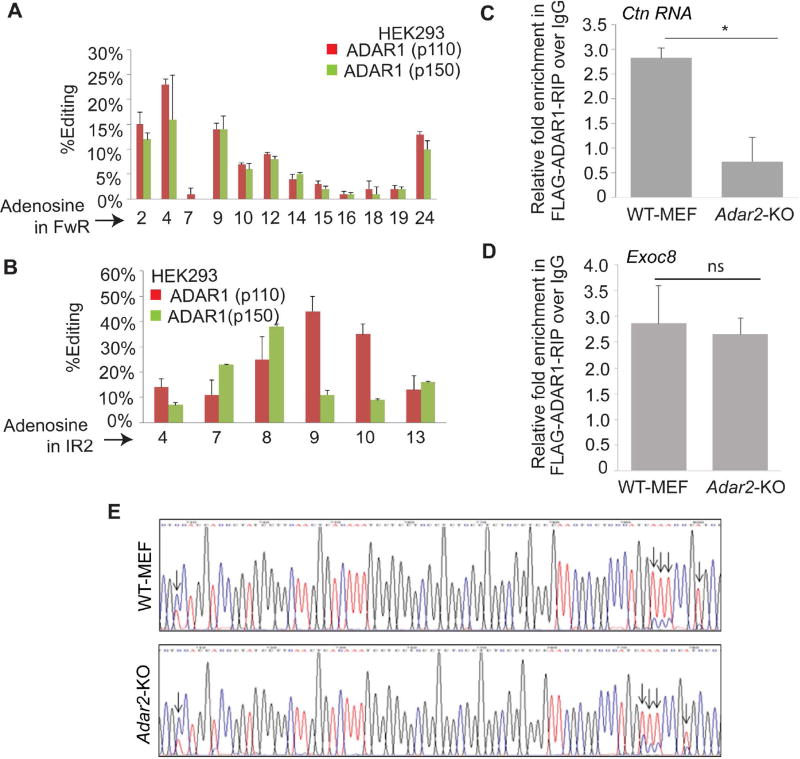
To further confirm the ability of ADAR1 and ADAR2 to edit Ctn RNA, we transiently transfected Ctn RNA alone or in combination with Flag-tagged Adar1-p110 or Adar1-p150 respectively, in HEK293 cells (Fig. S2). Previous studies have demonstrated that HEK293 cells have been used as a system to study A-to-I editing of substrates by exogenously expressing ADAR forms as these cells display low intrinsic A-to-I editing activity [45–48]. Therefore, we determined the editing profile of Ctn RNA in untransfected and ADAR1 transfected HEK293 cells (Fig S3). In cells where ADAR1 isoforms were exogenously expressed, several adenosine residues within the FwR and IR2 of Ctn RNA showed consistent A-to-I editing (Fig. S3, 2A–B). Taken together, our current results and previous findings show that both ADAR1 and ADAR2 edit specific sites within Ctn RNA [40].
Figure 2. ADAR1 edits Ctn RNA in presence of ADAR2.
(A) Graph showing % editing by ADAR1 (p110 and p150 isoforms of ADAR1) for each edited position (calculated as %G/G+A) within the (A) FwR and (B) IR2 in HEK293 cells that were transfected with Ctn RNA and Flag-Adar1-p110 or Flag-Adar1-p150. The X-axis shows the relative position of individual adenosines within the FwR or IR2. (C) Relative enrichment of Ctn RNA in FLAG-ADAR1-IP (FLAG-IP) over IgG in WT and Adar2-KO MEFs. (D) Relative enrichment of Ctn RNA in FLAG-ADAR1-IP (FLAG-IP) over IgG in WT and Adar2-KO MEFs. Normalization of RIP results was carried out by quantifying in parallel the relative levels of Gapdh in each IP sample. (E) Editing profile of Exoc8 in WT and Adar2-KO MEFs. Error bars in (A, B, C & D) represent mean ± SD of three independent experiments (biological replicates). *P<0.05; ns, not significant using Student’s t test.
Viewed in conjunction with our previous results, it appears that although Adar1- or Adar2-KO MEFs contain comparable levels of ADAR2 and ADAR1 respectively (with respect to WT cells; data not shown), individual ADARs fail to edit any of the sites within Ctn RNA in absence of the other ADAR [40]. We wanted to determine if ADAR1 requires ADAR2 to interact with Ctn RNA. To test this, we first performed FLAG-ADAR1 ribonucleoprotein immunoprecipitation (RIP) using an anti-FLAG antibody, followed by RT-qPCR analysis in WT and Adar2-KO MEFs. ADAR1 showed interaction with Ctn RNA only in WT, but not in Adar2-KO MEFs (Figs 2C, S4). On the other hand ADAR1 interacted with one of its known substrate transcripts, Exoc8 mRNA [49] both in WT and Adar2-KO cells (Figs. 2D). We also observed comparable levels of A-to-I editing of Exoc8 mRNA in both WT and Adar2-KO cells (Fig. 2E). These results indicate that cells require both endogenous ADAR1 and ADAR2 to efficiently edit Ctn RNA.
ADAR1 and ADAR2 interact in an RNA-dependent manner to edit common RNA substrates
The significant reduction in editing of Ctn RNA in both Adar1-KO and Adar2-KO MEFs, suggests that the coordinated action of both ADARs is essential for editing of Ctn RNA. For this to happen, ADAR1 and ADAR2 would have to interact with the same transcript in vivo. To determine the occurrence of such an association in mammalian cells, we co-transfected HEK293 cells with GFP-ADAR1 and FLAG-ADAR2, followed by GFP immunoprecipitation (IP) to determine their interaction. GFP IP revealed that ADAR1 and ADAR2 interact with each other (Fig. 3A–B). To ascertain if this interaction requires RNA, GFP-IP was conducted in the presence or absence of RNase A or ethidium bromide (EtBr) treatments, conditions that disassociate RBPs from RNAs [50]. RNase- and EtBr-treated extracts displayed reduced interaction between ADAR1 and ADAR2 (Fig 3A–B), indicating that most of the observed interaction between ADAR1 and ADAR2 require intact RNA. This is consistent with a recent study that revealed an RNA-dependent interaction between ADAR1 and ADAR2 in Caenorhabditis elegans [51]. Taken together, these results suggest that both ADAR1 and ADAR2 associate with shared RNA substrates, like Ctn RNA, to edit specific adenosines within the RNA.
Figure 3. ADAR1 and ADAR2 interact in an RNA-dependent manner and can potentially edit common substrates.
Western blot of immunoprecipitation (IP) experiments to determine the RNA-dependent interaction of GFP-ADAR1 and FLAG-ADAR2. ADAR1- or GFP-IP was performed in absence or presence of (A) RNAse A or (B) ethidium bromide (EtBr). HEK293 cell were transfected with GFP-Adar1 and Flag-Adar2. Numbers below each blot indicates ImageJ quantitation of decrease in Flag-ADAR2 levels in RNAse treated relative to RNAse untreated. (C) Pie-chart comparing different types or degrees of editing observed in the RNA-seq data of WT and Adar2-KO MEFs [40]. Heat map shows the degrees of editing of substrates that do not exhibit a complete loss of editing in absence of ADAR2 (representing potential overlapping ADAR1 and ADAR2 substrates) as observed in the RNA-seq data of WT and Adar2-KO MEFs [40]. Heat map was generated using Microsoft Excel 2016. (D) Hypothetical model showing different types of dynamic interactions of ADARs with substrates based on differing degrees of editing observed in the RNA-seq data of Adar2-KO compared to WT MEFs [40] (please see discussion for details).
To identify other potential RNA substrates that require positive or negative interaction of ADARs for editing, we analyzed previously published RNA sequencing data of WT and Adar2-KO MEFs [40]. While most RNAs were only edited in the WT and not in Adar2-KO MEFs (representing ADAR2-edited substrates), we observed unexpectedly that specific substrate RNAs showed differential editing in Adar2-KO MEFs (Fig. 3C) (please see “RNA Editing” in Methods section for detailed methodology). These included RNAs containing adenosine residues that were less edited in Adar2-KO, compared to WT (versus “no editing” in case of ADAR2-only edited substrates) and few transcripts that showed increased editing in Adar2-KOs (Fig. 3C). It is possible that these RNAs are initially edited by both ADAR1 and ADAR2 and the residual editing observed in Adar2-KO MEFs is due to editing by ADAR1 (Fig. 3C). The substrates that showed reduced editing in Adar2-KO MEFs showed differing degrees of editing indicative of heterogeneity in which a specific ADAR isoform dominates the interaction (Fig. 3C; heatmap, Table S1). These results suggest that potentially, ADAR1 and ADAR2 have overlapping substrates and in addition could positively or negatively influence each other’s affinities on a certain subset of substrates (Fig. 3D).
Both ADAR1 and ADAR2 promote the stability of Ctn RNA
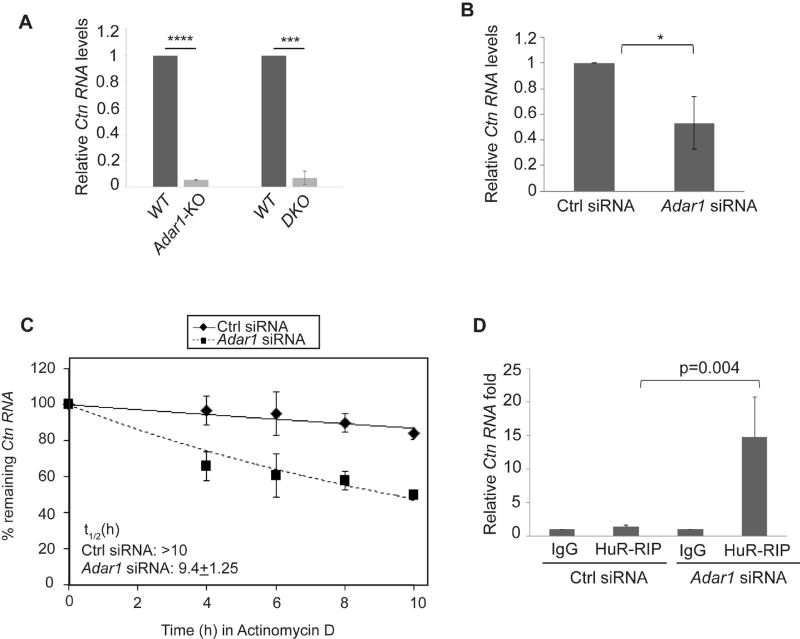
We have recently shown that ADAR2 promotes that stability of Ctn RNA by limiting its interaction with RNA decay-promoting proteins, HuR and PARN [40]. We wanted to test if ADAR1 functions similarly. To this end, we measured the total levels of Ctn RNA in WT, Adar1-KO or double-KO MEFs by RT-qPCR analysis and observed that Ctn RNA levels were significantly reduced in both in Adar1-KO and Adar1/2-double KO MEFs than in WT (Fig. 4A, Fig S5). Consistent with these observations, Ctn RNA levels were significantly lower in ADAR1-depleted, transformed MEFs than in control siRNA-treated cells (Fig. 4B). RNA-FISH analysis demonstrated that the majority of Ctn RNA in both WT and the residual Ctn RNA in Adar1-KO MEFs was retained in the nucleus (Fig. S6). Therefore, we conclude that the changes in Ctn RNA levels were not due to changes in its localization in Adar1-depleted cells. We also measured mCat2 RNA levels in WT and Adar1-KO MEFs by qRT-PCR using a primer set within the ORF region. (Figs S7, S5). We observed a small but significant reduction of mCat2 levels in Adar1-KO MEFs (Fig S7). However, the ORF primer is common to both Ctn RNA and mCat2 and the observed reduction may be due to lower Ctn RNA levels and not mCat2. As the previously demonstrated mechanism shows, in unstressed cells, cytoplasmic mCat2 levels are very low and is thus, possibly not detected [17]. RNA-FISH analysis also shows that majority of Ctn RNA is nuclear in both WT and Adar1-KO MEFs (Fig. S6). Therefore, while it is not possible to ascertain this conclusively, our results suggest that cytoplasmic mCat2 levels are not significantly altered in both WT and Adar1-KO MEFs based on the following; (a) the absence of any significant changes in the cytoplasmic amounts of Ctn RNA/mCat2, as observed by RNA-FISH and (b) the small change in total Ctn RNA/mCat2 levels, as observed by RT-qPCR. Furthermore, we determined the half-life (t1/2) of Ctn RNA in control and Adar1 siRNA-depleted cells in presence of the transcriptional inhibitor Actinomycin D. The results showed that in Adar1-depleted MEFs, Ctn RNA showed reduced stability (Fig. 4C). These results indicate that both ADAR1 and ADAR2 stabilize Ctn RNA.
Figure 4. ADAR1 stabilizes Ctn RNA.
(A) Relative levels of Ctn RNA in WT, Adar1-KO and DKO MEFs, determined by RT-qPCR using 3’UTR-1 primer (please see Fig S5). (B) Relative Ctn RNA levels in Ctrl and Adar1 siRNA treated transformed WT-MEFs determined by RT-qPCR using 3’UTR-1 primer. (C) Measurement of the stability of Ctn RNA by RT-qPCR in Ctrl and Adar1 siRNA treated transformed WT-MEFs in presence of the transcriptional inhibitor Actinomycin D (ActD) at indicated time points. Half-life of Ctn RNA (t1/2) in both cells has been indicated. (D) HuR-RIP followed by Ctn RNA RT-qPCR in Ctrl and Adar1 siRNA-treated transformed WT-MEFs. Error bars in (A, B, C & D) represent mean ± SD of three independent experiments (biological replicates). *P<0.05, ****P< 0.0001, ***P<0.001 using Student’s t test.
Next, we examined the interaction of HuR with Ctn RNA in the presence or absence of ADAR1. RIP analysis using an anti-HuR antibody in cells transfected with control and Adar1 siRNAs cells indicated that HuR displayed a robust interaction with Ctn RNA in Adar1-depleted MEFs (Fig. 4D). Thus, like ADAR2, ADAR1 too negatively regulates the interaction of HuR with Ctn RNA. Taken together, these results suggest that both ADAR1 and ADAR2 regulate Ctn RNA stability and the interaction with the RNA-destabilizing protein HuR.
ADAR1 levels are elevated in breast cancer subtypes
To gain insight into the relevance of ADARs in disease, we investigated the role of both ADAR1 and ADAR2 in breast cancer. Several reports including transcriptome-wide studies have shown that ADARs are de-regulated in different types of cancers [33–38]. Recent studies have shown that several types of cancers exhibit elevated RNA A-to-I editing [35, 38, 39]. This suggests that ADARs and their potential targets could be excellent candidate biomarkers of cancer progression [52]. A recent study has reported that the A-to-I editing frequency is increased in breast cancer (BC) cells, compared to normal cells, primarily due to the increased ADAR levels in BC cells [35]. In another study, Nakano et al, identified 26 RNA editing sites within the 3’UTR of Dihydrofolate reductase (DHFR) (a key molecule involved in tumor resistance to methotrexate), [39]. Here, the authors demonstrated that ADAR1 enhanced DHFR expression by editing the miR-25-3p and miR-125a-3p binding sites within the 3'-UTR of DHFR, thereby negatively regulating miRNA-mediated gene repression [39]. BC cells are not a single cell type, instead, they contain different molecular subtypes that differ in their patterns of gene expression, clinical features, responses to treatment and prognosis [53]. To investigate the contribution of ADARs in cancer progression in different breast cancer subtypes, we determined ADAR1 and ADAR2 mRNA levels in various samples of breast cancer subtypes, using the Cancer Genome Atlas (TCGA) RNA-seq data set. ADAR1 mRNA levels were elevated in all the different BC subtypes, including the basal subtype, indicating that it might play a role in different sub-types of breast cancers (Fig 5A and Fig S8A). In contrast to ADAR1 mRNA, ADAR2 mRNA, levels were reduced in all breast cancer subtypes compared to normal cells (Fig S8B–C). We also observed reduced ADAR3 (no reported editing activity) mRNA levels in all of the breast cancer subtypes compared to normal cells (Fig S8D). Therefore, ADAR1 mRNA but not ADAR2 or ADAR3 mRNA levels are elevated in breast cancer subtypes compared to normal cells. We performed immunoblot analysis to determine the levels of ADAR1 in several breast cancer cell lines (Fig 5B). ADAR1 levels were found to be significantly elevated in basal-like/TNBC (triple-negative breast cancer) cells such as HCC1937 (Fig 5B) [54].
Figure 5. ADAR1 expression is increased in different breast cancer subtypes.
(A) Box plot showing ADAR1 levels in various subtypes of breast cancer [TCGA_BRCA (gene expression RNAseq/polyA+ IlluminaHiSeq) obtained from The Cancer Genome Atlas]. Subtypes are based on PAM50_RNAseq [81]. Normalization of data was performed by RSEM [82]. Numbers above each datapoint indicate p-values calculated using Welch t-test between normal versus each subtype. (B) Western analysis of ADAR1 levels in breast cancer cell lines compared to normal cells (Me16c). Expression of ADAR1 measured at (C) RNA (by quantitative RT-PCR) and (D) protein level (by Western blotting) in M1-M3 cells (M1: MCF10A; M2: MCF10AT; M3: MCF10CA1h). B”-U2 snRNP was used as a loading control. (E) Distant metastasis free survival (DMFS) of basal subtype, ER/PR/HER2 negative breast cancer patients with low and high expression of ADAR1 (from Kaplan-Meier Plotter: http://kmplot.com/analysis/index.php?p=service&cancer=breast). Error bars in C represent mean ± SD of three independent experiments (biological replicates). *P<0.05 using Student’s t test.
Next, to determine whether increased levels of ADAR1 in TNBC cells correlate with tumorigenic properties, we measured ADAR1 mRNA and protein levels in an isogenic model breast cancer progression cell line series, including three cell lines named M1, M2 and M3 [55–58] (Fig 5C–D). These three isogenic cell lines are of basal-like/TNBC subtype and are derived from non-tumorigenic MCF10A mammary epithelial cells such as benign MCF10A (M1), transformed H-Ras-expressing MCF10A [MCF10AT (M2)] cells, and tumorigenic but low-metastatic MCF10CA1h (M3) cells (Fig. S8E). M2 cells were isolated from tumors in nude mice that were injected with H-Ras overexpressing M1 cells, whereas M3 cells were isolated from tumors developed in mouse xenografts after the nude mice were injected with M2 cells (Fig. S8E). Thus, M1-M3 system includes a cancer progression spectrum from a relatively normal breast epithelial cell line (M1) to a tumorigenic and low-metastatic breast cancer cell line (M3). Since these cell lines are of the same genetic lineage it enables us to measure ADAR1 levels in ‘matched’ normal (M1), transformed (M2) and primary tumor (M3) breast cancer cells. In this model, we observed elevated RNA and protein levels of ADAR1 in the M3 cell line, in comparison to normal (M1) or hyperproliferative, pre-malignant epithelial cells (M2) (Fig. 5C–D). Collectively, these results underscore the potential prognostic value and involvement of ADAR1 in driving breast cancer progression in basal-like TNBC cells. Further, Kaplan-Meier plotter analysis revealed that the degrees of distant metastasis-free survival (DMFS) were significantly reduced in patients with breast cancer who had higher levels of ADAR1 (Fig. 5E), suggesting that ADAR1 might play a role in breast cancer progression [59]. In contrast to ADAR1, ADAR2 did not show any significant differences between M1–M3 cells, indicating a specific role for ADAR1 in breast cancer (Fig S8F).
Discussion
RNA editing is an important post-transcriptional modification that regulates RNA localization, abundance, stability and function. However, detailed insights into how these proteins function as well as their role in disease is not well understood. Here, we demonstrate that nuclear-retained Ctn RNA is A-to-I edited in the 3’UTR and stabilized by both ADAR1 and ADAR2. By immunoprecipitation studies of ADARs and meta-analysis of published RNA-seq data of WT and Adar2-KO MEFs, we show that a subset of RNA substrates requires both ADAR1 and ADAR2 for their editing. Furthermore, our efforts to understand the role of ADARs in disease revealed that ADAR1 and not ADAR2 levels are increased in all breast cancer subtypes.
ADARs interact with each other, and exhibit overlapping substrate affinities and dynamic interactions
Many studies have demonstrated that A-to-I editing is widespread in the transcriptome [4–6, 8]. However, these studies have not assessed the contribution of each ADAR isoform in the editing of these RNAs. Of the three known forms of A-to-I editing enzymes, only ADAR1 and ADAR2 possess editing activity. Earlier studies have provided compelling data to demonstrate that ADAR1 and ADAR2 mainly function as homodimers [60–64]. However, other studies have indicated that they have the potential to heterodimerize as well [65]. These complex interactions merit further investigation as they could have important implications in understanding the function and therapeutic targeting of ADARs in disease.
An interesting finding of our study is the concerted action of both ADAR1 and ADAR2 in editing Ctn RNA. A previous study has pointed that in mouse brain both ADAR1 and ADAR2 differentially catalyzed the editing of independent adenosines within the 5HT2C receptor transcript without any cross-interference. [21]. In our study, depletion of either of the two ADARs compromises the overall editing within the 3’UTR of Ctn RNA. Furthermore, ADAR1 was unable to interact with or edit Ctn RNA in the absence of ADAR2, suggesting that ADAR2 influences ADAR1-mediated editing within Ctn RNA. Presently, it is not clear whether ADAR2 facilitates the association of ADAR1 with Ctn RNA or indirectly influences the editing potential of ADAR1 by regulating the activity of editing enhancers or repressors [66]. Since a double-stranded region is a common requirement for RNA editing by both ADARs, it is possible that the initial editing of a few sites by one of the ADARs could alter the double-stranded nature of the RNA substrate and thus facilitate the binding of the other ADAR subunit.
Alternatively, we cannot rule out the possibility of the involvement of ADAR1:ADAR2 heterodimers in editing Ctn RNA. The requirement of RNA for the interaction between ADAR1 and ADAR2 further strengthens the argument that both ADAR1 and ADAR2 could interact with the same RNA molecule. While ADARs have largely been shown to form homodimers, evidence of ADAR1:ADAR2 heterodimers exists in different cell lines. For example, in vivo FRET (fluorescence resonance energy transfer) studies using ADAR1 and ADAR2 fusion proteins reveal that intracellularly ADARs can form both homo- and heterodimers [65]. In HIV-infected 293T cells, ADAR2 was identified as an ADAR1-interacting partner [67]. Also, in high-grade astrocytomas, ADAR1 was shown to negatively regulate the editing activity of ADAR2 [68]. This study further confirmed the presence of ADAR2/ADAR1 heterodimers in astrocytoma cell lines [68].
It is known that the loss of editing activity of one ADAR is partially rescued by the overlapping activity of the other ADAR subunit [22, 69, 70]. On the other hand, the editing activity of ADARs was affected both in vitro and in vivo upon heterodimerization [61]. In the present study, the presence of RNAs with reduced editing in Adar2-KO MEFs (compared to WT) suggests that potentially ADAR1 partially edits some of the substrates in the absence of ADAR2. Similar to our study, Wang et al 2013 have also shown that some transcripts are edited by both ADARs [12]. This finding further supports the argument that editing of several of the RNA substrates in the transcriptome requires the interplay of both ADAR1 and ADAR2. Despite the documentation of heterodimerization of ADARs, effects on their editing-dependent and -independent functions have not been investigated.
Our study shows that both ADAR1 and ADAR2 regulate the stability of Ctn RNA as well as its ability to bind to the RNA-destabilizing protein HuR. Recent studies have shown that ADARs positively and negatively regulate the binding of RBPs to mRNA. For example, ADAR1 has been shown to preclude the binding of other 3’UTR-interacting RNA binding proteins such as CFIm68 and CstF64 to RNA [71]. Additionally, ADAR1 was reported to compete with Staufen-1 for binding to double-stranded regions within the 3’UTR of RNA and regulate the nuclear retention and stability of transcripts [72, 73]. ADAR1 also cooperates with HuR to stabilize transcripts [12, 30].
ADAR1 expression is elevated in specific breast cancer subtypes and stages
Sequencing analysis of metastatic lobular breast cancer has shown that ADAR is one of the top 5% of overexpressed proteins, and the only editing enzyme expressed at high levels [74]. By analyzing the transcriptome of 68 normal and cancerous breast tissues, Fumagalli and colleagues reported increased frequency of A-to-I editing in tumor breast tissues and in BC cell lines [35]. Another study, reported that in several cancers, including breast cancers, a significant number of RNA editing events are associated with clinically relevant sites such as those associated with drug sensitivity [75]. Elevated RNA editing in cancers also correlates with poor survival rates [38]. ADAR1 also regulates gene expression in breast cancer cells [39]. All these studies support the argument that deregulation of ADARs or their activity could contribute to BC progression.
Breast cancer is recognized as a heterogenous disease as it is composed of different subtypes with distinct morphological and molecular features and prognosis [76]. In the present study, we show that ADAR1, but not ADAR2, is overexpressed in all BC subtypes, compared to normal cells. ADAR1 shows elevated expression in basal-like breast cancers/TNBC that lack the expression of the immunohistochemical markers ER, PR, and HER2 receptor. TNBC are more difficult to treat, since most of the effective chemotherapeutic drugs target one of these three receptors [77]. Studies in the isogenic M1-M3 model BC cell line revealed that ADAR1 is overexpressed in highly tumorigenic but low-metastatic cells (M3) compared to pre-malignant or normal cells. These results indicate that ADAR1 could serve as a prognostic marker for BC progression and could play a role in BC progression. Interestingly, the increase in ADAR1 occurs at both RNA and protein levels, suggesting that the regulation happens at the transcriptional level. A recent study has shown that an increase in ADAR1-mediated editing of dihydrofolate reductase (DHFR), a key molecule that is involved in tumor resistance to methotrexate, leads to an increase in its expression in the BC cell line MCF-7 [39]. Furthermore, in our study, both ADAR1 and nuclear factor 90 (NF90) mRNA levels were found to be elevated in all BC subtypes, particularly in basal-like BC (Fig. S8G). Previously, ADAR1 has been shown to regulate gene expression by interacting with NF90 family of proteins [78–80]. The identification of other molecular targets and ADAR1-interacting partners that are affected by the increased expression of ADAR1 in BC cells will provide more mechanistic insights into the role of ADAR1 breast cancer progression.
Supplementary Material
Acknowledgments
We would like to thank Drs. K Nishikura (Wistar Institute, USA) for the FLAG-Adar1, FLAG-Adar2 plasmids and Marie Öhman (the WennerGren Institute, Stockholm University, Sweden) for the GFP-Adar1 plasmid. We would also like to thank Prasanth lab members for critical reading of this manuscript. We thank Dr. Ashish Lal (NCI) for sharing M1–M3 breast cancer cell lines.
Funding
This work was supported by grants from the National Institute of Health [1RO1GM088252 to K.V.P., 1RO1GM099669 to S.G.P.]; the American Cancer Society [RSG-11-174-01-RMC to K.V.P.]; the National Science Foundation [career, 1243372 to S.G.P. and EAGER to KVP]; and the National Institute on Aging Intramural Research Program, National Institutes of Health [Z01-AG000511 to J.H.Y. and M.G.]. The funders had no role in study design, data collection and analysis.
Abbreviations
- ADAR
Adenosine Deaminases acting on RNA
- Ctn RNA
Cat2 transcribed nuclear RNA
- IRAlus
inverted Alu sequences
- SINE
short interspersed element
- 3’UTR
3’untranslated region
- RIP
RNA Immunoprecipitation
- RT-qPCR
reverse transcription and quantitative PCR
- MEF
Mouse embryonic fibroblasts
- M1
MCF10A
- M2
MCF10AT
- M3
MCF10CA1h
- A-to-I
Adenosine-to-Inosine
- IR
Inverted repeat
- FwR
Forward Repeat
- WT
Wild-type
- KO
Knockout
- DKO
Double Knockout (Adar1/Adar2 Knockout)
Footnotes
Author Contributions
The study was designed by A.A. and K.V.P. A.A., S.G.P. & K.V.P. wrote the main manuscript. A.A., O.G., A.K., J.H.Y performed the experiments. M.J., J.H., M.G., & S.G.P provided essential reagents. All the authors have contributed in reviewing the manuscript.
References
- 1.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–46. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem. 2010;79:321–49. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hundley HA, Bass BL. ADAR editing in double-stranded UTRs and other noncoding RNA sequences. Trends Biochem Sci. 2010;35:377–83. doi: 10.1016/j.tibs.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazak L, Haviv A, Barak M, Jacob-Hirsch J, Deng P, Zhang R, Isaacs FJ, Rechavi G, Li JB, Eisenberg E, Levanon EY. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res. 2014;24:365–76. doi: 10.1101/gr.164749.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazak L, Levanon EY, Eisenberg E. Genome-wide analysis of Alu editability. Nucleic Acids Res. 2014;42:6876–84. doi: 10.1093/nar/gku414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DD, Kim TT, Walsh T, Kobayashi Y, Matise TC, Buyske S, Gabriel A. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–25. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, Olshansky M, Rechavi G, Jantsch MF. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol. 2004;22:1001–5. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- 8.Peng Z, Cheng Y, Tan BC, Kang L, Tian Z, Zhu Y, Zhang W, Liang Y, Hu X, Tan X, Guo J, Dong Z, Liang Y, Bao L, Wang J. Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat Biotechnol. 2012;30:253–60. doi: 10.1038/nbt.2122. [DOI] [PubMed] [Google Scholar]
- 9.Mallela A, Nishikura K. A-to-I editing of protein coding and noncoding RNAs. Crit Rev Biochem Mol Biol. 2012;47:493–501. doi: 10.3109/10409238.2012.714350. [DOI] [PubMed] [Google Scholar]
- 10.Ulbricht RJ, Emeson RB. One hundred million adenosine-to-inosine RNA editing sites: hearing through the noise. Bioessays. 2014;36:730–5. doi: 10.1002/bies.201400055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LL, Carmichael GG. Nuclear Editing of mRNA 3'-UTRs. Curr Top Microbiol Immunol. 2012;353:111–21. doi: 10.1007/82_2011_149. [DOI] [PubMed] [Google Scholar]
- 12.Wang IX, So E, Devlin JL, Zhao Y, Wu M, Cheung VG. ADAR regulates RNA editing, transcript stability, and gene expression. Cell Rep. 2013;5:849–60. doi: 10.1016/j.celrep.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel C, Veno MT, Ekdahl Y, Kjems J, Ohman M. A distant cis acting intronic element induces site-selective RNA editing. Nucleic Acids Res. 2012;40:9876–86. doi: 10.1093/nar/gks691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–7. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- 16.Chen LL, DeCerbo JN, Carmichael GG. Alu element-mediated gene silencing. EMBO J. 2008;27:1694–705. doi: 10.1038/emboj.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL. Regulating gene expression through RNA nuclear retention. Cell. 2005;123:249–63. doi: 10.1016/j.cell.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Capshew CR, Dusenbury KL, Hundley HA. Inverted Alu dsRNA structures do not affect localization but can alter translation efficiency of human mRNAs independent of RNA editing. Nucleic Acids Res. 2012;40:8637–45. doi: 10.1093/nar/gks590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vesely C, Tauber S, Sedlazeck FJ, Tajaddod M, von Haeseler A, Jantsch MF. ADAR2 induces reproducible changes in sequence and abundance of mature microRNAs in the mouse brain. Nucleic Acids Res. 2014;42:12155–68. doi: 10.1093/nar/gku844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vesely C, Tauber S, Sedlazeck FJ, von Haeseler A, Jantsch MF. Adenosine deaminases that act on RNA induce reproducible changes in abundance and sequence of embryonic miRNAs. Genome Res. 2012;22:1468–76. doi: 10.1101/gr.133025.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartner JC, Schmittwolf C, Kispert A, Muller AM, Higuchi M, Seeburg PH. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem. 2004;279:4894–902. doi: 10.1074/jbc.M311347200. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 23.Liddicoat BJ, Piskol R, Chalk AM, Ramaswami G, Higuchi M, Hartner JC, Li JB, Seeburg PH, Walkley CR. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science. 2015;349:1115–20. doi: 10.1126/science.aac7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pestal K, Funk CC, Snyder JM, Price ND, Treuting PM, Stetson DB. Isoforms of RNA-Editing Enzyme ADAR1 Independently Control Nucleic Acid Sensor MDA5-Driven Autoimmunity and Multi-organ Development. Immunity. 2015;43:933–44. doi: 10.1016/j.immuni.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannion NM, Greenwood SM, Young R, Cox S, Brindle J, Read D, Nellaker C, Vesely C, Ponting CP, McLaughlin PJ, Jantsch MF, Dorin J, Adams IR, Scadden AD, Ohman M, Keegan LP, O'Connell MA. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 2014;9:1482–94. doi: 10.1016/j.celrep.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, Dickerson JE, Bhaskar SS, Zampini M, Briggs TA, Jenkinson EM, Bacino CA, Battini R, Bertini E, Brogan PA, Brueton LA, Carpanelli M, De Laet C, de Lonlay P, del Toro M, Desguerre I, Fazzi E, Garcia-Cazorla A, Heiberg A, Kawaguchi M, Kumar R, Lin JP, Lourenco CM, Male AM, Marques W, Mignot C, Jr, Olivieri I, Orcesi S, Prabhakar P, Rasmussen M, Robinson RA, Rozenberg F, Schmidt JL, Steindl K, Tan TY, van der Merwe WG, Vanderver A, Vassallo G, Wakeling EL, Wassmer E, Whittaker E, Livingston JH, Lebon P, Suzuki T, McLaughlin PJ, Keegan LP, O'Connell MA, Lovell SC, Crow YJ. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet. 2012;44:1243–8. doi: 10.1038/ng.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi M, Suzuki T. Dyschromatosis symmetrica hereditaria. J Dermatol. 2013;40:336–43. doi: 10.1111/j.1346-8138.2012.01661.x. [DOI] [PubMed] [Google Scholar]
- 28.Hideyama T, Yamashita T, Aizawa H, Tsuji S, Kakita A, Takahashi H, Kwak S. Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol Dis. 2012;45:1121–8. doi: 10.1016/j.nbd.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 29.Shtrichman R, Germanguz I, Mandel R, Ziskind A, Nahor I, Safran M, Osenberg S, Sherf O, Rechavi G, Itskovitz-Eldor J. Altered A-to-I RNA editing in human embryogenesis. PLoS One. 2012;7:e41576. doi: 10.1371/journal.pone.0041576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stellos K, Gatsiou A, Stamatelopoulos K, Perisic Matic L, John D, Lunella FF, Jae N, Rossbach O, Amrhein C, Sigala F, Boon RA, Furtig B, Manavski Y, You X, Uchida S, Keller T, Boeckel JN, Franco-Cereceda A, Maegdefessel L, Chen W, Schwalbe H, Bindereif A, Eriksson P, Hedin U, Zeiher AM, Dimmeler S. Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nat Med. 2016;22:1140–1150. doi: 10.1038/nm.4172. [DOI] [PubMed] [Google Scholar]
- 31.Marcu-Malina V, Goldberg S, Vax E, Amariglio N, Goldstein I, Rechavi G. ADAR1 is vital for B cell lineage development in the mouse bone marrow. Oncotarget. 2016;7:54370–54379. doi: 10.18632/oncotarget.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Shoshan SO, Kagan P, Sultan M, Barabash Z, Dor C, Jacob-Hirsch J, Harmelin A, Pappo O, Marcu-Malina V, Ben-Ari Z, Amariglio N, Rechavi G, Goldstein I, Safran M. ADAR1 deletion induces NFkappaB and interferon signaling dependent liver inflammation and fibrosis. RNA Biol. 2016:1–16. doi: 10.1080/15476286.2016.1203501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song C, Sakurai M, Shiromoto Y, Nishikura K. Functions of the RNA Editing Enzyme ADAR1 and Their Relevance to Human Diseases. Genes (Basel) 2016;7 doi: 10.3390/genes7120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galeano F, Rossetti C, Tomaselli S, Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco CM, Locatelli F, Gallo A. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene. 2013;32:998–1009. doi: 10.1038/onc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fumagalli D, Gacquer D, Rothe F, Lefort A, Libert F, Brown D, Kheddoumi N, Shlien A, Konopka T, Salgado R, Larsimont D, Polyak K, Willard-Gallo K, Desmedt C, Piccart M, Abramowicz M, Campbell PJ, Sotiriou C, Detours V. Principles Governing A-to-I RNA Editing in the Breast Cancer Transcriptome. Cell Rep. 2015;13:277–89. doi: 10.1016/j.celrep.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salameh A, Lee AK, Cardo-Vila M, Nunes DN, Efstathiou E, Staquicini FI, Dobroff AS, Marchio S, Navone NM, Hosoya H, Lauer RC, Wen S, Salmeron CC, Hoang A, Newsham I, Lima LA, Carraro DM, Oliviero S, Kolonin MG, Sidman RL, Do KA, Troncoso P, Logothetis CJ, Brentani RR, Calin GA, Cavenee WK, Dias-Neto E, Pasqualini R, Arap W. PRUNE2 is a human prostate cancer suppressor regulated by the intronic long noncoding RNA PCA3. Proc Natl Acad Sci U S A. 2015;112:8403–8. doi: 10.1073/pnas.1507882112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Q, Crews LA, Barrett CL, Chun HJ, Court AC, Isquith JM, Zipeto MA, Goff DJ, Minden M, Sadarangani A, Rusert JM, Dao KH, Morris SR, Goldstein LS, Marra MA, Frazer KA, Jamieson CH. ADAR1 promotes malignant progenitor reprogramming in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2013;110:1041–6. doi: 10.1073/pnas.1213021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paz-Yaacov N, Bazak L, Buchumenski I, Porath HT, Danan-Gotthold M, Knisbacher BA, Eisenberg E, Levanon EY. Elevated RNA Editing Activity Is a Major Contributor to Transcriptomic Diversity in Tumors. Cell Rep. 2015;13:267–76. doi: 10.1016/j.celrep.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 39.Nakano M, Fukami T, Gotoh S, Nakajima M. A-to-I RNA Editing Up-regulates Human Dihydrofolate Reductase in Breast Cancer. J Biol Chem. 2017;292:4873–4884. doi: 10.1074/jbc.M117.775684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anantharaman A, Tripathi V, Khan A, Yoon JH, Singh DK, Gholamalamdari O, Guang S, Ohlson J, Wahlstedt H, Ohman M, Jantsch MF, Conrad NK, Ma J, Gorospe M, Prasanth SG, Prasanth KV. ADAR2 regulates RNA stability by modifying access of decay-promoting RNA-binding proteins. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkw1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaswami G, Li JB. RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Res. 2014;42:D109–13. doi: 10.1093/nar/gkt996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–93. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conrad NK, Mili S, Marshall EL, Shu MD, Steitz JA. Identification of a rapid mammalian deadenylation-dependent decay pathway and its inhibition by a viral RNA element. Mol Cell. 2006;24:943–53. doi: 10.1016/j.molcel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herb A, Higuchi M, Sprengel R, Seeburg PH. Q/R site editing in kainate receptor GluR5 and GluR6 pre-mRNAs requires distant intronic sequences. Proc Natl Acad Sci U S A. 1996;93:1875–80. doi: 10.1073/pnas.93.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melcher T, Maas S, Herb A, Sprengel R, Higuchi M, Seeburg PH. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J Biol Chem. 1996;271:31795–8. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- 47.Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379:460–4. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 48.Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riedmann EM, Schopoff S, Hartner JC, Jantsch MF. Specificity of ADAR-mediated RNA editing in newly identified targets. RNA. 2008;14:1110–8. doi: 10.1261/rna.923308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guetg C, Scheifele F, Rosenthal F, Hottiger MO, Santoro R. Inheritance of silent rDNA chromatin is mediated by PARP1 via noncoding RNA. Mol Cell. 2012;45:790–800. doi: 10.1016/j.molcel.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Washburn MC, Kakaradov B, Sundararaman B, Wheeler E, Hoon S, Yeo GW, Hundley HA. The dsRBP and inactive editor ADR-1 utilizes dsRNA binding to regulate A-to-I RNA editing across the C. elegans transcriptome. Cell Rep. 2014;6:599–607. doi: 10.1016/j.celrep.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rayon-Estrada V, Papavasiliou FN, Harjanto D. RNA Editing Dynamically Rewrites the Cancer Code. Trends Cancer. 2015;1:211–212. doi: 10.1016/j.trecan.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23(Suppl 2):S60–4. doi: 10.1038/modpathol.2010.33. [DOI] [PubMed] [Google Scholar]
- 54.Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35–48. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jadaliha M, Zong X, Malakar P, Ray T, Singh DK, Freier SM, Jensen T, Prasanth SG, Karni R, Ray PS, Prasanth KV. Functional and prognostic significance of long non-coding RNA MALAT1 as a metastasis driver in ER negative lymph node negative breast cancer. Oncotarget. 2016;7:40418–40436. doi: 10.18632/oncotarget.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol. 1996;148:313–9. [PMC free article] [PubMed] [Google Scholar]
- 57.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, Wolman SR, Heppner GH, Miller FR. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat. 2001;65:101–10. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 58.Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, Wakefield LM. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–24. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gyorffy B, Schafer R. Meta-analysis of gene expression profiles related to relapse-free survival in 1,079 breast cancer patients. Breast Cancer Res Treat. 2009;118:433–41. doi: 10.1007/s10549-008-0242-8. [DOI] [PubMed] [Google Scholar]
- 60.Cho DS, Yang W, Lee JT, Shiekhattar R, Murray JM, Nishikura K. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. J Biol Chem. 2003;278:17093–102. doi: 10.1074/jbc.M213127200. [DOI] [PubMed] [Google Scholar]
- 61.Gallo A, Keegan LP, Ring GM, O'Connell MA. An ADAR that edits transcripts encoding ion channel subunits functions as a dimer. EMBO J. 2003;22:3421–30. doi: 10.1093/emboj/cdg327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodman RA, Macbeth MR, Beal PA. ADAR proteins: structure and catalytic mechanism. Curr Top Microbiol Immunol. 2012;353:1–33. doi: 10.1007/82_2011_144. [DOI] [PubMed] [Google Scholar]
- 63.Poulsen H, Jorgensen R, Heding A, Nielsen FC, Bonven B, Egebjerg J. Dimerization of ADAR2 is mediated by the double-stranded RNA binding domain. RNA. 2006;12:1350–60. doi: 10.1261/rna.2314406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valente L, Nishikura K. RNA binding-independent dimerization of adenosine deaminases acting on RNA and dominant negative effects of nonfunctional subunits on dimer functions. J Biol Chem. 2007;282:16054–61. doi: 10.1074/jbc.M611392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chilibeck KA, Wu T, Liang C, Schellenberg MJ, Gesner EM, Lynch JM, MacMillan AM. FRET analysis of in vivo dimerization by RNA-editing enzymes. J Biol Chem. 2006;281:16530–5. doi: 10.1074/jbc.M511831200. [DOI] [PubMed] [Google Scholar]
- 66.Garncarz W, Tariq A, Handl C, Pusch O, Jantsch MF. A high-throughput screen to identify enhancers of ADAR-mediated RNA-editing. RNA Biol. 2013;10:192–204. doi: 10.4161/rna.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orecchini E, Doria M, Antonioni A, Galardi S, Ciafre SA, Frassinelli L, Mancone C, Montaldo C, Tripodi M, Michienzi A. ADAR1 restricts LINE-1 retrotransposition. Nucleic Acids Res. 2017;45:155–168. doi: 10.1093/nar/gkw834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cenci C, Barzotti R, Galeano F, Corbelli S, Rota R, Massimi L, Di Rocco C, O'Connell MA, Gallo A. Down-regulation of RNA editing in pediatric astrocytomas: ADAR2 editing activity inhibits cell migration and proliferation. J Biol Chem. 2008;283:7251–60. doi: 10.1074/jbc.M708316200. [DOI] [PubMed] [Google Scholar]
- 69.Farajollahi S, Maas S. Molecular diversity through RNA editing: a balancing act. Trends Genet. 2010;26:221–30. doi: 10.1016/j.tig.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–40. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bahn JH, Ahn J, Lin X, Zhang Q, Lee JH, Civelek M, Xiao X. Genomic analysis of ADAR1 binding and its involvement in multiple RNA processing pathways. Nat Commun. 2015;6:6355. doi: 10.1038/ncomms7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elbarbary RA, Li W, Tian B, Maquat LE. STAU1 binding 3' UTR IRAlus complements nuclear retention to protect cells from PKR-mediated translational shutdown. Genes Dev. 2013;27:1495–510. doi: 10.1101/gad.220962.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakurai M, Shiromoto Y, Ota H, Song C, Kossenkov AV, Wickramasinghe J, Showe LC, Skordalakes E, Tang HY, Speicher DW, Nishikura K. ADAR1 controls apoptosis of stressed cells by inhibiting Staufen1-mediated mRNA decay. Nat Struct Mol Biol. 2017 doi: 10.1038/nsmb.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, Delaney A, Gelmon K, Guliany R, Senz J, Steidl C, Holt RA, Jones S, Sun M, Leung G, Moore R, Severson T, Taylor GA, Teschendorff AE, Tse K, Turashvili G, Varhol R, Warren RL, Watson P, Zhao Y, Caldas C, Huntsman D, Hirst M, Marra MA, Aparicio S. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 75.Han L, Diao L, Yu S, Xu X, Li J, Zhang R, Yang Y, Werner HM, Eterovic AK, Yuan Y, Li J, Nair N, Minelli R, Tsang YH, Cheung LW, Jeong KJ, Roszik J, Ju Z, Woodman SE, Lu Y, Scott KL, Li JB, Mills GB, Liang H. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell. 2015;28:515–28. doi: 10.1016/j.ccell.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121:3786–8. doi: 10.1172/JCI60534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 78.Nie Y, Ding L, Kao PN, Braun R, Yang JH. ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing. Mol Cell Biol. 2005;25:6956–63. doi: 10.1128/MCB.25.16.6956-6963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartner JC, Walkley CR, Lu J, Orkin SH. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol. 2009;10:109–15. doi: 10.1038/ni.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iizasa H, Nishikura K. A new function for the RNA-editing enzyme ADAR1. Nat Immunol. 2009;10:16–8. doi: 10.1038/ni0109-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.