Abstract
While numerous changes in the GABA system have been identified in models of Fragile X Syndrome (FXS), alterations in subunits of the GABAA receptors (GABAARs) that mediate tonic inhibition are particularly intriguing. Considering the key role of tonic inhibition in controlling neuronal excitability, reduced tonic inhibition could contribute to FXS-associated disorders such as hyperactivity, hypersensitivity, and increased seizure susceptibility. The current study has focused on the expression and function of the δ subunit of the GABAAR, a major subunit involved in tonic inhibition, in granule cells of the dentate gyrus in the Fmr1 knockout (KO) mouse model of FXS. Electrophysiological studies of dentate granule cells revealed a marked, nearly four-fold, decrease in tonic inhibition in the Fmr1 KO mice, as well as reduced effects of two δ subunit-preferring pharmacological agents, THIP and DS2, supporting the suggestion that δ subunit-containing GABAARs are compromised in the Fmr1 KO mice. Immunohistochemistry demonstrated a small but statistically significant decrease in δ subunit labeling in the molecular layer of the dentate gyrus in Fmr1 KO mice compared to wildtype (WT) littermates. The discrepancy between the large deficits in GABA-mediated tonic inhibition in granule cells in the Fmr1 KO mice and only modest reductions in immunolabeling of the δ subunit led to studies of surface expression of the δ subunit. Cross-linking experiments followed by Western blot analysis demonstrated a small, non-significant decrease in total δ subunit protein in the hippocampus of Fmr1 KO mice, but a four-fold decrease in surface expression of the δ subunit in these mice. No significant changes were observed in total or surface expression of the α4 subunit protein, a major partner of the δ subunit in the forebrain. Postembedding immunogold labeling for the δ subunit demonstrated a large, three-fold, decrease in the number of symmetric synapses with immunolabeling at perisynaptic locations in Fmr1 KO mice. While α4 immunogold particles were also reduced at perisynaptic locations in the Fmr1 KO mice, the labeling was increased at synaptic sites. Together these findings suggest that, in the dentate gyrus, altered surface expression of the δ subunit, rather than a decrease in δ subunit expression alone, could be limiting δ subunit-mediated tonic inhibition in this model of FXS. Finding ways to increase surface expression of the δ subunit of the GABAAR could be a novel approach to treatment of hyperexcitability-related alterations in FXS.
Keywords: GABA receptor, Tonic inhibition, Dentate gyrus, Fmr1 gene, Fragile X mental retardation protein, Fragile X Syndrome
Introduction
Fragile X syndrome (FXS) is the most common form of inherited cognitive impairment in humans and results from loss of function of the Fmr1 gene that encodes the fragile X mental retardation protein (FMRP) (Kooy et al., 2000). This RNA-binding protein has many functions that include regulation of translation and transport of a subset of mRNAs into the dendrites and, through such functions, influences synapse development and plasticity (Bassell and Warren, 2008; Pfeiffer and Huber, 2009, for reviews). Loss of FMRP function affects multiple neurotransmitter and signaling systems, including the GABA system (D’Hulst and Kooy, 2009; Santoro et al., 2012). While numerous types of alterations in the GABA system have been reported (Braat and Kooy, 2015b; D’Hulst et al., 2009; Paluszkiewicz et al., 2011, for reviews), a reduction in GABAA receptor (GABAAR)-mediated tonic inhibition is particularly intriguing, both as a basic functional change in FXS and as a target for treatment. Tonic inhibition provides a powerful control of neuronal excitability (Brickley and Mody, 2012; Otis et al., 1991; Semyanov et al., 2004), and a decrease in tonic inhibition could contribute to the increased network excitability that is often observed in models of FXS (Gibson et al., 2008; Goncalves et al., 2013) and thus be associated with behavioral changes such as hyperactivity, hypersensitivity to sensory stimuli, and increased seizure susceptibility (Contractor et al., 2015).
Although several GABAAR subunits can be involved in tonic inhibition, the δ subunit is a critical subunit in several major brain regions, including the dentate gyrus, and expression of the δ subunit conveys special properties (Brickley and Mody, 2012). δ Subunit-containing GABAARs are located at extra- and perisynaptic sites, where they are ideally positioned to respond to ambient levels of GABA within the extracellular space or to spillover at the synapse; they have a high affinity for GABA and slow rates of desensitization; and, importantly, they are extremely sensitive to endogenous compounds such as neuroactive steroids (Saxena and Macdonald, 1994; Wei et al., 2003; Wohlfarth et al., 2002). As a result of these properties, δ subunit-containing GABAARs can be modulated continuously to help maintain the general level of excitability of neuronal networks (Belelli et al., 2009; Mtchedlishvili and Kapur, 2006; Olsen and Sieghart, 2008; Sun et al., 2004). Interestingly, the δ subunit mRNA was one of the first mRNAs to be identified as a direct target of FMRP (Miyashiro et al., 2003). If lack of FMRP leads to altered translation or transport of δ subunit mRNA or dysregulation of δ subunit expression and function, such changes could be associated with deficits in tonic inhibition and potentially constitute a key alteration in FXS.
Despite considerable interest in GABAARs in FXS, GABA-mediated tonic inhibition has been studied in only a limited number of brain regions, such as the subiculum and basolateral nucleus of the amygdala (Curia et al., 2009; Olmos-Serrano et al., 2010). Although tonic inhibition is prominent and mediated primarily by δ subunit-containing GABAARs in several major forebrain brain regions such as the dentate gyrus, alterations of δ-mediated tonic inhibition have not been studied in FXS in these regions. Altered tonic inhibition in the dentate gyrus could be quite important in FXS as this region serves as a gateway to the hippocampus and regulates the large amount of incoming information from the entorhinal cortex (Heinemann et al., 1992). Reduced control of granule cell activity in the dentate gyrus can lead to increased excitability throughout the hippocampal circuit and contribute to deficits in learning and memory as well as increased seizure susceptibility, conditions that are found in children with FXS (Berry-Kravis et al., 2010; Hagerman and Stafstrom, 2009; Musumeci et al., 1999).
The major goals of this study were to determine if tonic inhibition is impaired in dentate granule cells in the Fmr1 KO mouse model of FXS and if such changes are accompanied by alterations in the expression and localization of the δ subunit of the GABAAR in the dentate gyrus, using immunolabeling and biochemical methods. The findings suggest that decreased surface expression of the δ subunit, rather than a decrease in δ subunit expression alone, is likely limiting tonic inhibition in the dentate gyrus and add to the growing evidence for deficits in δ subunit-mediated tonic inhibition in FXS. Preliminary reports have been presented previously (Houser et al., 2014; Zhang et al., 2016).
Methods
Animals
Fmr1 KO male mice on a C57BL/6 background and their male wild-type (WT) littermates were used for all experiments. Fmr1 KO mice were originally obtained from Dr. William Greenough (University of Illinois at Urbana-Champaign) and a colony established at the University of California, Los Angeles. Genotypes were determined by PCR analysis of DNA extracted from tail samples. After weaning, mice were housed in groups of the same sex under standard laboratory conditions with a 12:12 h light-dark cycle. Animals for immunohistochemistry were studied from postnatal day (PN) 14–60, and those for electrophysiological, biochemical and electron microscopic studies were studied at 2–3 months of age. Data from 34 Fmr1 WT and 35 Fmr1 KO littermates were used in the study, and animal numbers are indicated for each experiment. All animal use protocols conformed to National Institutes of Health guidelines and were approved by the University of California, Los Angeles, Chancellor’s Animal Research Committee.
Immunohistochemistry
Tissue preparation for light microscopy
All mice used for light microscopic immunohistochemical studies were deeply anesthetized with sodium pentobarbital (90 mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde in 0.12 M phosphate buffer, pH 7.3. After 1 h in situ at 4°C, brains were removed and postfixed for 1 h. After rinsing, brains were cryoprotected in a 30% sucrose solution overnight and frozen in cryo-embedding compound on dry ice. Forebrain blocks containing the hippocampus were sectioned coronally at 30 µm with a cryostat.
Antisera and immunohistochemical methods
The following GABAAR subunit-specific antisera were used to localize δ and α4 subunits in the immunohistochemical studies: rabbit anti-δ subunit (1:1000; Millipore AB9752 or PhosphoSolutions 868-GDN, no longer commercially available) and rabbit anti-α4 subunit (1:1000; Millipore AB5457). The specificity of each antiserum was confirmed by a lack of immunohistochemical labeling in tissue from δ and α4 subunit KO animals, respectively (Peng et al., 2002; Peng et al., 2014). Prior to immunohistochemistry, free-floating sections were incubated in 1% H2O2 for 30 min and then processed with a water bath heating antigen-retrieval method to reduce endogenous peroxidase-like activity and enhance specific labeling of the receptor subunits (Peng et al., 2002). Briefly, the sections were heated to 90°C for 70 min in sodium citrate solution (pH 8.6). After cooling and rinsing in 0.1 M Tris buffered saline (TBS, pH 7.3), sections were processed for immunohistochemistry with standard avidin-biotin-peroxidase methods (Vectastain Elite ABC; Vector Laboratories) as described in detail previously (Peng et al., 2002; Peng et al., 2004).
Densitometry methods
The intensity of immunolabeling was analyzed with an Axioskop 2 microscope equipped with an AxioCam digital camera system and AxioVision 4.6 software (Zeiss). To evaluate possible differences in density of GABAAR subunit labeling in the Fmr1 WT and KO mice, sections from each animal at comparable levels of the hippocampus were processed in the same experimental run with identical conditions for each subunit. Linear black and white digital images of immunolabeling in the dentate molecular layer, from each side of the brain, were obtained under identical conditions on the same day with stabilized light levels for densitometric analysis (n=2–5 animals per group at each age; 4–6 samples per animal for each subunit). The entire molecular layer of the dentate gyrus was outlined in each image, and the densities of labeling (grey values) within this region were determined with morphometric AxioVision software. Data were analyzed with a nonparametric Wilcoxon Rank Sum Test, and p < 0.05 was considered statistically significant.
Immunogold Labeling for Electron Microscopy
Tissue preparation
Three pairs of Fmr1 WT and KO animals were prepared for postembedding immunogold labeling for the α4 and δ subunits of GABAARs, as described previously (Zhang et al., 2007). Following perfusion of the animals with 4% paraformaldehyde and 0.1% glutaraldehyde, brain sections were cut coronally at 0.5–1 mm with a razor blade, and small blocks of the dentate gyrus were trimmed from these sections. Specimens were cryoprotected, frozen at −190°C in a cryofixation unit (EM CPC; Leica Microsystems), and then transferred to a cryosubstitution unit (EM AFS; Leica), which was programmed for all subsequent steps (Zhang et al., 2007). Specimens were immersed in 4% uranyl acetate (Electron Microscopy Sciences), dissolved in anhydrous methanol for 24 h at −90°C, rinsed in methanol at −45°C, and infiltrated with Lowicryl HM20 resin (Electron Microscopy Sciences) for 48 h at −45°C. The resin was polymerized with ultraviolet light (360 nm) for 24 h at −45°C and then warmed in 4°C steps to 0°C.
Immunogold labeling
Ultrathin sections were cut on a microtome (Reichert-Jung), picked up on nickel mesh grids, and processed for immunogold labeling with previously described methods (Peng et al., 2014; Zhang et al., 2007). After appropriate pre-treatment, sections were incubated in primary antiserum, rabbit anti-δ subunit (1:100; AB9752, Millipore or 868-GDN, PhosphoSolutions, no longer commercially available) or rabbit anti-α4 subunit (1:200; AB5457, Millipore) in TBS containing 2% human serum albumin (HSA) for 18–24 h at room temperature. Sections were then incubated for 2.5 h in the secondary antisera, goat anti-rabbit IgG or F(ab’)2 fragment of goat-anti-rabbit IgG (Aurion; distributed by Electron Microscopy Sciences) conjugated to 10 nm colloidal gold particles, diluted 1:30 in 0.05 M TBS, pH 8.0, containing 2% HSA. After immunogold processing, sections were stained with uranyl acetate for 40 min and lead citrate for 4 min.
Quantitative analysis
Randomly selected series of α4 or δ subunit-labeled synaptic profiles within the molecular layer of the dentate gyrus were photographed with a JEOL 100CX II electron microscope at a primary magnification of 19,000x. The localization of colloidal gold particles was determined for each symmetric synapse in the photomicrographs. Symmetric synaptic contacts were operationally defined as regions with close apposition between an axon terminal and putative granule cell dendrites at which the presynaptic and postsynaptic membranes were precisely parallel. Such contacts generally included a thin postsynaptic density and some electron-dense material in the cleft between the membranes.
For analysis of δ subunit labeling in the Fmr1 WT and KO animals, synapses were classified as labeled (immunogold particles at perisynaptic or nearby extrasynaptic sites) or unlabeled (no immunogold labeling near the synapse). For analysis of α4 labeling, gold particle positioning along the synaptic membranes was classified as either perisynaptic or synaptic. Labeling was operationally defined as perisynaptic if the gold particles were located either directly at the ends of the synaptic contact, within 30 nm of the ends of the synaptic contact, or along the extrasynaptic membranes that extended up to 100 nm beyond the end of the synapse. Gold particles that were located at extrasynaptic sites farther than 100 nm from the ends of a synaptic contact were not included in this analysis. Labeling was classified as synaptic if gold particles were located directly at synaptic contacts, excluding the perisynaptic sites indicated above. Synaptic labeling was further described as within the center third or outer thirds of the synaptic density.
Electrophysiology
Tonic inhibition in granule cells of the dentate gyrus was studied with in vitro whole-cell recording in young adult (2 month) Fmr1 WT and KO littermates. To determine the contributions of the δ subunit to GABA-mediated tonic inhibition in the dentate granule cells, the effects of the δ subunit-selective agonist THIP (1 µm) and a δ subunit selective allosteric modulator DS2 (5 µm) were studied. Methods have been described in detail previously (Tong et al., 2015).
Brain slice preparation
Hippocampal slices were prepared from 2 month-old Fmr1 WT and KO littermates. To prepare slices, animals were deeply anesthetized and decapitated, and brains were placed in ice-cold, modified artificial cerebrospinal fluid (aCSF) containing 65 mm sucrose, 82.7 mm NaCl, 2.4 mm KCl, 0.5 mm CaCl2, 6.8 mm MgCl2, 1.4 mm NaH2PO4, 23.8 mm NaHCO3 and 23.7 mm D-glucose and saturated with 95% O2/5% CO2. This solution also served for cutting 300 µm-thick coronal or horizontal slices containing hippocampus and cortex using a VT-1000 vibratome (Leica). Brain slices were allowed to equilibrate for 30 min at 35°C and then switched to 21–23 °C (RT) in normal aCSF containing 126 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 2 mm CaCl2, 2 mm MgCl2, 26 mm NaHCO3 and 10 mm D-glucose, continuously bubbled with a mixture of 95% O2/5% CO2 gas. Kynurenic acid (2 mm) was included in the recording solution to block ionotropic glutamate receptors and isolate tonic GABA current.
Drug applications
The drugs used in the electrophysiology studies included SR95531, THIP and DS2 (all from Tocris Bioscience) and kynurenic acid (Sigma Aldrich). SR95531, THIP and DS2 were dissolved in DMSO and diluted 1:1000 into aCSF before use. For kynurenic acid, the powder was added to the fresh recording buffer at 2 mm working concentration each time.
Electrophysiological recording from brain slices
For in vitro slice studies of tonic inhibition, dentate gyrus granule cells were recorded in whole-cell mode using pipettes with a typical resistance of 4–5 MΩ when filled with internal solution containing 140 mm CsCl, 4 mm NaCl, 1 mm MgCl2, 1 mm QX-314, 10 mm HEPES, 0.1 mm EGTA, 2 mm Mg-ATP, 0.3 mm Na-GTP with pH set to 7.3. The initial studies of tonic current were performed in the absence of added GABA, since ambient GABA in healthy slice preparations was sufficient to evoke a distinct tonic baseline shift that could be detected when GABAARs were blocked with SR95531. Such conditions were considered the most physiological and avoided the possibility that adding GABA to the bath would desensitize the high affinity δ subunit-containing receptors (Bright and Smart, 2013). For subsequent pharmacological experiments, we added GABA (5 µM) to the bath in order to increase the size of the tonic current sufficiently to allow comparison of the effects of δ subunit-selective modulators in the Fmr1 WT and KO mice.
Neurons in all recordings were visualized with an iXon EMCCD camera (Andor Technology) and infrared optics on an upright epifluorescence microscope (Axioskop 2 FS, Zeiss). pCLAMP 8.2 software and an Axopatch-1D amplifier were used for electrophysiology (Axon Instruments). Data were filtered at 2 kHz and acquired at a sampling rate of 20 kHz. Solutions were continuously perfused at a rate of 2 ml/min. All the drugs used bath applications for at least 5 min to ensure the effect on the recorded slice.
Analysis of electrophysiological data
Tonic GABAAR-mediated current was defined as the steady-state current blocked by saturating concentrations of SR95531, and its magnitude was calculated by plotting all-point histograms of relevant 30 sec segments of data. Graphs for all studies were created in OriginPro 8 and assembled in CorelDraw 12. Normally distributed data were analyzed using unpaired Student’s two tailed t tests, with significance declared at p < 0.05. A nonparametric Mann-Whitney rank sum test was used to assess the statistical significance of data deviating from normality. Data are presented as mean ± SEM.
Immunoblotting
Measurement of surface receptor subunit expression by cross-linking
To determine cell surface protein levels from Fmr1 WT and KO mice, cross-linking experiments followed by Western blot analysis were performed, as described by Browning and colleagues for surface labeling of receptors in brain slices (Grosshans et al., 2002) and used previously by the current investigators (Liang et al., 2014; Lindemeyer et al., 2014; Lindemeyer et al., 2017). Briefly, 400 µm thick coronal hippocampal slices were prepared using a tissue chopper (VT1200S, Leica). Slices were incubated either in ice-cold aCSF composed of 124 mm NaCl, 3 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 1 mm KH2PO4, 10 mm D-glucose, 26 mm NaHCO3 and oxygenated with 95%O2/5% CO2, pH 7.4), or aCSF containing 1 mg/ml bis(sulfosuccinimidyl)suberate (BS3) (Pierce) at 4°C for 45 min, for measurements of total or internal protein, respectively. The cell-impermeable BS3 bi-functionally cross-links all surface proteins leading to large molecular weight aggregates which do not reliably migrate in the gel. Therefore, only internal proteins show up in the gel of the BS3 sample. The cross-link reaction was quenched with 20 mm Tris wash buffer, pH 7.6, and the slices were homogenized in homogenizing buffer containing 10 mm Tris, pH 8.0, 1 mm EDTA and 1% SDS. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce).
SDS-PAGE and Western blot analysis
Proteins were separated on SDS-polyacrylamide gels (Bio-Rad) using the Bio-Rad Mini-Protean 3 cell system. Proteins were transferred on a PVDF membrane (Bio-Rad) and blocked with 4% nonfat dry milk in TBS. Blots were incubated overnight at 4°C with primary polyclonal rabbit antibodies against the following: δ (aa1-44) (Jones et al., 1997); α4 (aa379-421) (Ebert et al., 1996); (both at 1 µg/ml, gift of W. Sieghart); α4 (1:1000; Millipore, AB5457); or mouse monoclonal β-actin (1: 1000; Sigma Aldrich, A2228), followed by HRP-conjugated secondary antibodies (1:5000; Rockland) for 2 h at room temperature. Bands were detected using ECL detection kit (GE Healthcare) and exposed to X-ray films (MidSci).
Data analysis
Protein signals were analyzed by densitometry using ImageQuant5.2 (Molecular Dynamics). For cross-linking experiments, the signal intensity of bands was normalized to the corresponding β-actin signal after background subtraction. Surface protein levels were calculated by subtraction of the internal protein signal (from BS3-treated slices) from the respective total protein signal (from untreated slices). Statistical comparisons were made by nonparametric Mann-Whitney tests, with p < 0.05 considered statistically significant. All values are shown as mean ± SEM with n representing the number of experiments.
Results
δ Subunit labeling is slightly lower in the dentate gyrus of Fmr1 KO mice
Patterns of δ subunit immunoperoxidase labeling in the dentate gyrus were compared in Fmr1 WT and KO littermates at PN 14, 21, 28, 35 and 60. In WT animals, the normal pattern of δ subunit labeling in the hippocampus was evident as early as PN14, although the immunoreactivity appeared slightly lower at the young ages and progressively increased at older ages (Fig. 1). Labeling was highest in the molecular layer of the dentate gyrus, where the δ subunit is normally located on dendrites of dentate granule cells (Fig. 1). Very low levels of labeling were found in the hilus and CA3 region, with slightly higher labeling in CA1.
Fig. 1.
Immunolabeling of the δ subunit in the dentate gyrus is slightly lower in Fmr1 KO mice than in WT littermates at PN14-PN60. (A,C,E,G) In WT animals, the δ subunit is strongly expressed in the molecular layer of the dentate gyrus (M). Labeling is very low in the hilus (H) and CA3, but slightly higher in CA1. Similar patterns of labeling are evident from PN14 to PN60, but the level of δ subunit labeling in the molecular layer increases throughout this period and labeling of interneurons appears strongest at PN14. (B,D,F,H) In Fmr1 KO animals, the patterns of δ subunit labeling closely resemble those of the WT littermates at each age. However, slightly lower levels of labeling are found in the dentate molecular layer in the KO animals. (I) Densitometry confirmed small but significant differences in labeling intensity in the molecular layer at PN35 and borderline differences at PN60. Mean intensities of labeling for WT and KO littermates at four ages are included in the bar graph. However, only data from animals at PN35 and PN60 were analyzed statistically due to limited numbers of littermates at younger ages. *p = 0.03. Scale bar = 150 µm (A–H).
The broad patterns of labeling were similar in WT and KO mice at all ages studied (illustrated for PN 14, 21, 35 and 60 in Fig. 1). However, the levels of labeling in the molecular layer of the dentate gyrus appeared consistently lower in the KO animals when compared to their WT littermates, although the differences were slight (Fig. 1). Densitometric analyses of the immunohistochemical labeling in the mice at PN 35 (n = 4 WT and 4 KO) and PN 60 (n = 3 WT and 4 KO) confirmed lower levels of labeling in the Fmr1 KO animals compared to their WT littermates (PN 35, 15.1% lower, p = 0.03; PN 60, 15.2% lower, p = 0.06; Wilcoxon Rank Sum Test) (Fig. 1).
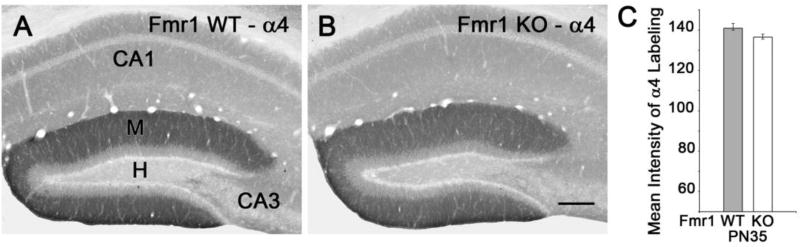
Immunolabeling for the α4 subunit in WT animals closely resembled that of the δ subunit in the hippocampus (Fig. 2; compare with Fig. 1). No differences in the patterns or levels of α4 labeling were observed in WT and Fmr1 KO mice, and densitometric analysis revealed no significant differences (Fig. 2).
Fig. 2.
Immunolabeling of the α4 subunit in the dentate gyrus is similar in Fmr1 WT and KO mice at PN35. (A,B) α4 labeling is highest in the molecular layer (M) of the dentate gyrus, and the level of α4 labeling in the molecular layer is similar in WT and KO littermates. The pattern of α4 labeling in the dentate gyrus closely resembles that of the δ subunit (see Fig. 1E,F). (C) Densitometry demonstrated no significant difference in the mean intensity of α4 labeling in the molecular layer of WT and KO littermates. Scale bar = 150 µm (A,B).
These immunohistochemical findings raised questions about the functional effects of the limited decreases in δ subunit expression on tonic inhibition in dentate granule cells.
δ subunit-containing GABAAR-mediated tonic inhibition is severely reduced in Fmr1 KO mice
To determine if tonic inhibition was altered in the dentate granule cells, we made whole-cell recordings from these neurons in young adult (2 month) Fmr1 WT and KO littermates. We found significantly lower GABAAR-mediated tonic currents in Fmr1 KO mice (31.1 ± 2.4 pA in WT mice vs 6.7 ± 1.4 pA in KO littermates (p < 0.0001) (Fig. 3A,B). Similar differences were found in tonic inhibitory conductance (11.4 ± 1.3 S/F in WT mice and 2.4 ± 0.5 in KO mice; p < 0.0001) (Fig. 3B). These marked differences in tonic current and conductance were observed without any change in membrane properties as no differences were observed in either membrane resistance or membrane capacitance in the two groups of mice (Fig. 3C).
Fig. 3.
GABAAR-mediated tonic current in dentate granule cells is significantly lower in Fmr1 KO mice than in WT littermates. (A) Example traces from WT (top) and Fmr1 KO (bottom) dentate gyrus granule cells demonstrate a large tonic current that is sensitive to the GABAAR antagonist SR 95531 in WT but not in the KO cell. To the right are all points histograms of the current levels prior to (black) and following application of SR 95531 (grey). (B) Summary of the mean ± SEM levels of SR 95531-sensitive tonic current (left) and conductance (right) in WT and KO conditions. Significant reductions in current and conductance were observed in the KO relative to WT cells. (C) No significant differences were observed in basic membrane properties of membrane resistance (left) or capacitance (right) when comparing WT and KO granule cells.
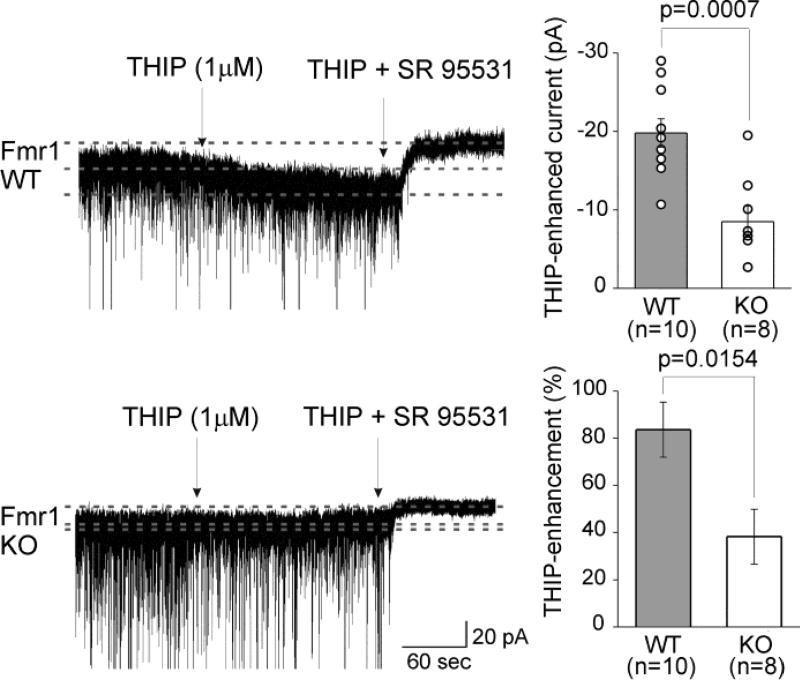
To further probe the function of δ subunit-containing GABAARs in these animals, we next tested the efficacy of THIP, a δ subunit-preferring agonist at low concentrations (Jia et al., 2005; Meera et al., 2011). In WT animals, THIP (1 µM) substantially increased tonic current, as expected, in animals with normal levels of δ subunit-containing receptors (Fig. 4). In contrast, in Fmr1 KO mice, THIP-activated currents were small, consistent with low levels of δ subunit-containing GABAARs (−19.8 ± 1.8 pA in WT and −8.5 ± 2.0 pA in Fmr1 KO mice, p = 0.0007) (Fig. 4). Normalized to baseline tonic current, the THIP-activated current was approximately 2.2-fold larger in WT than in Fmr1 KO mice (84% enhancement in WT vs 38% enhancement in KO mice; p = 0.0154) (Fig. 4).
Fig. 4.
Activation of GABAAR-mediated current by the δ subunit-selective agonist THIP is significantly decreased in Fmr1 KO mice. Example traces shown on the left indicate the current activated by application of 1 µM THIP, an agonist selective for δ subunit-containing GABAARs. A recording from a WT granule cell is shown on the top and a KO granule cell on the bottom. To the upper right are summaries of the levels of current activated by THIP in each condition (mean ± SEM). Open symbols indicate individual cells. On the lower right is a summary of the amount, expressed as a percentage, of additional current activated by THIP relative to the SR 95531-sensitive tonic current level prior to THIP.
To further evaluate the δ subunit contribution to the lower tonic current in the Fmr1 KO mice, we studied the effects of the δ subunit-selective GABAAR positive allosteric modulator DS2 (Jensen et al., 2013; Wafford et al., 2009). The DS2-enhanced current was significantly larger in the WT than in the Fmr1 KO mice (−19.0 ± 3.2 pA in WT and −9.3 ± 2.0 pA in KO mice; p = 0.019) (Fig. 5). This represented a 3-fold greater enhancement in the WT compared to that in the Fmr1 KO mice (95% enhancement in WT vs 32% enhancement in KO mice; p = 0.0126) (Fig. 5).
Fig. 5.
Enhancement of GABA-mediated tonic current by the δ subunit-selective positive allosteric modulator DS2 is significantly decreased in Fmr1 KO mice. Example traces shown on the left indicate the current activated by application of DS2, a positive modulator selective for δ subunit-containing GABAARs. A recording from a WT granule cell is shown on the top and a KO granule cell on the bottom. To the upper right are summaries of levels of current increase observed in DS2 in each condition (mean ± SEM). Open symbols indicate individual cells. On the lower right is a summary of the percent increase in SR 95531-sensitive tonic current following DS2 application.
Together these electrophysiological findings suggest that δ subunit-containing GABAAR-mediated tonic inhibition is severely reduced in dentate granule cells in the Fmr1 KO mice compared to that in WT littermates. The substantial decrease in tonic inhibition differed markedly from the limited decreases in δ subunit expression in the immunohistochemical experiments. These discrepant findings suggested that additional alterations, beyond a basic reduction in the δ subunit protein, could be contributing to the functional changes in tonic inhibition. This led to immunoblotting experiments to evaluate potential differences in both total and surface protein levels in WT and Fmr1 KO littermates.
Cell surface protein levels of the δ subunit are significantly reduced in Fmr1 KO mice
To determine the total and surface expression of the δ subunit, we conducted cross-linking experiments followed by Western blot analysis. We found a 31.4% but non-significant (p = 0.1267, Mann-Whitney test) decrease in total δ subunit protein in the Fmr1 KO mouse (Fig. 6). However, a fourfold decrease in surface expression of the δ subunit was found in the Fmr1 KO mice (p = 0.0067, Mann-Whitney test) (Fig. 6). Because the α4 subunit is a major partner of the δ subunit in the forebrain (including dentate granule cells), the levels of α4 subunit were also studied. The level of total α4 protein in the KO animals did not differ significantly from that in the WT mice (p > 0.999, Mann-Whitney test) (Fig. 6). Likewise, no significant differences were found in levels of α4 subunit surface protein between WT and KO mice (p > 0.999, Mann-Whitney test) (Fig. 6). These findings suggested that the differences in δ subunit-mediated tonic inhibition could be related to reduced surface expression of the δ subunit in the Fmr1 KO mouse, and this led to questions about the subcellular localization of both the δ and α4 subunits.
Fig. 6.
Surface expression of the GABAAR δ subunit is decreased in Fmr1 KO mice. (A) Representative Western blots for δ (left panel) and α4 (right panel) after cell surface cross-linking (t = amount of total protein; int = amount of internal protein). The respective β-actin signals are shown below. (B) Quantification of the total and surface δ subunit levels. Surface protein levels were calculated by subtracting internal protein signal from the total protein signal. A four-fold decrease in surface expression of the δ subunit was found in the KO mice. (C) Quantification of the total and surface α4 subunit levels of WT vs KO. No differences in total or surface expression of α4 subunit protein were found in WT and KO mice. Data are mean ± SEM, n = 5–8 mice/group. * p < 0.01, Mann-Whitney test between KO and WT.
Immunogold labeling of the δ subunit is severely reduced near synaptic contacts on dentate granule cells in Fmr1 KO mice
Light microscopic studies did not allow a distinction between localization of the subunits within the cytoplasm or near synaptic sites on the surface of the granule cell dendrites. Postembedding immunogold labeling was thus used to determine if there were alterations in the subcellular localization of the δ and α4 subunits. After immunolabeling for the δ subunit, symmetric synapses in the dentate molecular layer were identified, and the presence or absence of immunogold particles was determined at each symmetric synapse that exhibited a clear postsynaptic density. In WT littermates (n = 3 mice; 183 total synapses), immunogold particles were found in perisynaptic locations in 83.8% of the synapses, while 16.2% of symmetric synapses were unlabeled (Fig. 7). In contrast, in the Fmr1 KO mice (n = 3 mice; 212 total synapses), only 24.6% of the symmetric synapses showed immunogold labeling along the plasma membrane at perisynaptic sites, and 75.4% of the symmetric synapses showed no immunogold labeling either at or near the synaptic contacts (Fig. 7). Differences in numbers of labeled and unlabeled synapses in the two groups of animals were highly significant (Student’s t test, p < 0.001).
Fig. 7.
Immunogold labeling of the δ subunit is decreased along the dendritic plasma membrane in Fmr1 KO mice. (A,C) In Fmr1 WT mice, δ subunit immunogold labeling is localized predominantly at perisynaptic locations (arrows) near symmetric synapses (open arrowheads; T = axon terminal). (B,D) In Fmr1 KO littermates, many symmetric synapses (open arrowheads) lack δ subunit immunogold labeling at perisynaptic sites. Immunogold particles were occasionally observed within the cytoplasm of granule cell dendrites (arrow in panel D). (E) Quantitative analysis in WT animals demonstrated δ subunit immunogold labeling at perisynaptic locations in a high percentage of symmetric synapses (identified as labeled synapses). In contrast, a high percentage of symmetric synapses in Fmr1 KO mice lacked labeling at the synaptic membrane (identified as unlabeled synapses). Scale bars = 0.1 µm (A–D).
These findings are consistent with the biochemical findings of significantly lower surface expression of the δ subunit in the Fmr1 KO mice and demonstrate that a reduction occurs at perisynaptic sites where δ subunit-containing receptors are normally located and mediate tonic inhibition.
Immunogold labeling of the α4 subunit is retained at the cell surface but is altered in synaptic location in Fmr1 KO mice
The α4 subunit is a major partner of the δ subunit in dentate granule cells, and the severe decrease in surface labeling of the δ subunit led to questions about the localization of the α4 subunit. Thus similar immunogold labeling was used to determine the localization of the α4 subunit. In Fmr1 WT littermates, 78.8% of the symmetric synapses (n = 3 mice; 101 total synapses) showed immunogold labeling at perisynaptic or extrasynaptic locations, while 21.2% exhibited labeling directly at the synaptic contacts, with labeling at either the center third (19.5%) or outer third (1.7%) (Fig. 8). In contrast, in the Fmr1 KO mouse, 80.3% of the symmetric synapses (n = 3 mice; 116 total synapses), showed α4 subunit labeling directly at the synaptic contacts, at either the center third (71.9%) or outer third (8.4%) (Fig. 8). α4 subunit labeling at perisynaptic or nearby extrasynaptic locations was found in only 19.7% of the total symmetric synapses in Fmr1 KO mice (Fig. 8). These findings suggest that, while surface expression of the δ subunit is altered in the Fmr1 KO mice, surface labeling of the α4 subunit is maintained, but the location of the α4 subunit is altered, possibly due to lack of δ subunit partnership at perisynaptic sites.
Fig. 8.
Immunogold labeling of the α4 subunit is decreased at perisynaptic locations but increased at synaptic sites in Fmr1 KO mice. (A,C) In WT mice, labeling for the α4 subunit was found primarily at perisynaptic (arrow in panel A) or extrasynaptic (arrow in panel C) locations near symmetric synapses (open arrowheads). (B,D) In Fmr1 KO animals, α4 subunit immunogold labeling (arrows) was frequently found directly at synaptic contacts (open arrowheads). (E) In a high percentage of symmetric synapses in WT mice, α4 immunogold labeling was found at nonsynaptic locations. In contrast, in Fmr1 KO mice, a high percentage of symmetric synapses exhibited α4 immunogold labeling directly at synaptic contacts. (F) Immunogold labeling of the α4 subunit is found predominantly at perisynaptic locations in WT mice but near the center of symmetric synapses in Fmr1 KO mice. Lower percentages of synapses were labeled at extrasynaptic sites or within the outer third of synaptic contacts in both groups of animals. Scale bars = 0.1 µm (A–D).
Discussion
This study provides the first evidence for decreased neuronal cell surface expression of the δ subunit of the GABAAR in a model of FXS. The major findings of the study are that 1) δ subunit-mediated tonic inhibition in dentate granule cells is substantially lower in Fmr1 KO mice than in WT littermates; 2) surface expression in the δ subunit is reduced in the dentate gyrus of the Fmr1 KO mice, as demonstrated by both biochemical and immunogold labeling studies; and 3) the substantial decrease in δ subunit surface expression contrasts with the limited decrease in total δ subunit protein and immunolabeling in the dentate molecular layer. Together the findings suggest that the decrease in tonic inhibition in the dentate gyrus is due primarily to a decrease in surface expression of δ subunit-containing receptors rather than a reduction in δ subunit protein alone.
The marked reduction in tonic inhibition in dentate granule cells in this model of FXS could have important functional effects as the granule cells control the massive input from the entorhinal cortex and can thus limit the information that reaches the hippocampus (Dengler and Coulter, 2016; Leutgeb et al., 2007). This filtering function of the dentate gyrus provides a critical early step in information processing within the hippocampal formation. Thus, reduced tonic inhibitory control of dentate granule cells could negatively impact learning and memory as well as increase seizure susceptibility and levels of anxiety (Lee et al., 2016; Maguire et al., 2005; Whissell et al., 2013). A decrease in tonic inhibition has been found previously in other brain regions in mouse models of FXS, including the subiculum (Curia et al., 2009), basolateral nucleus of the amygdala (Martin et al., 2014; Olmos-Serrano et al., 2010), and the CA1 region of the hippocampus (Sabanov et al., 2016). The current findings support the suggestion that a decrease in tonic inhibition could be a common finding in FXS, occurring across multiple brain regions where the deficits could contribute to several behavioral features of FXS, including hyperexcitability, hypersensitivity, and increased seizure susceptibility (Martin et al., 2014; Paluszkiewicz et al., 2011; Whissell et al., 2015). The current electrophysiological studies also confirm that the altered tonic inhibition in dentate granule cells of the Fmr1 KO mouse results from deficits in δ subunit-containing GABAARs, as demonstrated by reduced responses to both THIP, a δ subunit-preferring agonist at low (µm) concentrations (Jia et al., 2005; Meera et al., 2011), and DS2, a δ subunit-selective positive allosteric modulator of GABAARs (Jensen et al., 2013; Wafford et al., 2009).
While alterations in the α5 subunit cannot be ruled out as contributing to the decrease in tonic inhibition in the dentate gyrus, the α5 subunit is most highly expressed in the hippocampus (Houser and Esclapez, 2003), with comparatively low levels in the dentate gyrus, where tonic inhibition is mediated primarily by the δ subunit (Glykys et al., 2008). In addition, no change in α5 subunit mRNA levels were found in the hippocampus of the Fmr1 KO mouse (Sabanov et al., 2016).
Additional factors such as the levels of GABA in the extracellular space can also influence tonic inhibition (Glykys and Mody, 2007b), and previous studies have suggested decreases in the availability of GABA in the amygdala of Fmr1 KO mice, changes that could reduce both phasic and tonic inhibition (Olmos-Serrano et al., 2010). However, direct measurement of GABA levels in the extracellular space, particularly near synaptic sites, remains technically challenging and can be influenced by many factors in vitro and in vivo (Glykys and Mody, 2007a; Patel et al., 2016). Thus, currently, altered surface expression of δ subunit-containing receptors remains the most likely contributor to decreased tonic inhibition in the dentate gyrus of the Fmr1 KO mouse.
Alterations in the δ subunit of the GABAAR in FXS has been suspected for some time, following the discovery that the δ subunit mRNA is one of the direct targets of FMRP, as demonstrated initially by antibody-positioned RNA amplification methods (Miyashiro et al., 2003) and subsequently by electrophoretic mobility shift assays (Braat et al., 2015). Lower levels of δ subunit mRNA have also been found in Fmr1 KO mice in several forebrain regions, including the cerebral cortex, subiculum and hippocampus (Braat and Kooy, 2015a; D’Hulst et al., 2006; Gantois et al., 2006; Sabanov et al., 2016).
Descriptions of decreased δ subunit mRNA have led to the frequent assumption that the δ protein is also decreased. However, studies of the δ subunit protein have been limited, and the findings have differed among brain regions and ages. Decreases in the δ subunit protein in Fmr1 KO mice have been found in preparations of the entire forebrain at PN12, but not at PN5 or in adults (Adusei et al., 2010); in the subiculum in young adult animals (Curia et al., 2009); and in the hippocampus at PN22 (Sabanov et al., 2016).
No detailed immunohistochemical studies of δ subunit expression in the Fmr1 KO mouse have been published, but, based on previous biochemical studies and the electrophysiological findings in the current study, a substantial decrease in δ subunit labeling was expected. Surprisingly, δ subunit labeling in the dentate gyrus was only slightly, though consistently, lower in the Fmr1 KO mice when compared with WT littermates. Likewise, only a small, statistically nonsignificant, decrease in total δ subunit protein was detected in the current biochemical studies.
A previous study of tonic inhibition and δ subunit expression in the subiculum of Fmr1 KO mice found remarkably similar discrepancies between a very large decrease in tonic inhibition in subicular neurons (~91% lower than in WT mice) and a more limited underexpression of the δ protein in this region (~28% lower) (Curia et al., 2009). Surface expression of the δ subunit was not determined in the previous study. However, based on the similarities to the present findings, it is plausible that a decrease in surface expression of the δ subunit also occurs in the subiculum of Fmr1 KO mice and, potentially, other brain regions where δ subunit-containing GABAARs mediate tonic inhibition.
Significantly lower δ subunit protein expression was recently found in the hippocampus of Fmr1 KO mice at PN22 (Sabanov et al., 2016), and it is likely that the brain samples included the dentate gyrus. δ Subunit levels were determined in a Triton-insoluble fraction which could have been enriched in membranes, allowing detection of significant differences between KO and WT mice. However, total and surface protein levels were not distinguished.
The lower surface expression of the δ subunit in the Fmr1 KO mice led to questions about the expression and localization of the α4 subunit of the GABAAR, as the α4 subunit is the normal partner of the δ subunit in dentate gyrus granule cells and is also normally localized primarily at perisynaptic locations on granule cell dendrites (Liang et al., 2006; Zhang et al., 2007). No differences were observed in light microscopic immunohistochemical labeling of the α4 subunit, and no significant changes in α4 subunit levels were found in biochemical studies of either total or surface expression of this subunit in the hippocampus of the Fmr1 KO mice. Consistent with such findings, immunogold labeling was evident along the plasma membrane of granule cell dendrites. However, the location of the α4 subunit was altered in the Fmr1 KO mice. Rather than being localized at the normal perisynaptic location in dentate granule cells, immunogold labeling of the α4 subunit was found directly at synaptic contacts. A similar change in α4 subunit localization was found previously in a rat hyperexcitability model of alcohol withdrawal syndrome in which δ subunit-mediated tonic inhibition was also reduced (Liang et al., 2006). Such changes in α4 subunit localization, presumably related to the reduced surface expression of the δ subunit, may reflect a change in α4 subunit partnership within GABAARs, from predominant partnership with the δ subunit at perisynaptic locations to increased partnership with the γ2 subunit directly at synaptic contacts.
This idea is based on previous suggestions that, during development, the δ and γ2 subunits compete with each other for partnership with α4 in GABAARs (Tretter et al., 2001). In the forebrain, including the dentate gyrus, partnership of the δ and α4 subunits normally predominates (Sur et al., 1999), and the two subunits have very similar regional and cellular localizations (Peng et al., 2002; Pirker et al., 2000). However, when the δ subunit is deficient, as in the δ subunit KO mouse, expression of the γ2 subunit increases (Peng et al., 2002), consistent with the formation of α4/β/γ2 GABAARs. Also, in a mouse model of epilepsy in which δ subunit expression is decreased during the chronic period, the levels of both α4 and γ2 are increased (Peng et al., 2004; Rajasekaran et al., 2010), possibly resulting in increased partnership of these subunits. Similar decreases in δ subunit expression and associated increases in α4 and γ2 expression have been observed following acute and chronic alcohol administration in rodent models of alcohol withdrawal (Lindemeyer et al., 2014; Shen et al., 2011). In all of these models in which the δ and α4 subunits are altered, network excitability was increased (Houser et al., 2012; Olsen and Spigelman, 2012; Spigelman et al., 2003).
Somewhat surprisingly, an associated increase in α4 subunit expression was not detected in the Fmr1 KO mouse, as had been observed in the animal models with increased network excitability described above. One possible explanation is that the increase in α4 subunit expression is most pronounced when there is a decrease in total δ subunit levels, as occurs in the pilocarpine model of recurrent seizures (Joshi et al., 2017; Peng et al., 2004) and after withdrawal from chronic intermittent ethanol treatment (Cagetti et al., 2003), rather than when the alterations are predominantly in surface expression of the δ subunit, as in the current study.
The basic mechanisms underlying the decrease in surface expression of the δ subunit are unknown. The reduced surface expression could be related to deficits in activity-dependent transport of FMRP and its δ subunit mRNA cargo to local translation sites near GABAergic synapses. Indeed, the δ subunit mRNA was one of the select FMRP targets for which stimulus-induced dendritic mRNA transport was impaired in cultured hippocampal neurons from Fmr1 KO mice (Dictenberg et al., 2008). Alternatively, the lack of surface expression could reflect either a deficit in trafficking of the δ subunit-containing receptors to the dendritic surface or increased endocytosis of these receptors (Gonzalez et al., 2012; Shen et al., 2011). Interestingly, alterations of several G quartet-containing mRNAs that are part of the FMRP-mRNP (messenger-ribonucleoprotein) complex in vivo, such as MAP1B, a microtubule protein, and NAP-22, a protein involved in synaptic maturation (Brown et al., 2001; Darnell et al., 2001) could potentially contribute to deficits in transport of GABAARs within neurons and maintenance of GABAergic synapses.
While the basic mechanisms could be related directly to the δ subunit, alterations in associated subunits, such as altered phosphorylation of the β3 subunit, as observed in Fmr1 KO mice (Vien et al., 2015), could also play a role. The possibility of identifying the underlying mechanisms and finding ways to increase surface expression of δ subunit-containing GABAARs is particularly intriguing since the current findings indicate that a substantial amount of δ subunit protein is present in the neurons but fails to reach its normal localization at the dendritic surface. Interestingly, there are other examples of decreased surface expression of membrane-associated proteins in Fmr1 KO mice, including the AMPA receptor subunit GluR1 in the lateral amygdala, in this case possibly related to excessive mGluR signaling (Suvrathan et al., 2010).
The identification of deficits in tonic inhibition in an additional brain region provides further support for the development of pharmacological agents that could selectively enhance tonic inhibition. Despite lower δ subunit expression in the dentate gyrus, a δ subunit-preferring agonist, THIP, and a δ subunit-selective positive allosteric modulator, DS2, increased tonic inhibition in dentate granule cells of Fmr1 KO mice, although the enhancement was less than that in WT granule cells. Likewise, in previous studies, THIP augmented tonic inhibition and reduced hyperexcitability of principal cells of the basolateral nucleus of the amygdala of Fmr1 KO mice in brain slices (Olmos-Serrano et al, 2010) and also reduced some behavioral deficits, including hyperexcitability, in vivo (Olmos-Serrano et al., 2011). At low (micromolar) concentrations, THIP could be exerting its effects primarily at remaining δ subunit-containing nonsynaptic receptors to increase tonic inhibition. However, at higher concentrations, THIP could be activating other GABAARs that do not contain the δ subunit.
Neurosteroids are also major positive allosteric modulators of GABAARs and many, such as tetrahydrodeoxycorticosterone (THDOC), have high efficacy at nonsynaptic δ subunit-containing receptors (Stell et al., 2003; Wohlfarth et al., 2002). However, all neuroactive steroids, including allopregnanolone and a synthetic analog, ganaxolone, can modulate both nonsynaptic and synaptic GABAARs (Carter et al., 1997; Reddy and Estes, 2016). In previous behavioral studies in Fmr1 KO mice, acute administration of ganaxolone prevented audiogenic seizures (Heulens et al., 2012) and reduced repetitive, perseverative behavior (Braat et al., 2015). Enhanced tonic inhibition could have contributed to the behavioral changes, but increased phasic inhibition at synaptic receptors could also play a role. Ganaxolone is currently in a phase II clinical trial for treatment of children with Fragile X (ClinicalTrials.gov Identifier NCT01725152), and THIP is also in a trial for Angelman Syndrome (ClinicalTrials.gov Identifier NCT02996305).
The search for synthetic neurosteroids with selective actions at nonsynaptic GABAARs has led to the development of a new positive allosteric modulator of GABAARs, SGE-812 (Martin et al., 2016). This compound is highly selective for δ/α4 subunit-containing GABAARs at low concentration and increased tonic inhibition in principal neurons of the basolateral nucleus of the amygdala in slices from Fmr1 KO mice (Martin et al., 2016). Whether such synthetic neuroactive steroids are acting at remaining perisynaptic α4/δ-expressing GABAARs or potentially at other α4-containing receptors is unclear. However, such GABAAR modulators hold promise for the treatment of FXS by increasing tonic inhibition at δ or α4 subunit receptors and thus providing a more limited and targeted approach to GABAAR-mediated treatment.
Beyond the direct effects of neuroactive steroids as positive allosteric modulators of GABAARs, some neurosteroids such as THDOC can increase the trafficking and membrane insertion/stabilization of α4 subunit-containing GABAARs in hippocampal neurons, apparently through protein kinase C (PKC)-dependent phosphorylation of the α4 and β3 subunits (Abramian et al., 2014; Abramian et al., 2010; Adams et al., 2015). Recently, allopregnanolone and another new synthetic neuroactive steroid, SGE-516, were also found to increase the surface expression of α4/β/δ subunit-expressing receptors in dentate granule cells, possibly involving PKC-dependent phosphorylation of the β3 subunit (Modgil et al., 2017). Thus some endogenous and synthetic neurosteroids (although not ganaxolone) could enhance tonic inhibition by both direct modulatory effects on nonsynaptic GABAARs and enhanced trafficking and surface expression of these receptors (Modgil et al., 2017). Considering the current findings of lower surface expression of the δ subunit in Fmr1 KO mice, it would be particularly interesting to learn if such compounds can increase δ subunit-containing GABAARs at nonsynaptic sites along the membrane and enhance tonic inhibition in this mouse model of FXS.
Findings of this study suggest that altered surface expression of the δ subunit of the GABAAR, rather than a decrease in δ subunit expression alone, is likely limiting tonic inhibition in the dentate gyrus in this model of FXS. Whether deficits in surface expression are confined to the δ subunit or could be found for additional receptor proteins that require trafficking to and insertion at the membrane surface in FXS remains to be determined. The findings add to the growing number of GABA system alterations that have been identified in models of FXS and suggest novel treatment approaches for this disorder.
Highlights.
Tonic inhibition is reduced in the dentate gyrus in a model of Fragile X Syndrome.
Surface expression of GABAAR δ subunits is significantly reduced in this FXS model.
Labeling of the δ subunit is reduced at perisynaptic locations in this FXS model.
Decreased surface expression of the δ subunit limits tonic inhibition in this model.
Acknowledgments
We thank Werner Sieghart (Medical University of Vienna, Austria) for the generous gift of GABAAR subunit antibodies.
Funding Sources
This work was supported by the National Institutes of Health Grants HD067225 and NS075245 (to C.R.H.) and AA021213 (to R.W.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc. Natl. Acad. Sci. USA. 2014;111:7132–7137. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J. Biol. Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Thomas P, Smart TG. Modulation of neurosteroid potentiation by protein kinases at synaptic- and extrasynaptic-type GABAA receptors. Neuropharmacology. 2015;88:63–73. doi: 10.1016/j.neuropharm.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology. 2010;59:167–171. doi: 10.1016/j.neuropharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am. J. Intellect. Dev. Disabil. 2010;115:461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- Braat S, D’Hulst C, Heulens I, De Rubeis S, Mientjes E, Nelson DL, Willemsen R, Bagni C, Van Dam D, De Deyn PP, Kooy RF. The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle. 2015;14:2985–2995. doi: 10.4161/15384101.2014.989114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat S, Kooy RF. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron. 2015a;86:1119–1130. doi: 10.1016/j.neuron.2015.03.042. [DOI] [PubMed] [Google Scholar]
- Braat S, Kooy RF. Insights into GABAAergic system deficits in fragile X syndrome lead to clinical trials. Neuropharmacology. 2015b;88:48–54. doi: 10.1016/j.neuropharm.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Smart TG. Methods for recording and measuring tonic GABAA receptor-mediated inhibition. Front Neural Circuits. 2013;7:193. doi: 10.3389/fncir.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol. Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J. Pharm. Exp. Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Contractor A, Klyachko VA, Portera-Cailliau C. Altered neuronal and circuit excitability in fragile X syndrome. Neuron. 2015;87:699–715. doi: 10.1016/j.neuron.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb. Cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Heulens I, Brouwer JR, Willemsen R, De GN, Reeve SP, De Deyn PP, Hassan BA, Kooy RF. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Res. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Kooy RF. Fragile X syndrome: from molecular genetics to therapy. J. Med. Genet. 2009;46:577–584. doi: 10.1136/jmg.2008.064667. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Dengler CG, Coulter DA. Normal and epilepsy-associated pathologic function of the dentate gyrus. Prog. Brain Res. 2016;226:155–178. doi: 10.1016/bs.pbr.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev. Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert V, Scholze P, Sieghart W. Extensive heterogeneity of recombinant γ-aminobutyric acidA receptors expressed in α4β3γ2-transfected human embryonic kidney 293 cells. Neuropharmacology. 1996;35:1323–1330. doi: 10.1016/s0028-3908(96)00062-7. [DOI] [PubMed] [Google Scholar]
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D’Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABAA receptor subunit δ in the fragile X knockout mouse model. Neurobiol. Dis. 2006;21:346–357. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007a;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J. Physiol. 2007b;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat. Neurosci. 2013;16:903–909. doi: 10.1038/nn.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Moss SJ, Olsen RW. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors. J. Neurosci. 2012;32:17874–17881. doi: 10.1523/JNEUROSCI.2535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci. STKE. 2002:l8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Stafstrom CE. Origins of epilepsy in fragile X syndrome. Epilepsy Curr. 2009;9:108–112. doi: 10.1111/j.1535-7511.2009.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. In: Ribak CE, Gall CM, Mody I, editors. The Dentate Gyrus and Its Role in Seizures (Epilepsy Res. Suppl.7) Elsevier; Amsterdam: 1992. pp. 273–280. [PubMed] [Google Scholar]
- Heulens I, D’Hulst C, Van Dam D, De Deyn PP, Kooy RF. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav. Brain Res. 2012;229:244–249. doi: 10.1016/j.bbr.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Downregulation of the α5 subunit of the GABAA receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus. 2003;13:633–645. doi: 10.1002/hipo.10108. [DOI] [PubMed] [Google Scholar]
- Houser CR, Tong X, Cetina Y, Huang CS, Otis TS, Peng Z. Altered tonic inhibition in the dentate gyrus in a mouse model of fragile X syndrome. Soc. Neurosci. Abstr. 2014 699.02. [Google Scholar]
- Houser CR, Zhang N, Peng Z. Alterations in the distribution of GABAA receptors in epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Oxford University Press; New York: 2012. pp. 532–544. [Google Scholar]
- Jensen ML, Wafford KA, Brown AR, Belelli D, Lambert JJ, Mirza NR. A study of subunit selectivity, mechanism and site of action of the δ selective compound 2 (DS2) at human recombinant and rodent native GABAA receptors. Br. J. Pharmacol. 2013;168:1118–1132. doi: 10.1111/bph.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J. Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJH, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J. Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Williamson J, Kapur J. Neurosteroid-sensitive o-GABAA receptors: a role in epileptogenesis? Epilepsia. 2017;58:494–504. doi: 10.1111/epi.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy RF, Willemsen R, Oostra BA. Fragile X syndrome at the turn of the century. Mol. Med. Today. 2000;6:193–198. doi: 10.1016/s1357-4310(00)01674-9. [DOI] [PubMed] [Google Scholar]
- Lee V, MacKenzie G, Hooper A, Maguire J. Reduced tonic inhibition in the dentate gyrus contributes to chronic stress-induced impairments in learning and memory. Hippocampus. 2016;26:1276–1290. doi: 10.1002/hipo.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Liang J, Marty VN, Mulpuri Y, Olsen RW, Spigelman I. Selective modulation of GABAergic tonic current by dopamine in the nucleus accumbens of alcohol-dependent rats. J. Neurophysiol. 2014;112:51–60. doi: 10.1152/jn.00564.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J. Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, Spigelman I. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem. Res. 2014;39:1162–1170. doi: 10.1007/s11064-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemeyer AK, Shen Y, Yazdani F, Shao XM, Spigelman I, Davies DL, Olsen RW, Liang J. α2 subunit-containing GABAA receptor subtypes are upregulated and contribute to alcohol-induced functional plasticity in the rat hippocampus. Mol. Pharmacol. 2017;92:101–112. doi: 10.1124/mol.116.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Martin BS, Corbin JG, Huntsman MM. Deficient tonic GABAergic conductance and synaptic balance in the fragile X syndrome amygdala. J. Neurophysiol. 2014;112:890–902. doi: 10.1152/jn.00597.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BS, Martinez-Botella G, Loya CM, Salituro FG, Robichaud AJ, Huntsman MM, Ackley MA, Doherty JJ, Corbin JG. Rescue of deficient amygdala tonic γ-aminobutyric acidergic currents in the Fmr-/y mouse model of fragile X syndrome by a novel γ-aminobutyric acid type A receptor-positive allosteric modulator. J. Neurosci. Res. 2016;94:568–578. doi: 10.1002/jnr.23632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J. Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Modgil A, Parakala ML, Ackley MA, Doherty JJ, Moss SJ, Davies PA. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology. 2017;113:314–322. doi: 10.1016/j.neuropharm.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol. Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, De Sarro GB, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40:1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Corbin JG, Burns MP. The GABAA receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev. Neurosci. 2011;33:395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J. Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ -aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Spigelman I. GABAA receptor plasticity in alcohol withdrawal. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Oxford University Press; New York: 2012. pp. 562–573. [Google Scholar]
- Otis TS, Staley K, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev. Neurosci. 2011;33:349–364. doi: 10.1159/000329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Bright DP, Mortensen M, Frolund B, Smart TG. Context-dependent modulation of GABAAR-mediated tonic currents. J. Neurosci. 2016;36:607–621. doi: 10.1523/JNEUROSCI.2047-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J. Comp. Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J. Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Chandra D, Homanics GE, Olsen RW, Houser CR. Altered localization of the δ subunit of the GABAA receptor in the thalamus of α4 subunit knockout mice. Neurochem. Res. 2014;39:1104–1117. doi: 10.1007/s11064-013-1202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Joshi S, Sun C, Mtchedlishvilli Z, Kapur J. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol. Dis. 2010;40:490–501. doi: 10.1016/j.nbd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes WA. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol. Sci. 2016;37:543–561. doi: 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabanov V, Braat S, D’Andrea L, Willemsen R, Zeidler S, Rooms L, Bagni C, Kooy RF, Balschun D. Impaired GABAergic inhibition in the hippocampus of Fmr1 knockout mice. Neuropharmacology. 2016;116:71–81. doi: 10.1016/j.neuropharm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu. Rev. Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J. Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J. Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol. Pharmacol. 2011;79:432–442. doi: 10.1124/mol.110.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABAA receptor δ subunit. J. Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol. Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA. 2010;107:11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Peng Z, Zhang N, Cetina Y, Huang CS, Wallner M, Otis TS, Houser CR. Ectopic expression of α6 and δ GABAA receptor subunits in hilar somatostatin neurons increases tonic inhibition and alters network activity in the dentate gyrus. J. Neurosci. 2015;35:16142–16158. doi: 10.1523/JNEUROSCI.2853-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Höger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABAA receptor δ subunit gene leads to an up-regulation of γ2 subunit-containing receptors in cerebellar granule cells. J. Biol. Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- Vien TN, Modgil A, Abramian AM, Jurd R, Walker J, Brandon NJ, Terunuma M, Rudolph U, Maguire J, Davies PA, Moss SJ. Compromising the phosphodependent regulation of the GABAAR β3 subunit reproduces the core phenotypes of autism spectrum disorders. Proc. Natl. Acad. Sci. USA. 2015;112:14805–14810. doi: 10.1073/pnas.1514657112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Lecker I, Wang DS, Yu J, Orser BA. Altered expression of δGABAA receptors in health and disease. Neuropharmacology. 2015;88:24–35. doi: 10.1016/j.neuropharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Rosenzweig S, Lecker I, Wang DS, Wojtowicz JM, Orser BA. γ-aminobutyric acid type A receptors that contain the δ subunit promote memory and neurogenesis in the dentate gyrus. Ann. Neurol. 2013;74:611–621. doi: 10.1002/ana.23941. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J. Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Lindemeyer AK, Peng Z, Cetina Y, Huang CS, Olsen RW, Houser CR. Altered surface expression of delta subunit-containing GABAA receptors in a mouse model of Fragile X syndrome. Soc. Neurosci. Abstr. 2016;296:18. [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J. Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]