Biochemistry. In the article “Unexpected genetic and structural relationships of a long-forgotten flavoenzyme to NAD(P)H:quinone reductase (DT-diaphorase),” by Qinjian Zhao, Xiao Ling Yang, W. David Holtzclaw, and Paul Talalay, which appeared in number 5, March 4, 1997, of Proc. Natl. Acad. Sci. USA (94, 1669–1674), Fig. 6 was incorrectly reproduced. The two parts were abutted when they should not have been. A corrected figure is shown below.
Figure 6.
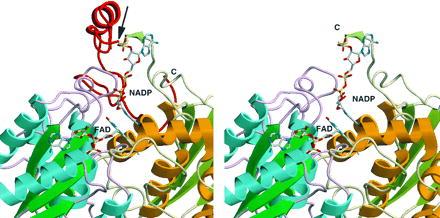
Three-dimensional structure of rQR1 in complex with FAD and NADP+ (12). The C-terminal 43 amino acids (232–274) are shown in red (Left), and have been truncated at the arrow (Right). Different colors of the backbone indicate the two different subunits. It can be seen that the truncated portion of QR2 is critical for binding of the pyrophosphate moiety of the NAD(P)H cofactor. Note that the contact regions between the enzyme and FAD are presumably preserved in QR2 (Right), consistent with the tight binding of FAD. The structural comparison between rQR1 and hQR2 is appropriate since the homology between rQR1 and hQR1 is very high (85% identity).


