Abstract
Although the absence of the age-regulating klotho protein causes klotho-deficient mice to rapidly develop cognitive impairment and increasing klotho enhances hippocampal-dependent memory, the cellular effects of klotho that mediate hippocampal-dependent memory function are unknown. Here we show premature aging of the klotho-deficient hippocampal neurogenic niche as evidenced by reduced numbers of neural stem cells, decreased proliferation, and impaired maturation of immature neurons. Klotho-deficient neurospheres show reduced proliferation and size that is rescued by supplementation with shed klotho protein. Conversely, 6 month old klotho overexpressing mice exhibit increased numbers of neural stem cells, increased proliferation, and more immature neurons with enhanced dendritic arborization. Protection from normal age-related loss of object location memory with klotho overexpression and loss of spatial memory when klotho is reduced by even half suggest direct, local effects of the protein. Together these data show that klotho is a novel regulator of postnatal neurogenesis affecting neural stem cell proliferation and maturation sufficient to impact hippocampal-dependent spatial memory function.
Keywords: postnatal neurogenesis, hippocampus, neural stem cell, aging, cognition
Introduction
Klotho (KL) deficient, knockout mice (KO) have a shortened lifespan of ~8 weeks and undergo rapid body-wide deterioration consistent with premature aging(Kuro-o et al. 1997) whereas KL overexpression extends mouse lifespan (Kuro-o et al. 1997; Kurosu et al. 2005). KL is highly expressed by the kidney where transmembrane KL regulates phosphate homeostasis (Kurosu et al. 2006). Shedding of KL allows it to circulate throughout the body (Shiraki-Iida et al. 1998; Imura et al. 2004) to act as a sialidase (Cha et al. 2008) or an inhibitor of several signaling pathways (Kurosu et al. 2005; Liu et al. 2007; Doi et al. 2011; Zhou et al. 2013). Considerable progress has been made toward understanding the functional relevance of KL in peripheral systems but very little is known about the specific cellular or molecular actions of KL within the brain.
The brain expresses all forms of KL protein: transmembrane, shed, and secreted (Imura et al. 2004; Clinton et al. 2013; Masso et al. 2015). KL expression is downregulated with age (Duce et al. 2008; Yamazaki et al. 2010; King et al. 2012) and with neurodegenerative disease (Semba et al. 2014) suggesting that KL sensitive pathways and processes are impaired both with normal aging and disease development. Supporting this idea, KO mice rapidly develop cognitive impairment (Nagai et al. 2003) but without gross anatomical abnormalities and only subtly increased apoptosis (Shiozaki et al. 2008) and oxidative stress (Nagai et al. 2003), and decreased synaptic protein expression (Shiozaki et al. 2008). KL overexpressing mouse (OE) brains have no reported cellular changes although neurons are more resistant to oxidative stress (Zeldich et al. 2014; Brobey et al. 2015). Still, KL overexpression enhances cognitive function concomitant with higher levels of NMDA GluN2B glutamate receptor subunits (Dubal et al. 2014), the subunits known to promote synaptic plasticity (Tang et al. 1999). Overexpression also protects against the development of Alzheimer’s disease-like synaptic change and cognitive impairment (Dubal et al. 2015). In humans, a polymorphism that naturally increases levels of serum KL correlates with increased cognition, suggesting that KL may be important for cognitive reserve (Deary et al. 2005; Dubal et al. 2014; Yokoyama et al. 2015).
Since hippocampal immature postnatal-born neurons preferentially express the GluN2B subunit of the NMDA receptor (Ge et al. 2007) and KL-deficiency causes premature aging of peripheral stem cell niches (Liu et al. 2007), herein we assessed the contribution of KL to hippocampal postnatal neurogenesis. We measured the effects of both body-wide, global KL-deficiency and overexpression on neurogenesis at ages before and after onset of cognitive changes as previously reported (Nagai et al. 2003; Dubal et al. 2014). We show that total KL-deficiency causes a premature neurogenic aging-like effect wherein decreased neurogenic capacity begins prior to the onset of cognitive impairment or peripheral organ system failure. Remarkably, KL overexpression does not affect the niche during early life, but adults show delayed age-related loss of neurogenesis and protection from age-related decline of spatial discrimination. Together these results suggest that KL is a key component of the postnatal stem cell niche and that age-related downregulation of KL expression may contribute to hippocampal decline.
Materials and Methods
1.1 Animals
Procedures were approved by the UAB IACUC. Global body-wide KL-deficient mice (KO; 129S1/SvImJ) and global body-wide KL-overexpressing mice (OE; C57BL/6J) lines were obtained from M. Kuro-o (University of Texas Southwestern, Dallas, TX), POMC-GFP(Overstreet et al. 2004) (C57BL/6J) from L. Overstreet-Wadiche (University of Alabama at Birmingham, Birmingham AL) and Nestin-GFP (Mignone et al. 2004) (C57BL/6J) from G. Enikolopov (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). WT (average weight: 3 week,11.6g; 7 week, 20.4g) and KO (average weight: 3 week,10g; 7 week, 8.1g) mice were generated by breeding heterozygotes (KL +/−, HET). KO mice die naturally at ~8 weeks of age and thus brains are harvested at or before 7 weeks as detailed. To ensure equivalent KL overexpression, mice carrying the KL overexpression cassette are bred to WT mouse from the same line. Mice were weaned on postnatal day 21. All mice were group housed and had free access to food and water at 26.6°C with humidity maintained above 40%. KO mice were supplemented with Bacon Softies or Nutra-gel (BioServ, Frenchtown, NJ). As mouse neurogenesis is not gender specific (Lagace et al. 2007), male and female mice were utilized. BrdU was intraperitoneal injected (50mg/kg) either 1x or 4x (2 hours apart) and brains collected after 30 minutes, 24 hours, 1, 2 or 3 weeks.
1.2 Immunohistochemistry (IHC)
1.2.1 Basic IHC
Tissue was collected after transcardial perfusion with Tyrode’s solution (137 mM NaCl, 2.7 mM KCL, 1mM MgCl2, 1.8mM CaCl2, 0.2mM Na2HPO4, 12mM NaHCO3, and 5.5 mM glucose) and 4% paraformaldehyde. Serial 30μm, free-floating coronal sections representing 1/6th of each brain were permeabilized in Tris-buffered saline containing TritonX-100 (TBST; 50mM Tris with 0.9% NaCl, and 0.5% TritonX-100), incubated with 0.3% H2O2, and blocked with 10% horse serum/TBST. When BrdU detection was not required, primary antibodies were incubated in 1% horse serum/TBST for 48 hours. Primary antibodies Ki67 (1:500, anti-rabbit, Abcam Cambridge, MA ab15580), BLBP (1:300, anti-rabbit, EMD Millipore, Billerica, MA ABN14), Dcx (1:50, anti-goat, Santa Cruz Biotechnology, Santa Cruz, CA, sc-8066, or 1:200, anti-rabbit, Abcam ab18723), GFP (green fluorescent protein, 1:100, anti-rabbit, Novus NB600-308), GFAP (glial fibrillary acidic protein, 1:500, anti-rabbit, Dako Z033429), Sox 2 (sex determining region box 2, 1:100, anti-goat, Santa Cruz sc-17320 or anti-rabbit 1:100, R and D Systems), NeuN (1:300, anti-rabbit, Millipore ABN78), ND1 (1:50, anti-goat, Millipore AB15580), or S100β (1:400, anti-rabbit, Dako, Santa Clara CA Z0311) were used. Secondary antibodies conjugated to Alexa 488 or 594 (Life Technologies, Grand Island, NY) were used to detect NeuN, Ki67 and BLBP antibodies. Dcx and Sox2 were detected with biotin conjugated secondary (Southern Biotech, Birmingham, AL) and visualized with streptavidin 488, 594, or 674 (Vector Labs, Burlingame, CA). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI, Life Technologies) and mounted in Prolong Gold anti-fade mounting media (Life Technologies). ND1 was detected using diaminobenzidine (DAB)(King et al. 2008; King et al. 2011). GFP and GFAP co-localization with KL was detected using 10μm thick paraffin sections and anti-KL antibody (R and D Systems, Minneapolis, MN AF1819) as reported(Maltare et al. 2014). This KL antibody is directed against the amino terminus of the KL protein and thus detects all protein forms.
1.2.2 BrdU IHC
After incubating with the co-labeling primary antibody, sections were washed with 2N HCl at room temperature, borate neutralized (0.1M boric acid, pH 8.5) and incubated with BrdU antibody (1:50, anti- rat, Santa Cruz sc-56258). Biotinylated secondary and streptavidin 594 detected BrdU. Nuclei were labeled with Hoechst (BD Biosciences, San Jose, CA).
1.2.3 Microscopy and quantification
All quantification was performed by genotype blind researchers. Stereology with optical fractionator software (Stereo Investigator Version 9, MicroBrightField Inc., Germany) and a Zeiss Axio Imager (Zeiss, Oberkochen, Germany) microscope fitted with a motorized stage and video camera (AxioCam MRc5, Zeiss)(West et al. 1991) was used to estimate sub-granular zone (SGZ) proliferating cells (Ki67 quantifications) every 6th section from −1.34mm to −2.10mm from bregma (dissector height:15μm with a 5μm guard zone; fixed counting frame of 80×80μm with a sampling grid size of 75×75 μm resulted in 100–200 sites/brain). For all other quantification, images were collected with an Olympus BX53 fluorescent (Center Valley, PA) and the average total number of cells in 3 sections of dorsal hippocampus (1/6th of each brain processed, −1.34mm to −2.10mm from bregma) reported (Gilley et al. 2011; Okamoto et al. 2011). Within this same range, maturation stage was defined similar to Plumpe et. al(Plumpe et al. 2006) counting 100 cells unless 3 bregma levels was exceeded first (3 bregma levels counted for 7 week KO and 6 month WT). KL dentate co-localization was imaged using confocal Zeiss laser scanning LSM510 microscope (Zeiss, Oberkochen, Germany).
1.2.4 Dendrite analysis
Neuronal morphology of POMC-GFP WT immature neurons was traced from confocal image stacks using Neurolucida and measurement of total dendrite length was determined by furthest Sholl radius containing measurable dendrite length (v7. MicroBrightField, Williston, VA) (Pugh et al. 2011). Cells with obvious truncation were excluded from analysis.
1.3 Nissl Stain
After cresyl violet acetate incubation, slides were dH2O washed and dehydrated with graded ethanol and xylenes. Volume was estimated in 1/6th of each brain used the Cavalieri estimator and Stereo-Investigator software (−1.22mm to −3.88mm from bregma). When dentate was estimated, total volume, dorsal (−1.22mm to −2.18mm from bregma) and ventral (−2.20 to −3.88mm from bregma) was measured.
1.4 Neurosphere (NSP) Assays
1.4.1 Isolation
Progenitors were isolated from 3 week old hippocampi using 0.05% trypsin. NSPs were grown on non-tissue culture treated plastics with NeuroCult proliferation media (Stem Cell Technologies; Seattle, WA) containing 10ng/ml bFGF, (ProSpec, East Brunswick, NJ), 10ng/ml EGF (ProSpec), and 2ug/ml heparin (Fisher Scientific). NSPs achieved sufficient confluency for assays ~2 weeks after isolation and all assays were completed within 5 passages.
1.4.2 Primary and Secondary NSP assays
NSPs were dissociated using Accutase (Fisher Scientific). Reliable survival after plating of 1–5000 cells/well established a minimum of 500 cells/well for viability. 96-well plates received 500 cells/well in complete media with or without 100ng/mL recombinant mouse KL (R&D Systems). Ten days later primary NSP number/well and diameter (20–150μm) were counted. The average/well reported. Following evaluation, primary NSPs were collected, dissociated, and re-plated at 500 cells/well. Secondary sphere number and size were evaluated 10 days later. Images of both primary and secondary NSPs was taken with an Evos FL Cell Imaging System and the diameter of each sphere measured using ImageJ (National Institutes of Health, Bethesda MD).
1.4.3 Proliferation Assay
NSPs were dissociated and plated at 150,000 cells/well on Matrigel (Fisher Scientific) coated coverslips. The next day, half the medium was replaced with 2x EdU (Fisher Scientific) in complete medium. EdU (5-ethynyl-2′-deoxyuridine) was detected 24 hours later using the manufacturer’s Click-iT protocol and IHC as in 1.2(Zeng et al. 2010; Qu et al. 2013; Logan et al. 2015).
1.5 qPCR
RNA was extracted from flash frozen brain, isolated hippocampi, or NSP cultures using RNA STAT-60 (Tel-Test Inc., Friendswood, TX) and cDNA was generated using iScript RT Supermix (Biorad, Hercules, CA) per manufacturer’s protocols. Messenger RNA (mRNA) was measured by primer/probe duplex qPCR with SsoFast Probes Supermix (Biorad) and Prime time qPCR assays to the mouse 18s ribosomal subunit (Rn18s: Assay ID Mm.PT.49.3175696.g, IDT, Coralville, IA) and mouse klotho (assay ID Mm.PT.49.11505558; IDT) on a StepOne qPCR system (Applied Biosystems, Foster City, CA). The KL primers will detect both secreted and transmembrane forms of KL. Fold change relative to adult brain was calculated using the ΔΔCt method(Clinton et al. 2013).
1.6 Behavior
1.6.1 Design
Researchers conducting behavior assays were blind to genotype from habituation through scoring. While the OE mice are physically indistinguishable from WT, the 7 week KO mice are smaller than WT. However, mice are coded to prevent genotypic awareness and several lines of transgenic mice for other studies were tested on behaviors concurrently with mice in this study to avoid direct comparison of 7 week KO and WT mice. Mice were habituated to the researcher for 3 consecutive days. Groups of mice were processed sequentially through open field, object location, and context-dependent fear conditioning tasks.
1.6.2 Open Field
On test day mice were placed in the center of the open field apparatus (43×43×30cm plexiglass box). Photo beam detectors quantified all activity for 30 min (ENV-515 software, Med Associates, St. Albans, VT).
1.6.3 Object location
Objects of the same height: star shaped plastic bath toys and Lego towers were mounted on metal washers to prevent tipping. Objects were compared using naïve mice to ensure objects elicited a similar level of exploratory interest. Experimental mice were habituated to a white plexiglass testing chamber (39×19×21cm); containing only black tape marking the north facing wall and fresh bedding material for 2 consecutive days. On training day, 2 identical objects were placed on the same side of the box as the black tape and mice were allowed to freely explore for 10 minutes(Haettig et al. 2011). 24 hours later, mice were returned to the box for 5 minutes where one object was displaced to the center, back of the box (see Figure 6E for schematic). Mouse behavior was recorded (TopScan, Clever Sys 2.0, Reston, VA). Videos of task performance were manually scored. Interaction was judged to occur if mice were observed to be facing and sniffing the within 2cm of a given object. Mice had to explore each object for at least 5 seconds during the training phase and 15 seconds during testing to be included; no mice failed minimum interaction requirements. During training, mice would be excluded if they displayed object/side preference greater than 20%; no mice required exclusion. Percent discrimination was calculated as % of time spent with the moved object – time spent with the non-moved object/total time (Wang et al. 2014).
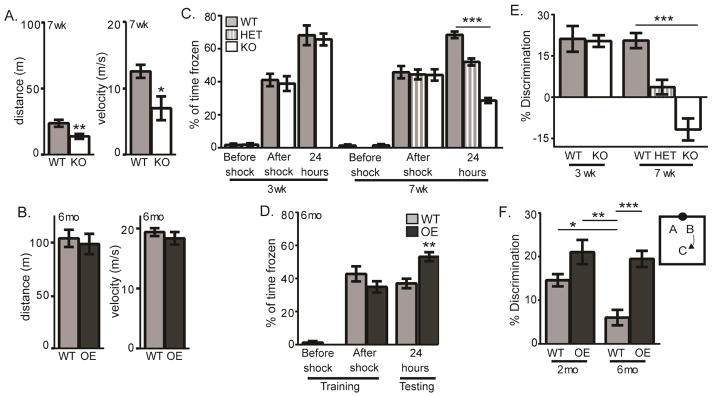
Figure 6. KL regulates spatial discrimination.
A.7 week old WT and KO open field total distance traveled (meters) and velocity (meters/second). B. 6 month WT and OE open field as in A. C. 3 week WT and KO and 7 week WT, HET, and KO context-dependent fear conditioning quantified as % of time spent freezing on training day before and after foot shock and testing day, 24 hours after shock when mice were returned to the same context. D. 6 month WT and OE context-dependent fear conditioning as in C. E. Percent discrimination of 3 week WT and KO and 7 week WT, HET, and KO object location. F. Diagram depicts experimental design of object location task. Quantification of % discrimination of WT and OE mice at 2 and 6 months old. (n=10–11/genotype; mean +/− S.E.M.; T-tests A–G, ANOVA with Tukey’s post hoc analysis H; *p<0.01, **p<0.004, ***p<0.0001)
1.6.4 Context-Dependent Fear Conditioning
Mice were habituated to the testing room for 2 days. On training day, mice were placed in an operant chamber inside an isolation box (Med Associates) for 5 minutes. During that time, mice freely explored for the first 2 minutes. Subsequently, a series of 3, 1 second, 0.5mA shocks were delivered 1x/minute. Mice remained in the chamber for 2 minutes after the last shock. 24 hours after training, mice were tested by return to the same chamber for 5 minutes. All training and testing was recorded by automated video tracking system (Med Associates). Percent of time spent freezing was manually scored by measuring freezing behavior in 5 second intervals.
1.7 Statistics
All data are reported as mean +/− standard error of the mean (S.E.M). Statistical significance is noted if groups differ by p-value less than 0.05 as determined using GraphPad Prism (GraphPad version 6, La Jolla, CA) using Students T-test or one-way ANOVA as noted in each legend. For qPCR, Dunnett’s analysis was conducted to compare each time point to the adult P56. Chi squared test was performed for 24 hour post-injection BrdU/BLBP and BrdU/S100β due to a low number of cells co-labeling with BrdU.
Results
2.1 KL regulates postnatal neurogenesis
Hippocampal development begins around embryonic day 14 and is not complete until the dentate gyrus (dentate) is fully formed ~2 weeks after birth (Li and Pleasure 2005; Li and Pleasure 2014). Thereafter, the dentate subgranular zone (SGZ) is the only hippocampal neurogenic region. Mouse brain KL expression is first detected during late embryonic development (Takeshita et al. 2004). The cerebrospinal fluid generating choroid plexus cells express the highest levels of KL (Kuro-o et al. 1997; Li et al. 2004) but lower level expression is detected in neurons throughout the brain (Kuro-o et al. 1997; Clinton et al. 2013; Li et al. 2017). We measured KL’s expression profile during dentate development using mRNA isolated from wild-type (WT) hippocampi embryonic day 17 (E17) to 8 weeks of age. Using a primer/probe set to allow detection of all KL forms, we found that hippocampus achieved adult level KL expression by 3 weeks of age (Figure 1A), consistent with the reported expression of KL mRNA and protein in mature neurons (Clinton et al. 2013; Li et al. 2017).
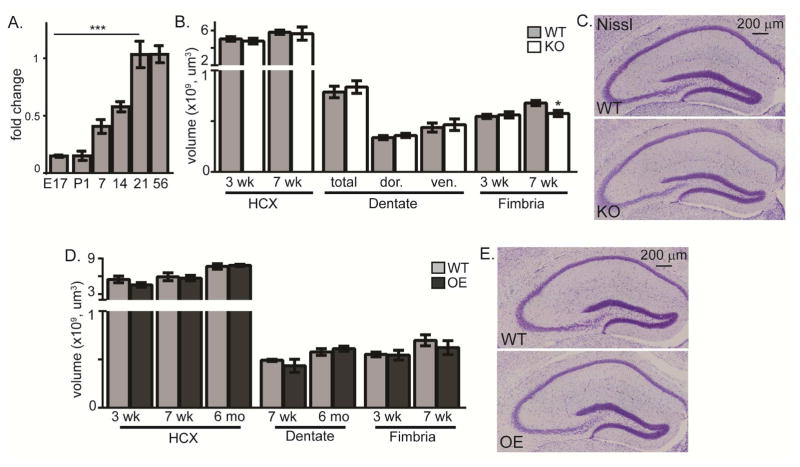
Figure 1. KL does not affect hippocampal development.
A. Brain KL qPCR fold change after normalization to 18s ribosomal subunit and adult brain KL (P56). KL was detected at embryonic (E) day 17 and postnatal (P) days 1, 7, 14, 21, and 56. B. Quantification of 3 and 7 week WT and KO hippocampus (HCX) and fimbria volume and 7 week dentate gyrus (−1.22mm to −3.88mm from bregma; dor (dorsal:−1.22mm to −2.18mm from bregma), ven (ventral: −2.20 to −3.88mm from bregma)). C. Representative 7 week WT and KO Nissl stain. Scale bar is 200μm. D. Quantification of 3 and 7 week WT and OE hippocampus (HCX) and fimbria volume and HCX and dentate volume at 7 weeks and 6 months, as in B. E. Representative 7 week WT and OE Nissl. (n=6; mean +/− S.E.M.; T-test: *p<0.05; mRNA: ANOVA, Dunnett’s post hoc analysis,***p<0.0003 relative to P56).
KL-deficiency is not reported to disrupt embryonic development. To determine if KL expression affects initial hippocampal or dentate development, we measured hippocampal volume of 3 and 7 week old WT and KO mice. We confirmed the described fimbria volume decrease in 7 week old KO mice (Chen et al. 2013) (Figure 1B). Meanwhile, the volume of the WT and KO hippocampi were indistinguishable at 3 weeks, when initial dentate genesis is complete (Nicola et al. 2015), and at 7 weeks when KO mice are near terminal (Figure 1B,C). Although KL is overexpressed in the hippocampus of OE mice (Dubal et al. 2014; Li et al. 2017), we found no hippocampal or fimbria volume change at 3 or 7 weeks of age nor were changes detected into adulthood (6 months) (Figure 1D, E). These data show that gross hippocampal development is not affected by either increasing or decreasing KL protein expression. Herein, the term postnatal neurogenesis is used to distinguish developmental neurogenesis, from ongoing neurogenesis at time points we evaluated on/after postnatal day 21 and selected based on the individual mouse model’s lifespan and cognitive status.
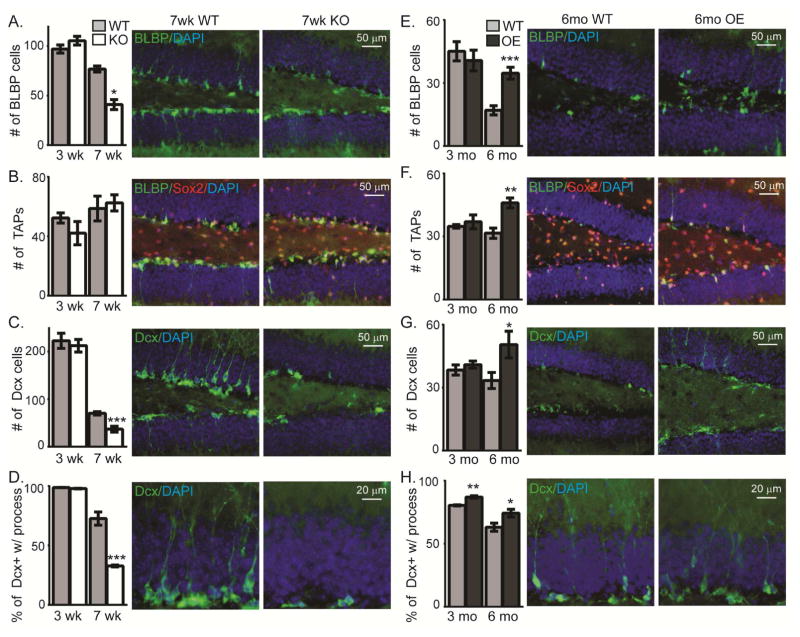
To assess KO and OE postnatal neurogenesis, we measured dorsal hippocampal neurogenic cell populations before and after the onset of reported cognitive change (Nagai et al. 2003; Dubal et al. 2014) (KO at 3 and 7 weeks, white bars throughout; OE at 3 and 6 months, black bars throughout) using strain-relevant WT (grey bars throughout; strain KO:129S1/SvImJ, OE:C57BL/6J) mice to control for strain-specific differences in total neurogenic cell number (Kempermann et al. 1997). At 3 weeks, post-weanling KO mice show no change in the number of stem cells (brain lipid binding protein, BLBP), transient amplifying progenitors (TAP: Sox2+/BLBP−), or immature neurons (doublecortin, Dcx) (Figure 2A–C). However, by 7 weeks KO mice show a robust loss of stem cells and immature neurons (Figure 2A–C). Additionally, maturation of the immature neurons appears to be reduced, as when their dendritic processes were measured, the percent of cells with a process extending into the granule cell layer was less (Figure 2D). Meanwhile, while young adult OE mice showed no neurogenic changes at 3 months, all cell populations (stem cells, TAPs, and immature neurons) were increased at 6 months (Figure 2E–G). Furthermore, there were a greater percentage of immature neurons with a process extending to the granule cell layer suggesting enhanced dendritic maturation (Figure 2H). Although WT immature neuron total dendrite length was not different between sexes (Supplemental Figure 1A), to determine if sex was a factor in the KL models, KO and OE cell population counts were separated based on sex. Neither the total number of stem cells nor immature neurons showed sex-specific effects (Supplemental Figure 1B, C). These data suggest that mouse KL-deficiency causes collapse of the neurogenic niche but KL overexpression is protective against normal age-related decline of neurogenic capacity.
Figure 2. KL is required for the maintenance of neurogenic cell populations.
WT and KO (3 and 7weeks) or WT and OE (3 and 6 month) neurogenic cell populations are the average total cell number across 3 bregma levels/animal (−1.34mm to −2.10mm) of dorsal hippocampal SGZ. Quantification (graph) and representative images (7 weeks for KO, 6 month for OE) for: A,E. Radial-like glial stem cells (brain lipid binding protein, BLBP; green), B,F. Transient amplifying progenitors (TAPs; Sox2+ (red) and BLBP- (green)), and C,G. Immature neurons (doublecortin, Dcx; green). Scale bars represents 50 μm. D,H. Immature neuron arborization as the percent of immature neurons with a process extending into the granule cell layer. Scale bar represents 20μm. DAPI used to label cell nuclei. (n=3–6 for OE; all others n= 8; mean +/− S.E.M.; T-test: *p<0.05, **p<0.001, ***p<0.0003)
2.2 KL regulates neuronal progenitor proliferation
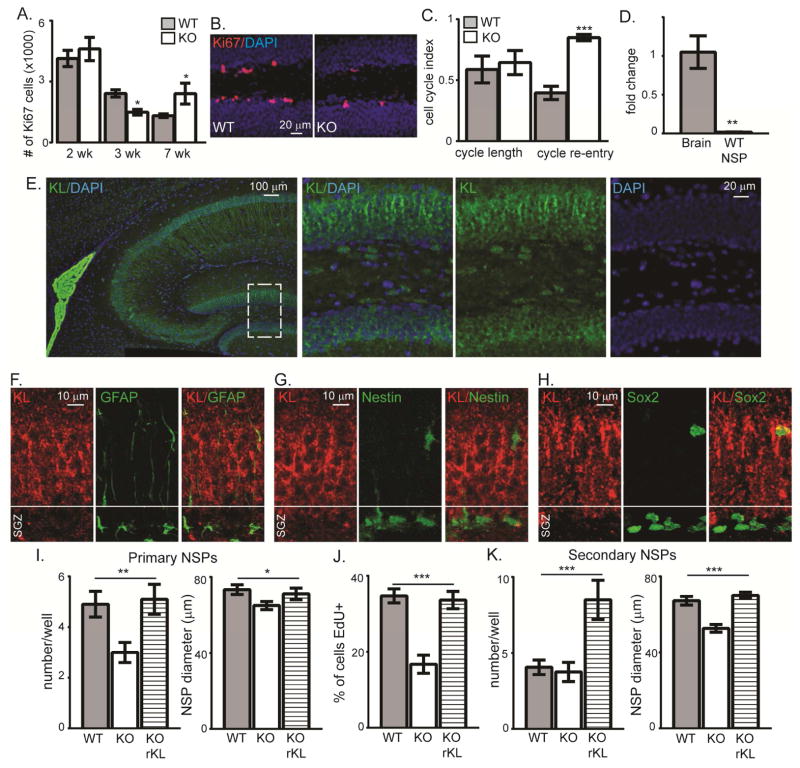
Outside of the brain, loss of some adult KO stem cell populations is attributed to accelerated stem cell proliferation and premature depletion of the stem cell pool (Liu et al. 2007). We measured the number of SGZ proliferating cells (Ki67+) and at 2 weeks KO showed no change (Figure 3A). By 3 weeks the KO SGZ showed decreased numbers of proliferating cells, the earliest reported KO brain phenotype (Figure 3A, B). Peripheral stem cells show increased proliferation (Liu et al. 2007), suggesting that KL action on neural progenitors may be unique. Decreased proliferation could result from altered cell cycle dynamics and as KL is an age-regulating protein decreased proliferation could be an indicator of premature aging of the neurogenic niche. Although aging does not change cell cycle length (Olariu et al. 2007), it can cause progenitors to reenter the cell cycle more frequently (Stoll et al. 2011). Thus we examined KO cell cycle length and re-entry at 3 weeks (Qu et al. 2013) after a single injection of 5-bromo-2′-deoxyuridine (BrdU). As observed with aged stem cells, we measured increased cell cycle re-entry with no cell cycle length change (Figure 3C). Interestingly, at 7 weeks when fewer stem cells and immature neurons were counted (Figure 2A, C), more KO SGZ cells are proliferating which may reflect the earlier increased cell cycle re-entry (Figure 3A). Thus, KL-deficiency affects progenitor cell cycle dynamics causing 3 week KO progenitors to appear phenotypically similar to those in aged brain.
Figure 3. KL-deficiency reduces proliferation of neurogenic precursors.
A,B. WT and KO 2, 3, and 7 week stereological quantification and representative 3 week IHC of SGZ proliferating cells (Ki67). Cell nuclei labeled with DAPI. Scale bar represents 20μm. C. 3 week old WT and KO mice received 1 BrdU injection to measure cell cycle length (BrdU++Ki67+/BrdU+) and cell cycle re-entry (BrdU++Ki67+/Ki67+). D. Adult hippocampus and 14 day in vitro, primary neurosphere WT qPCR normalized to the 18S ribosomal subunit and adult hippocampus (HCX). E. Choroid plexus and hippocampal representative WT KL IHC (green) and DAPI (blue) (left) with white dashed box indicating location of higher magnification images (right). Scale bar represents 100μm or 20μm. F. Representative confocal Z stack WT SGZ images of KL (red) and GFAP (green). Nuclei not shown for clarity and scale bar represents 10 μm. G. Representative confocal Z stacks WT SGZ images of KL (red) and Nestin-GFP (green) from Nestin-GFP WT mice. Nuclei not shown for clarity and scale bar represents 10 μm. H. Representative confocal Z stacks WT SGZ images of KL (red) and Sox2 (green) from WT mice. Nuclei not shown for clarity and scale bar represents 10 μm. I. Ten days after plating 500 cells/well, the number and diameter (μm) of WT/KO spheres was measured. Separate wells of KO cells received recombinant mouse KL (rKL) at plating. J. EdU was added to the media overly laying adherent cells and proliferation measured as the % of cells that were EdU+. K. Primary spheres (C) were re-plated as single cells to measure secondary sphere number and diameter (μm) 10 days later. (n=4–6 in vivo; NSPs: n=3 independent NSP preparations; F,H are the average of all wells counted +/− S.E.M., G is the average of 4 fields per coverslip; T-test (A,C,D): **p<0.004, ANOVA (F,G,H, I): *p<0.05 (multiple comparison test part F, diameter WT vs KO), **p<0.005, ***p<0.0001)
KL modulation of peripheral stem cell proliferation is attributed to the action of circulating KL since peripheral stem cells do not express KL protein (Liu et al. 2007). Since KL is not detected until late embryonic development (Takeshita et al. 2004), it is unlikely that neural stem cells express KL protein. Consistent with previous reports (Clinton et al. 2013; Li et al. 2017), the highest expression of KL is measured in choroid plexus cells but mature neuronal layers of the hippocampus, including the dentate also express KL protein (Figure 3E). KL expression is prominent in mature neuronal processes as evidenced by a gradient of increasing KL expression with lowest KL reactivity occurring in SGZ (Figure 3E). Consistent with low or no SGZ KL expression, confocal microscopy shows no KL co-localization in either Nestin-GFP, Sox2, or GFAP reactive cells most likely to be astrocytes, stem cells, or TAPS when localized to the SGZ (Figure 3F,G, H). However, as KL protein expression is lower ain hippocampus relative to choroid plexus and KL expressing cells are in close apposition to KL+ SGZ cells, we used qPCR to more specifically measure KL expression by neurogenic precursors. We detected no KL mRNA from stem cells and TAPs isolated from 3 week old mice and cultured as neurospheres (NSP) (Gil-Perotin et al. 2013) (Figure 3D), further supporting a lack of KL expression in early neural stem cells/progenitors.
Since neurons are bathed cerebrospinal fluid that contains shed KL (Imura et al. 2004; Yamazaki et al. 2010; Semba et al. 2014; Kunert et al. 2016; Shardell et al. 2016), these data suggest that non-cell autonomous action of shed KL affects early progenitors. Although not taking into account quiescent cells or possible NSP fusion events (Reynolds and Rietze 2005), primary NSPs estimate the in vivo potential of cells to exhibit stem cell traits (Pastrana et al. 2011). When we plated primary NSPs at the same density, KO NSPs produced fewer, smaller spheres that were less proliferative than WT controls (Figure 3I,J), confirming our in vivo decreased proliferation (Figure 3A). If NSPs expressed KL this would suggest a cell intrinsic mechanism (Gilley et al. 2011), however; our results show that the absence of KL prior to NSP plating degrades progenitor potential even before major phenotypic differences occur for the mouse. To test if the loss of stem cell potential was permanent, we added recombinant, shed KL to primary KO NSP media and induced rescue of KO NSP size, number, and proliferation (Figure 3I,J). Using secondary NSPs, we next tested self-renewal. Although secondary KO NSPs self-renewal was not different, forming the same number of spheres as WT, the size of KO spheres was smaller (Figure 3K). This suggests there is no change in stem:TAP ratio (Piccin and Morshead 2011) but rather altered cell activity with enhanced KO NSP quiescence. Adding recombinant shed KL rescued size and stimulated greater self-renewal (Figure 3K). OE mice have broad KL overexpression directed by the human elongation factor 1α promoter (Kurosu et al. 2005). We found that NSPs cultured from OE mice express KL (Supplemental Figure 2A). However, expression does not affect baseline measures of the primary NSP number, diameter, or the proliferation of OE spheres, relative to WT (Supplemental Figure 2B, C, D). Together these data suggest that KL is a direct and non-cell autonomous regulator of progenitor proliferation and that addition of shed KL can decrease stem cell quiescence.
2.3 KL regulates progression of progenitors into mature neurons
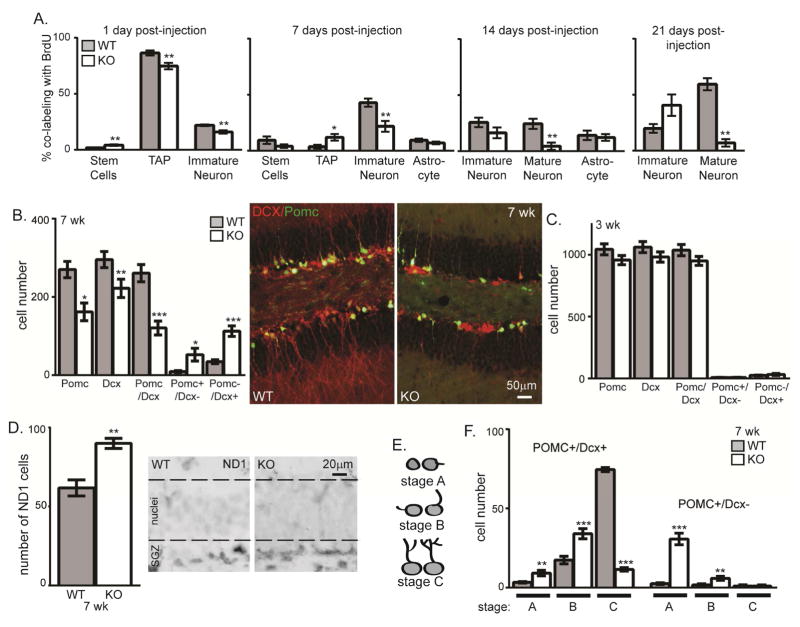
KO proliferation is first affected at 3 weeks when short-lived mice (death at ~8 weeks) are young enough to survive assessment of cell fate after proliferation. To track fate, 3 week old KO mice were injected 4 times with BrdU and were sacrificed 1, 7, 14, or 21 days later. Terminal differentiation to astrocytes (S100β) did not change at any time point (Figure 4A). As expected, 1 day post-injection WT and KO highly proliferative TAPs (Sox2+/BLBP−) incorporate BrdU more than stem cells (BLBP) (Figure 4A). KO brains showed fewer co-labeled TAPs as measured by either TAP markers or the earliest expression of Dcx (Figure 4A, 1 day). As cells matured over the subsequent 3 weeks, the pattern established day 1 continued with fewer immature neurons co-labeling with Dcx at 1 week post-injection and fewer mature neurons labeled with NeuN 2 and 3 weeks post-injection (Figure 4A). These results show that over the course of neuronal commitment and maturation, the absence of KL causes a failure of progenitors to progress to mature neurons.
Figure 4. Klotho-deficiency impairs neuronal maturation.
A. At 3 weeks of age, WT and KO mice were injected 4x with BrdU. Brains were collected 1, 7, 14, and 21 days post-injection. IHC was performed to detect BrdU co-localization with each neurogenic cell type. The percent of BrdU containing cells that co-expressed each cell-type specific protein was quantified by averaging the total number of cells across 3 bregma levels as above. % of BrdU labeled cells also expressing BLBP (stem cell), Sox2+/BLBP- (TAP), Dcx (immature neuron), S100β (astrocytes), and NeuN (mature neurons). B. 7 week quantification of the total number of POMC+, DCX+, POMC+/DCX+, POMC+/Dcx−, or POMC−/Dcx+ cells in 3 bregma levels and KO/POMC-GFP reporter mouse representative 7 week WT and KO images (Dcx (red) and POMC (green)). DAPI not shown for clarity of neuronal arbors. Scale bars represent 50μm. C. 3 week quantification of the total number of POMC+ cells, DCX+, POMC+/DCX+, POMC+/Dcx−, or POMC−/Dcx+ cells in 3 bregma levels. D. 7 week representative images and quantification of SGZ cells expressing NeuroD1 (ND1) protein, dashed line added to delineate granule cell layer from SGZ. E. Morphology schematic used to quantify maturation stage. F. 7 week quantification of number of POMC+/DCX+ or POMC+/Dcx− cells by maturation stage. (n=6 POMC/DCX, n= 3 for ND1; mean +/− S.E.M.; T-test: *p<0.05, **p<0.007, ***p<0.0004; chi-square: A stem cells and astrocyte).
To assess maturation dynamics more precisely than allowed by the broad window of Dcx and NeuN expression alone, we bred the KO mouse to POMC-GFP reporter mice wherein dentate neurons that are 1–2 weeks post-mitotic express GFP (Overstreet et al. 2004). We quantified the total number of cells expressing POMC-GFP, Dcx, and co-labeling for both proteins. Dcx is expressed from early neural progenitor commitment through the early integration phases of new neuron maturation (Brown et al. 2003; Rao and Shetty 2004). POMC expression occurs during a shorter window within the period of Dcx expression (Overstreet et al. 2004) (Figure 7A). While 3 week brains showed no change of these immature cell groups, by 7 weeks all three cell groups were decreased in KO mice, consistent with a loss of immature neurons over time (Figure 4B,C). Whereas 7 week, WT brains contain a small number of cells expressing only Dcx (Figure 4B), in the KO brain the number Dcx only cells increased ~3× and POMC-GFP only cells increased ~5× (Figure 4B). This suggests an accumulation of immature cells at the earliest stages of neuronal differentiation, an idea we sought to further test by quantifying the number of cells expressing neuroD1 (ND1), a transcription factor required for granule cell differentiation (Gao et al. 2009). Despite the dramatic reduction of Dcx-expressing immature neurons, the number of ND1 expressing cells was increased (Figure 4D). This could explain why the number of 7 week KO TAPs is not decreased even though both stem cells and immature neurons are less numerous (Figure 2B). Together these data suggest that the absence of KL not only affects progenitor proliferation but also impairs new neuron maturation at early stages of neuronal commitment.
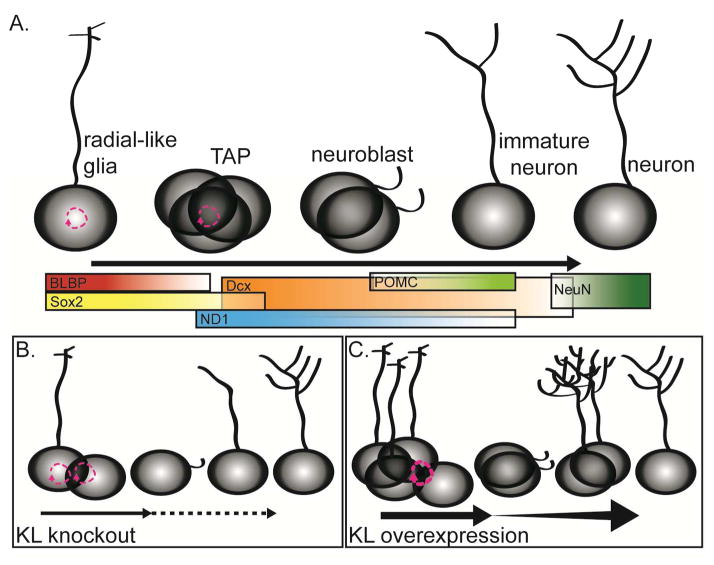
Figure 7. KL regulates postnatal neurogenesis.
A. Postnatal neurogenesis occurs as stem cells progress through a series of protein expression and morphological changes to mature into neurons. B. In brains without KL, with time, there is a loss of the stem/proliferative pool of cells but increased cell cycle re-entry consistent with a prematurely aging neurogenic niche. Decreased proliferation, decreased number of stem cells and immature neurons were measured, as was delayed maturation of immature neurons. C Overexpression of KL increases the number of stem cells/proliferative cells still present at 6 months. Increased proliferation and enhanced maturation allow a greater number of highly arborized immature neurons to persist in the dentate long after normal age-related downregulation of postnatal neurogenesis.
To further quantify maturation, we classified immature neurons by maturation stage (Plumpe et al. 2006). Stage A cells are adjacent to the SGZ and have no or only a small process extending perpendicular into the granular cell layer. Stage B cells have a process that is beginning to grow toward or into the granule cell layer. Stage C cells are the most mature characterized by processes that extended through the granule cell layer with branching into the molecular layer (Figure 4E). Most WT POMC+/Dcx+ immature neurons are mature stage C cells but KO immature neurons are stage A and B cells (Figure 4F). The rare POMC+/Dcx− cells were nearly exclusively all stage A or B cells (Figure 4F). Decreased KO dendritic complexity (Figure 2D,4F) suggests impaired maturation of immature neurons that resembles the phenotype of immature neurons from aged brain (Rao et al. 2005). KL is also important for oligodendrocyte maturation (Chen et al. 2015; Zeldich et al. 2015), suggesting that trophic activity of KL could be required throughout the brain or that different forms of KL protein could have independent effects on different cell populations. Together data from the KO shows that the absence of immature neurons at 7 weeks (Figure 2A) is the product of progenitors failing to proceed through stages of differentiation and maturation causing suppression of neurogenesis.
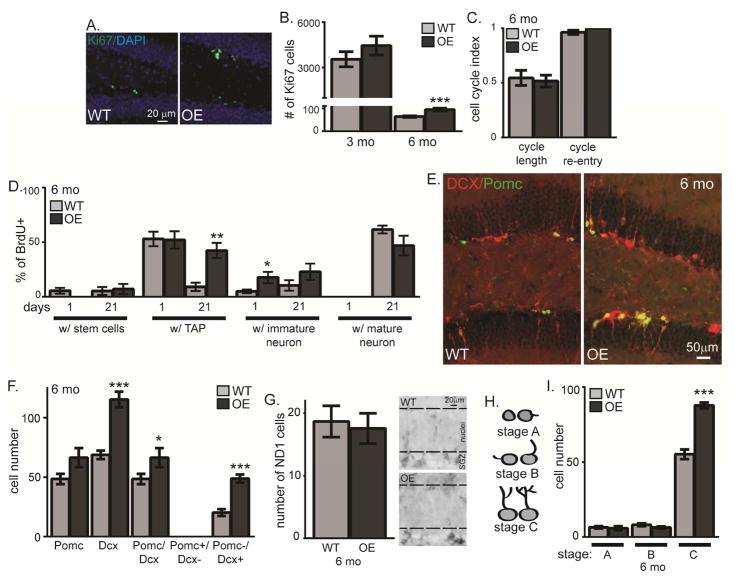
The OE neurogenic niche shows several inverse neurogenic phenotypes, although the timing of effects is distinct between the two KL models. Previous OE phenotypes are reported only after 4–6 months of age (Dubal et al. 2014; Dubal et al. 2015). Likewise, we found OE SGZ showed no proliferation change (Ki67) at 3 months but increased proliferation without an effect on cell cycle by 6 months (Figure 5A–C). Although neurogenesis is age-downregulated (Rao et al. 2006; Ben Abdallah et al. 2010) and thus very few cells incorporated BrdU at 6 months, we tracked cell fate after proliferation as above. Consistent with increased proliferation, the number of BrdU cells co-labeling as TAPs was increased 3 weeks post-injection in the 6 month OE (Figure 5D). More cells expressing the immature neuron protein Dcx were counted 1 day post-injection with a trend toward more 3 weeks later and with no change in mature neurons (Figure 5D). Together with the increase in total stem cells, TAPS, and immature neurons (Figure 2), these results show that KL overexpression enhances proliferation and may affect maturation of adult-born neurons.
Figure 5. KL overexpression enhances neuronal maturation.
A,B. WT and OE 6 month IHC for proliferating cells (Ki67), 3 and 6 month stereological quantification. Cell nuclei labeled with DAPI. Scale bar represents 20μm. C. 6 month old mice received 1 BrdU injection to measure cell cycle length (BrdU++Ki67+/BrdU+) and cell cycle re-entry (BrdU++Ki67+/Ki67+). D. 6 month old WT and OE mice were injected 4x with BrdU. Brains were collected 1 day and 3 weeks (21 days) post-injection. The percent of BrdU cells that co-expressed each cell-type specific protein were quantified as in Figure 4: stem cells (BLBP+), TAPs (Sox2+/BLBP−), immature neurons (Dcx+), and mature neurons (NeuN+). E. OE/POMC-GFP reporter mouse representative 6 month WT and OE images (Dcx (red) and POMC (green)). DAPI not shown for clarity of neuronal arbors. Scale bars represent 50μm. F. 6 month quantification of the total number of POMC+, DCX+, POMC+/DCX+, POMC+/Dcx−, or POMC−/Dcx+ cells. G. 6 month representative images and quantification of SGZ neuroD1+ cells (ND1) with dashed line added to delineate SGZ from granule cell body layer. H. Morphology schematic used to quantify maturation stage. I. 6 month quantification of number of cells POMC+/DCX+ or POMC+/Dcx− by maturation. (n=4–6 POMC/DCX, n= 3 for ND1; mean +/− S.E.M.; T-test: *p<0.05, **p<0.007, ***p<0.0004)
To further test whether KL overexpression alters maturation of new neurons, we measured OE/POMC-GFP immature neurons at 3 and 6 months of age. Neither cell number nor maturation stage changed at 3 months (not shown). At 6 months, the number of OE Dcx+ cells was robustly increased (Figure 5E,F). Unlike the KO, POMC+ only and POMC+/Dcx+ cell groups did not follow the same pattern as Dcx+ alone and were not as robustly increased (Figure 5F). This could suggest that KL overexpression increases the number of either early and/or late stage immature neurons. To differentiate these possibilities, we quantified cells expressing early neuronal commitment protein ND1 and found no change (Figure 5G). Rather, maturational staging of Dcx+ cells confirmed an effect on late immature, stage C neurons, with more in the OE (Figure 5H,I). Thus, while KL overexpression has no effect on cells in young adults, by 6 months it promotes new neuron generation and enhances dendritic arborization.
2.4 KL regulates spatial memory function
Intact neurogenesis is required for normal dentate function, as selectively manipulating the number of adult born neurons is sufficient to alter the performance of spatial discrimination tasks that have long been associated with dentate gyrus function in pattern separation (Aimone et al. 2014). We thus sought to determine whether KO or OE neurogenic changes are functionally relevant by testing mice on behavioral tasks well established to be affected by altered neurogenesis. We first assessed the mice’s motor performance since 7 week old KO mice are small and kyphotic, in terminal decline (Kuro-o et al. 1997). Open-field assessment showed that 7 week KOs were slower and thus traveled less distance than WT (Figure 6A). Consistent with previous data (Dubal et al. 2014), 6 month old OE mice showed no open-field differences (Figure 6B). But both models are sufficiently mobile to perform behavioral testing at ages when we measure neurogenic changes.
To validate previous reports of hippocampal-dependent memory impairment/enhancement (Nagai et al. 2003; Dubal et al. 2014) we tested context-dependent fear conditioning. During training, all mice froze post-shock (Figure 6C,D). When returned to the same context 24 hours later, 3 week KO mice performed equivalently to WT mice but 7 week old KO mice froze less than WT mice (Figure 6C). As cognitive impairment measured at 7 weeks could be caused by terminal decline of the KO (Kuro-o et al. 1997), we also tested 7 week old KL-heterozygotic mice (HET) that are physically indistinguishable from WT. KL HET mice show decreased freezing consistent with cognitive impairment 24 hours after training, although impairment was less pronounced than KO mice (Figure 6C). Although KL heterozygotes do not develop the severe symptoms of loss of KL from renal systems (Kuro-o et al. 1997), they do display lung and heart abnormalities most likely resulting from decreased shed KL (Suga et al. 2000; Sato et al. 2005; Ishii et al. 2008). Meanwhile, consistent with the previous report (Dubal et al. 2014), 6 month OE mice froze more than WT (Figure 6D). These data confirm the previous reports showing an age-dependent, hippocampal functional role for KL (Nagai et al. 2003; Dubal et al. 2014).
Context-dependent fear conditioning requires the hippocampus and can be affected by selective manipulation of adult neurogenesis but is not an exclusively hippocampal task (Saxe et al. 2006; Farioli-Vecchioli et al. 2008; Ko et al. 2009; Goodman et al. 2010; Maren et al. 2013). To further test hippocampal dentate function, we evaluated object location task performance, which is well-established to require the dentate and postnatal neurogenesis (Soule et al. 2008; Goodman et al. 2010; Wimmer et al. 2012; Dees and Kesner 2013). When mice were free to explore two identical objects, they showed no side or object preference on training day but when one of the two objects was moved from the north wall, to the center and back of the box (Figure 6F diagram), 7 but not 3 week old KO mice explored the moved object less and may have shown a preference for the non-moved object (Figure 6E). Again, loss of even half normal KL level at 7 weeks was sufficient to impair cognitive function of HET mice (Figure 6E), confirming that poor task performance does not result from systemic decline but rather is specific for KL deficiency. In contrast, 6 month old OE mice explored the moved object more than WT, consistent with enhanced dentate function (Figure 6F). Object recognition is stable with increasing mouse age but object location memory diminishes(Wimmer et al. 2012). Increasing circulating KL correlates with increased executive function across human lifespan (Dubal et al. 2014; Yokoyama et al. 2015). To determine if OE mice showed enhanced discrimination across lifespan, we also tested 2 month olds. At 2 months WT and OE mice performed significantly better than 6 month old WT mice show no difference relative to 6 month old OE mice (Figure 6F). Thus, while WT mouse performance decreased with age, 6 month old OE mice maintained young-like function (Figure 6F) at an age when enhanced neurogenesis is also measured (Figure 2,5). Sex-specific spatial memory task performance differences were not detected (Supplemental Figure 1D, E). Together these data suggest that KL-mediated control of the neurogenic niche is associated with altered function of the dentate and KL protects against age-related functional decline.
Discussion
3.1 KL regulation of adult neurogenesis
Postnatal-born neurons arise from SGZ radial-like glial cells, divide, and proceed through a series of commitment steps where sequential protein expression promotes morphological development into mature granule neurons (Figure 7A). Here we show that across shortened lifespan, KO mice have decreased numbers of stem cells and immature neurons (Figure 2, 7B). Decreased proliferation of progenitors and increased cell cycle reentry are the first detectable alteration and precedes loss of neurogenic cell populations and cognitive impairment (Figure 2,3,6). KL-deficiency causes delayed TAP/early immature neuron maturation (Figure 4). While our data support a non-cell autonomous role for KL on early progenitors (Figure 3), mature neurons express KL RNA and protein (Kuro-o et al. 1997; Clinton et al. 2013; Li et al. 2017) and it is possible that immature neurons could require KL expression for maturation. Cellular changes to the KO neurogenic niche are consistent with the functional loss of spatial discrimination over lifespan (Figure 6) given the prior work showing that altering the number of immature neurons impairs spatial memory(Marin-Burgin and Schinder 2012). Together the KO neurogenic niche recapitulates some characteristics of the aged dentate suggesting that KL could be involved in regulating niche aging.
Overexpression of KL induced several inverse effects on neurogenesis. The effect of KL overexpression was not robust until 6 months, consistent with previously observed OE brain phenotypes (Dubal et al. 2014). At this time, KL overexpression protected from age-related loss of spatial discrimination (Figure 6). Increased spatial discrimination (Figure 6) occurred at the same time as increased proliferation (Figure 5), more stem cells, TAPs, and immature neurons (Figure 2) and enhanced immature neuron maturation (Figure 5, 7C). Taken together these data provide evidence that KL expression level regulates proliferation and maturation of hippocampal neurogenic precursors.
It is important to note that in both model systems, KL is globally manipulated across lifespan. In the KL-deficient mice, terminal decline of the body could impact brain phenotypes as is observed in human disease (Hermann et al. 2014; Picano et al. 2014). In the KL overexpressing model, overexpression in cells that do not normally make KL could cause non-physiological effects to be measured. As well in both model systems, all forms of the KL protein are increased or decreased and it is likely that transmembrane, shed, and secreted forms of KL have unique and even organ-specific mechanisms of action. Even with these considerations noted; however, our results are the first to show inverse effects of KL on a distinct neuronal population that occur at the same time as cognitive effects of the protein begin to be observed. These argue for a direct role of KL within the brain that could be important for healthy brain function. As KL is age-downregulated body-wide (Duce et al. 2008; King et al. 2012) and the polymorphism that increases serum KL correlates with increased cognitive function (Dubal et al. 2014) it is increasingly important to elucidate the cell-type specific functions of KL and determine whether KL could be targeted to support healthy brain aging or as a biomarker of neurodegenerative disease.
3.2 The temporal development of KL brain phenotypes
To date most KO studies have utilized end of life brains as KO mice have lifespan of only ~8 weeks. We show that KL reaches adult levels of expression at 3 weeks of age (Figure 1). At this time we report reduced neurogenesis and altered stem cell potential (Figure 3). These data are among the earliest KO reported changes. Other 3 week KO studies found increased serum Vitamin D (Yoshida et al. 2002; Tsujikawa et al. 2003), increased skin wnt reporter activation (Liu et al. 2007), and increased lung apoptotic and proliferative indexes (Ishii et al. 2008). Since skin and lung do not express KL (Kuro-o et al. 1997), these data implicate the importance of shed KL. We found that early SGZ progenitors do not express KL but that even when they do, as observed in our cultured OE progenitors, KL expression has no cell autonomous effect (Figure 3, Supplemental Figure 2). Rather the addition of shed KL to cultured progenitor media is sufficient to rescue proliferation defects and promote self-renewal (Figure 3). Although current genetic models do not allow direct assessment of shed KL under conditions of normal renal, transmembrane KL protein, our results show that isolated NSPs robustly respond to exogenous shed KL. Our data also show that KO line heterozygote mice develop hippocampal-dependent cognitive impairment even though half of normal KL is sufficient to maintain renal function, again implicating the importance of shed KL (Suga et al. 2000; Sato et al. 2005; Ishii et al. 2008). Thorough understanding of the direct functions of shed KL is important since targeting a single circulating protein to systemically improve health would be therapeutically useful.
Our data suggest that KL effects are restricted to postnatal neurogenic populations. A pronounced transition occurs between postnatal week 2 and 3 when the hippocampal neurogenic niche takes on the morphologic and protein expression pattern of the adult structure (Nicola et al. 2015) and development optimized processes are replaced by adult patterns of circuit function(Ben-Ari 2001). We show steadily increased KL expression that peaks during postnatal week 3 (Figure 1A) which is also when we measure the first KO cellular phenotypes. These potentially suggest that KL is required for neurogenesis when the brain transitions to adult functionality. We speculate that in the early postnatal environment optimized for neural network formation, redundant mechanisms ensure appropriate neuronal proliferation and maturation even in the absence of KL. However, postnatal and adult brains are less optimized for neurogenesis and the loss of redundancy reveals a requirement for KL regulation.
It is also intriguing that the neurogenic and cognitive consequences of KL overexpression are not evident until ~6 months of age (Figure 2,5,6). This could be an effect of the model as OE mice overexpress only ~1.5x normal KL (Kurosu et al. 2005). However, the cognitive enhancing human polymorphism, KL-VS, also arises with mild serum KL elevation (Dubal et al. 2014) and minor polymorphic KL changes correlate with human disease risk (Arking et al. 2002; Kawano et al. 2002; Arking et al. 2005; Deary et al. 2005; Tsezou et al. 2008). As such, mild KL increase or decrease may be sufficient to affect brain function especially with increasing age.
3.3 KL function in the brain
The first examination of the OE brain revealed increased expression of hippocampal GluN2B subunits and enhanced dentate LTP (Dubal et al. 2014). GluN2B is the immature NMDA receptor subunit that decreases markedly after birth (Shipton and Paulsen 2014). Adult born neurons preferentially express GluN2B beginning after commitment to a neuronal fate (Nacher et al. 2007) and these receptors participate in establishment of the first excitatory synapses on adult born neurons (Chancey et al. 2013), underlying enhanced long-term potentiation of immature adult born neurons (Ge et al. 2007). Thus, increased GluN2B and synaptic plasticity reported in the OE dentate could result from enhanced numbers of immature neurons (Figure 2,5). Alternatively, since proliferation and maturation of adult born neurons maybe be influenced by NMDA receptors, KL-mediated altered expression of GluN2B subunits could contribute to neurogenic changes (Nacher and McEwen 2006). If increased GluN2B expression is widespread and not restricted to immature neurons, KL mediated upregulation could impact many forms of hippocampal plasticity.
As knowledge of the role of brain KL increases, it is important to determine which form(s) of the protein mediate cellular and cognitive effects and whether effects are mediated by similar mechanism to those reported peripherally. Until such definition is possible, care must be taken to interpret data where all forms of KL are globally manipulated. The mouse brain is the only organ with all forms of KL protein (Kuro-o et al. 1997; Shiraki-Iida et al. 1998; Imura et al. 2004; Li et al. 2004; Clinton et al. 2013; Masso et al. 2015). Various protein forms, expressed in multiple cell types and circulating in cerebrospinal fluid throughout the brain, provide the potential that KL has widespread actions that likely affect cognition by multiple mechanisms. Our data showing that KL affects dentate neurogenesis with the expected behavioral consequences is likely just one of the mechanisms that contribute to KL’s cognitive role. However, as mild life-long overexpression is sufficient to protect against aging-related loss of spatial discrimination and neurogenic aging (Figure 2,6), it is important to determine whether either sustaining KL expression or sustaining expression of specific forms of the protein with age or in old brain protects against age-related cognitive decline.
Supplementary Material
A. 8 week old WT POMC-GFP immature neurons were traced to compare total dendrite length between male and female mice. B. Figure 2 counts of BLBP+ cells in 7 week WT/KO and 6 month WT/OE brains split by sex. C. Figure 2 counts of DCX+ cells in 7 week WT/KO and 6 month WT/OE brains split by sex. D. Figure 6 context-dependent fear conditioning percent of time spent freezing 24 hours after training split by sex using 7 week old WT/KO and 6 month old WT/OE mice. E. Figure 6 object location percent discrimination split by sex using 7 week old WT/KO and 6 month old WT/OE mice. (n=3–5/genotype, except E, n=14/sex; mean +/− S.E.M., T-test, no significant differences detected)
A. Adult hippocampus and 14 day in vitro, primary neurosphere WT/OE qPCR normalized to the 18S ribosomal subunit and adult hippocampus (HCX). B,C. Ten days after plating 500 cells/well, the number and diameter (μm) of WT/OE spheres was measured. D. EdU was added to the media overly laying adherent cells and proliferation measured as the % of cells that were EdU+. (NSPs: n=3 independent NSP preparations; B,C are the average of all wells counted +/− S.E.M., ANOVA (A):***p<0.0001, T-test (B,C,D))
Highlights.
Klotho-deficiency decreases hippocampal neural progenitor proliferation and maturation.
Klotho overexpression enhances neural progenitor proliferation and maturation.
While klotho-deficiency rapidly impairs cognitive function, overexpression protects from loss of spatial discrimination.
Acknowledgments
We would like to thank: Drs. Irene Masiulis Bowen and Amelia Eisch, University of Texas Southwestern, for advice on BrdU co-labeling protocols, Dr. Andrew Kennedy, University of Alabama at Birmingham, for behavioral assay training, and Dr. Anita Hjelmeland for use of the Evos Cell Imaging System. Work was funded in part by NIH R00AG034989 and R56AG052936 (GDK) and NIH T32NS061788 (AML).
Abbreviations
- BLBP
Brain lipid binding protein
- BrdU
5-bromo-2′-deoxyuridine
- DAPI
4′,6-diamidino-2-phenylindole
- Dcx
doublecortin
- E17
embryonic day 17
- EdU
5-ethynyl-2′-deoxyuridine
- EGF
epidermal growth factor
- bFGF
basic fibroblast growth factor
- GFP
green fluorescent protein
- GFAP
glial fibrillary acidic protein
- HCX
hippocampus
- IHC
immunohistochemistry
- KL
Klotho
- KO
klotho-deficient knock out mice
- LTP
long term potentiation
- mRNA
messenger ribonucleic acid
- ND1
neuroD1
- NMDA
N-Methyl-D-Aspartate
- NSP
neurosphere
- OE
klotho overexpressing mice
- POMC
proopiomelanocortin
- qPCR
quantitative polymerase chain reaction
- rKL
recombinant shed klotho
- SEM
standard error of the mean
- SGZ
subgranular zone
- SOX2
sex determining region-Y box 2
- TAP
transient amplifying progenitor
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Li Y, et al. Regulation and function of adult neurogenesis: from genes to cognition. Physiological reviews. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Atzmon G, et al. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96(4):412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- Arking DE, Krebsova A, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 2002;99(2):856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends in neurosciences. 2001;24(6):353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, et al. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiology of aging. 2010;31(1):151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Brobey RK, German D, et al. Klotho Protects Dopaminergic Neuron Oxidant-Induced Degeneration by Modulating ASK1 and p38 MAPK Signaling Pathways. PloS one. 2015;10(10):e0139914. doi: 10.1371/journal.pone.0139914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, et al. Transient expression of doublecortin during adult neurogenesis. The Journal of comparative neurology. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cha SK, Ortega B, et al. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105(28):9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, et al. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(15):6614–6622. doi: 10.1523/JNEUROSCI.0781-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Li H, et al. The anti-aging and tumor suppressor protein Klotho enhances differentiation of a human oligodendrocytic hybrid cell line. Journal of molecular neuroscience: MN. 2015;55(1):76–90. doi: 10.1007/s12031-014-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Sloane JA, et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(5):1927–1939. doi: 10.1523/JNEUROSCI.2080-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Glover ME, et al. Expression of klotho mRNA and protein in rat brain parenchyma from early postnatal development into adulthood. Brain research. 2013;1527:1–14. doi: 10.1016/j.brainres.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Harris SE, et al. KLOTHO genotype and cognitive ability in childhood and old age in the same individuals. Neurosci Lett. 2005;378(1):22–27. doi: 10.1016/j.neulet.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Dees RL, Kesner RP. The role of the dorsal dentate gyrus in object and object-context recognition. Neurobiology of learning and memory. 2013;106:112–117. doi: 10.1016/j.nlm.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Doi S, Zou Y, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286(10):8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Yokoyama JS, et al. Life extension factor klotho enhances cognition. Cell reports. 2014;7(4):1065–1076. doi: 10.1016/j.celrep.2014.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu L, et al. Life Extension Factor Klotho Prevents Mortality and Enhances Cognition in hAPP Transgenic Mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(6):2358–2371. doi: 10.1523/JNEUROSCI.5791-12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce JA, Podvin S, et al. Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 2008;56(1):106–117. doi: 10.1002/glia.20593. [DOI] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, et al. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS biology. 2008;6(10):e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nature neuroscience. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, et al. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Perotin S, Duran-Moreno M, et al. Adult neural stem cells from the subventricular zone: a review of the neurosphere assay. Anatomical record. 2013;296(9):1435–1452. doi: 10.1002/ar.22746. [DOI] [PubMed] [Google Scholar]
- Gilley JA, Yang CP, et al. Developmental profiling of postnatal dentate gyrus progenitors provides evidence for dynamic cell-autonomous regulation. Hippocampus. 2011;21(1):33–47. doi: 10.1002/hipo.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Trouche S, et al. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171(3):769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, et al. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learning & memory. 2011;18(2):71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Kribben A, et al. Cognitive impairment in chronic kidney disease: clinical findings, risk factors and consequences for patient care. Journal of neural transmission. 2014;121(6):627–632. doi: 10.1007/s00702-014-1160-z. [DOI] [PubMed] [Google Scholar]
- Imura A, Iwano A, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565(1–3):143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- Ishii M, Yamaguchi Y, et al. Airspace enlargement with airway cell apoptosis in klotho mice: a model of aging lung. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63(12):1289–1298. doi: 10.1093/gerona/63.12.1289. [DOI] [PubMed] [Google Scholar]
- Kawano K, Ogata N, et al. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17(10):1744–1751. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, et al. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(19):10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Kroeger KM, et al. Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Molecular therapy: the journal of the American Society of Gene Therapy. 2008;16(4):682–690. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Muhammad AK, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther. 2011;19(10):1793–1801. doi: 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Rosene DL, et al. Promoter methylation and age-related downregulation of Klotho in rhesus monkey. Age. 2012;34(6):1405–1419. doi: 10.1007/s11357-011-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HG, Jang DJ, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Molecular brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert SK, Hartmann H, et al. Klotho and fibroblast growth factor 23 in cerebrospinal fluid in children. Journal of bone and mineral metabolism. 2016 doi: 10.1007/s00774-016-0746-y. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, et al. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17(3):175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Li G, Pleasure SJ. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Developmental neuroscience. 2005;27(2–4):93–99. doi: 10.1159/000085980. [DOI] [PubMed] [Google Scholar]
- Li G, Pleasure SJ. The development of hippocampal cellular assemblies. Wiley interdisciplinary reviews Developmental biology. 2014;3(2):165–177. doi: 10.1002/wdev.127. [DOI] [PubMed] [Google Scholar]
- Li Q, Vo HT, et al. Klotho regulates CA1 hippocampal synaptic plasticity. Neuroscience. 2017;347:123–133. doi: 10.1016/j.neuroscience.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SA, Watanabe M, et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29(4):91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Logan TT, Rusnak M, et al. Runx1 promotes proliferation and neuronal differentiation in adult mouse neurosphere cultures. Stem cell research. 2015;15(3):554–564. doi: 10.1016/j.scr.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Maltare A, Nietz AK, et al. Development and characterization of monoclonal antibodies to detect klotho. Monoclonal antibodies in immunodiagnosis and immunotherapy. 2014;33(6):420–427. doi: 10.1089/mab.2014.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, et al. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews Neuroscience. 2013;14(6):417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behavioural brain research. 2012;227(2):391–399. doi: 10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Masso A, Sanchez A, et al. Secreted and Transmembrane alphaKlotho Isoforms Have Different Spatio-Temporal Profiles in the Brain during Aging and Alzheimer’s Disease Progression. PloS one. 2015;10(11):e0143623. doi: 10.1371/journal.pone.0143623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, et al. Neural stem and progenitor cells in nestin-GFP transgenic mice. The Journal of comparative neurology. 2004;469(3):311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Nacher J, McEwen BS. The role of N-methyl-D-asparate receptors in neurogenesis. Hippocampus. 2006;16(3):267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, et al. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144(3):855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada K, et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17(1):50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- Nicola Z, Fabel K, et al. Development of the adult neurogenic niche in the hippocampus of mice. Frontiers in neuroanatomy. 2015;9:53. doi: 10.3389/fnana.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Inoue K, et al. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25(10):3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, et al. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. The Journal of comparative neurology. 2007;501(4):659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, et al. A transgenic marker for newly born granule cells in dentate gyrus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(13):3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Silva-Vargas V, et al. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell stem cell. 2011;8(5):486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picano E, Bruno RM, et al. Cognitive impairment and cardiovascular disease: so near, so far. International journal of cardiology. 2014;175(1):21–29. doi: 10.1016/j.ijcard.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Piccin D, Morshead CM. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem cells. 2011;29(3):528–538. doi: 10.1002/stem.589. [DOI] [PubMed] [Google Scholar]
- Plumpe T, Ehninger D, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC neuroscience. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh P, Adlaf E, et al. Enhanced integration of newborn neurons after neonatal insults. Front Neurosci. 2011;5:45. doi: 10.3389/fnins.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q, Sun G, et al. Wnt7a regulates multiple steps of neurogenesis. Molecular and cellular biology. 2013;33(13):2551–2559. doi: 10.1128/MCB.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, et al. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. The European journal of neuroscience. 2005;21(2):464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, et al. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging cell. 2006;5(6):545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. The European journal of neuroscience. 2004;19(2):234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nature methods. 2005;2(5):333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Sato I, Miyado M, et al. NADH dehydrogenase activity and expression of mRNA of complex I (ND1, 51kDa, and 75kDa) in heart mitochondria of klotho mouse. Okajimas folia anatomica Japonica. 2005;82(2):49–56. doi: 10.2535/ofaj.82.49. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Moghekar AR, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neuroscience letters. 2014;558:37–40. doi: 10.1016/j.neulet.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shardell M, Semba RD, et al. Plasma Klotho and Cognitive Decline in Older Adults: Findings From the InCHIANTI Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71(5):677–682. doi: 10.1093/gerona/glv140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki M, Yoshimura K, et al. Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience. 2008;152(4):924–941. doi: 10.1016/j.neuroscience.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Shipton OA, Paulsen O. GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369(1633):20130163. doi: 10.1098/rstb.2013.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki-Iida T, Aizawa H, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424(1–2):6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- Soule J, Penke Z, et al. Object-place recognition learning triggers rapid induction of plasticity-related immediate early genes and synaptic proteins in the rat dentate gyrus. Neural plasticity. 2008;2008:269097. doi: 10.1155/2008/269097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll EA, Habibi BA, et al. Increased re-entry into cell cycle mitigates age-related neurogenic decline in the murine subventricular zone. Stem cells. 2011;29(12):2005–2017. doi: 10.1002/stem.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga T, Kurabayashi M, et al. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. American journal of respiratory cell and molecular biology. 2000;22(1):26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Fujimori T, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109(14):1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tsezou A, Furuichi T, et al. Association of KLOTHO gene polymorphisms with knee osteoarthritis in Greek population. J Orthop Res. 2008;26(11):1466–1470. doi: 10.1002/jor.20634. [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, et al. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Molecular endocrinology. 2003;17(12):2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- Wang W, Pan YW, et al. Genetic activation of ERK5 MAP kinase enhances adult neurogenesis and extends hippocampus-dependent long-term memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(6):2130–2147. doi: 10.1523/JNEUROSCI.3324-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, et al. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. The Anatomical record. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wimmer ME, Hernandez PJ, et al. Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiology of aging. 2012;33(9):2220–2224. doi: 10.1016/j.neurobiolaging.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Imura A, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochemical and biophysical research communications. 2010;398(3):513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama JS, V, Sturm E, et al. Variation in longeivity gene KLOTHO is associated with greater cortical volumes. Annals of Clinical and Translational Neurology. 2015;2(3):215–230. doi: 10.1002/acn3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujimori T, et al. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology. 2002;143(2):683–689. doi: 10.1210/endo.143.2.8657. [DOI] [PubMed] [Google Scholar]
- Zeldich E, Chen CD, et al. The Anti-Aging Protein Klotho Enhances Remyelination Following Cuprizone-Induced Demyelination. Journal of molecular neuroscience: MN. 2015;57(2):185–196. doi: 10.1007/s12031-015-0598-2. [DOI] [PubMed] [Google Scholar]
- Zeldich E, Chen CD, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. The Journal of biological chemistry. 2014;289(35):24700–24715. doi: 10.1074/jbc.M114.567321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Pan F, et al. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain research. 2010;1319:21–32. doi: 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li Y, et al. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. Journal of the American Society of Nephrology: JASN. 2013;24(5):771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. 8 week old WT POMC-GFP immature neurons were traced to compare total dendrite length between male and female mice. B. Figure 2 counts of BLBP+ cells in 7 week WT/KO and 6 month WT/OE brains split by sex. C. Figure 2 counts of DCX+ cells in 7 week WT/KO and 6 month WT/OE brains split by sex. D. Figure 6 context-dependent fear conditioning percent of time spent freezing 24 hours after training split by sex using 7 week old WT/KO and 6 month old WT/OE mice. E. Figure 6 object location percent discrimination split by sex using 7 week old WT/KO and 6 month old WT/OE mice. (n=3–5/genotype, except E, n=14/sex; mean +/− S.E.M., T-test, no significant differences detected)
A. Adult hippocampus and 14 day in vitro, primary neurosphere WT/OE qPCR normalized to the 18S ribosomal subunit and adult hippocampus (HCX). B,C. Ten days after plating 500 cells/well, the number and diameter (μm) of WT/OE spheres was measured. D. EdU was added to the media overly laying adherent cells and proliferation measured as the % of cells that were EdU+. (NSPs: n=3 independent NSP preparations; B,C are the average of all wells counted +/− S.E.M., ANOVA (A):***p<0.0001, T-test (B,C,D))