Abstract
Airway remodeling is a hallmark of bronchial asthma. Our group has previously reported that the thymic stromal lymphopoietin (TSLP), an airway epithelial-derived cytokine, has a central role in the pathogenesis of airway remodeling, and that toll-like receptor (TLR) 4 signaling in epithelial cells may trigger T-helper 2 (Th2) immune responses by overexpression of TSLP. However, it is currently unclear whether TLR4 is a target in the treatment of airway remodeling in asthma. The present study established a house dust mite (HDM)-induced chronic asthmatic model in female BALB/c mice and treated the HDM-exposed mice with 3 mg/kg TAK242, as a TLR4 antagonist, 30 min prior to HDM challenge for up to 2 weeks. General structural changes in the airways were subsequently evaluated and the levels of TSLP in the bronchoalveolar lavage fluid (BALF) and interleukin (IL)-4, IL-13 and interferon (IFN)-γ in the blood serum were determined. Results indicated that TAK242 treatment markedly reduced pathological changes in the airways of HDM-induced asthmatic mice, as demonstrated by reductions in airway wall thickening, peribronchial collagen deposition and subepithelial fibrosis. Furthermore, airway hyperresponsiveness to inhaled methacholine and the levels of TSLP in the BALF and IL-4, IL-13 and IFN-γ in the peripheral blood were significantly reduced by TAK242 treatment (P<0.05). Furthermore, the shift in the IFN-γ/IL-4 ratio induced by HDM treatment was significantly reversed following TAK242 pretreatment, which indicated that TAK242 modulated Th1/Th2 immune homeostasis in the chronic asthma mouse model. The present findings in a chronic asthma mouse model suggest that TAK242 may be an efficient treatment for airway remodeling, possibly through the inhibition of TSLP overexpression and Th2 airway inflammation.
Keywords: airway remodeling, bronchial asthma, mouse model, toll-like receptor 4 antagonist, thymic stromal lymphopoietin
Introduction
Allergic asthma is among the most common chronic lung diseases worldwide (1), of which airway remodeling is the predominant feature and primary cause of asthma-related disability and mortality (2,3). However, the development of effective therapeutic agents for the treatment of airway remodeling is in its infancy (4). The current therapeutic agents used for asthma therapy include glucocorticoids, which are only effective in inhibiting airway inflammation and have a limited effect in treating airway remodeling, which is among the underlying reasons for drug-resistant asthma (5,6). The identification of novel strategies that overcome the pathological airway structural changes in asthma is required. However, the mechanisms underlying the airway structural changes in asthma are not well understood and require further investigation.
It has recently been indicated that bronchial epithelial cells are critical in driving naïve T cell differentiation towards T-helper 2 (Th2) cells through the activation of dendritic cells (7–10). This is mediated by toll-like receptor 4 (TLR4) signaling upon the interaction of epithelial cells and environmental aero-antigens, including house dust mites (HDMs), which are the most common allergen known to induce asthma (11,12). The activation of TLR4 has been demonstrated to stimulate the expression of multiple epithelium-derived cytokines, including thymic stromal lymphopoietin (TSLP) and interleukins (ILs)-25 and −33 (13,14). Notably, our group has previously reported that TSLP signaling in airway epithelial cells may serve a central role in initiating airway remodeling by stimulating the Th2 cell response (15). Therefore, targeting of TLR4 signaling may be an effective strategy to treat airway remodeling in asthma. TAK242, as a specific TLR4 antagonist that was initially developed for sepsis therapy, is a potent anti-inflammatory drug (16,17). However, the efficacy of this drug in the treatment of airway remodeling in asthma remains unknown. By establishing a HDM-induced chronic asthma mouse model, the present study investigated whether TAK242 was able to reducing pathological airway structural changes. Furthermore, the present study evaluated whether TAK242 may exert its effects by inhibiting TSLP signaling and reversing Th2/Th1 skewing in mice with chronic asthma. The outcomes of the present study may indicate a novel strategy for the treatment of chronic asthma and improve understanding of asthma pathogenesis.
Materials and methods
Animals and reagents
A total of 15 female BALB/c mice (6–8 weeks old; weighing 18±2 g) were purchased from Shanghai SLAC Laboratory Animal Center (Shanghai, China). The animals were housed under specific pathogen-free conditions under a 12-h light/dark cycle with a constant temperature of 20°C and humidity of 55%. No dietary restriction was applied. All procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Sun Yat-sen University (Guangzhou, China). Purified HDM whole-body extract was purchased from Greer Laboratories, Inc. (Lenoir, NC, USA) and dissolved in saline for intranasal instillation. TAK242 powder was purchased from Takeda Pharmaceutical Company (Osaka, Japan) and dissolved in dimethyl sulfoxide (DMSO) prior to use. A mouse TSLP ELISA kit was purchased from R&D Systems, Inc. (cat. no. MTLP00; Minneapolis, MN, USA), and IL-4, IL-13 and interferon (IFN)-γ ELISA kits were purchased from eBioscience; Thermo Fisher Scientific, Inc. (cat. nos. BMS613, BMS6015, and BMS606, respectively; Waltham, MA, USA). The present study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University.
HDM-induced chronic asthma model
Mice were randomly divided into saline (negative control), HDM-exposed and TAK242 treatment groups (5 mice/group). The HDM-induced chronic asthma model was established as described previously (15,18) with slight modifications (Fig. 1). Briefly, following anesthesia with 2.0% isoflurane (Baxter, Deerfield, IL, USA) using a Matrx Animal Anesthesia Ventilator System (Matrx VIP3000; Midmark Corporation, Dayton, OH, USA), the mice were challenged by intranasal instillation with 15 µg HDM (in 10 µl saline) every day for 3 consecutive days followed by a 4 day rest period, which was repeated over the course of 5 weeks. From week 4 onwards, 3 mg/kg TAK242 or an equivalent volume of DMSO was administered by intraperitoneal injection 30 min prior to HDM exposure to the TAK242 treatment group or HDM group, respectively. The negative control group was exposed to 10 µl saline instead of HDM.
Figure 1.
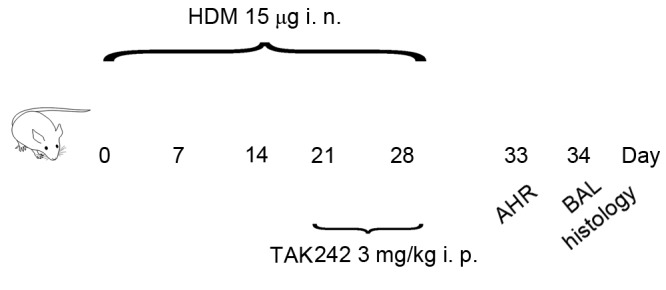
Establishment of HDM-induced chronic asthma model in mice. BALB/c mice were challenged by i.n. HDM on 3 consecutive days per week for 5 weeks. The negative control group was exposed to saline instead of HDM. From week 4 onwards, TAK242 or dimethyl sulfoxide was administered by i.p. injection 30 min prior to HDM exposure in the TAK242 treatment group or HDM group, respectively. On day 32 AHR to methacholine was assessed. For histological analysis, BALF and lung tissue were harvested on day 33 following the sacrifice of mice. HDM, house dust mites; AHR, airway hyperresponsiveness; BALF, bronchoalveolar lavage fluid; i.n., intranasal; i.p., intraperitoneal.
Whole-body plethysmograph
On day 32, 24 h after the final HDM intranasal administration, airway hyperresponsiveness (AHR) was measured using whole-body plethysmography (Buxco Electronics, Inc., Wilmington, NC, USA), as described previously (15). The mice were placed in a chamber for acclimatization and the basal enhanced pause (Penh) values were recorded. Following acclimatization, saline was nebulized as a control in all groups, which was followed by administration of increasing concentrations (6.25, 12.5, 25, 50 and 100 mg/ml) of methacholine (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany), administered at 5-min intervals. Penh values were recorded as testing values. The final Penh for each concentration was calculated using the following formula: 100 × (testing Penh-basal Penh)/basal Penh.
Bronchoalveolar lavage fluid (BALF) collection
Mice were sacrificed with an overdose of anesthetic (by inhalation of 5.0% isoflurane) 24 h after the assessment of AHR. The left major bronchus was tied and inserted with a 24-guage needle. The BALF was harvested using a 1 ml saline perfusion, which was repeated 3 times. Following collection, the BALF was centrifuged at 600 × g for 10 min at 4°C and the supernatant was stored at −80°C for TSLP measurement using ELISA kit following the manufacturer's instructions.
Cell counting
Cells in the BALF were collected via centrifugation (600 × g, 10 min at 4°C) and then resuspended with 0.5 ml of PBS. 0.1 ml of the cell suspension was used for total cell counting in a hemocytometer. The remaining cell suspension was smeared onto the glass slides. The Wright-Giemsa staining were performed on the dry-out slides and the eosinophils were identified and counted in every 400 cells (percentage of eosinophils=numbers of eosinophils/400×100%) (15).
Lung histology
The left lung lobes of the mice were harvested, fixed in 4% neutral buffered formalin at room temperature overnight and embedded in paraffin. Lung sections were prepared by sectioning the tissue into 5-µm thick sections. Hematoxylin and eosin staining was performed to evaluate the general structural changes of the airways, and periodic acid-Schiff and Masson's trichrome staining were performed on the lung sections to visualize goblet cells and collagen deposition, respectively, using a light microscope. The goblet cell coverage and peribronchial collagen thickness were measured as described previously (15).
Peripheral blood collection and ELISA
After the mice were sacrificed, a capillary was inserted into the medical canthus of the eye with slight pressure. The peripheral blood was harvested through the capillary tube to obtain a total volume of 0.8–0.9 ml. Following clot formation, the blood was centrifuged at 600 × g for 10 min at 4°C and the serum was stored at −80°C to measure cytokine levels. ELISA was performed to determine the levels of IL-4, IL-13 and IFN-γ in the peripheral blood following the manufacturer's instructions.
Statistical analysis
Data were analyzed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA) and presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance for multiple comparisons followed by a Fisher's least significant difference post hoc test with homoscedasticity, or a Welch's approximate t-test followed by a Dunnett's T3 test with heterogeneity of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
TAK242 treatment inhibits airway structural changes in HDM-exposed mice
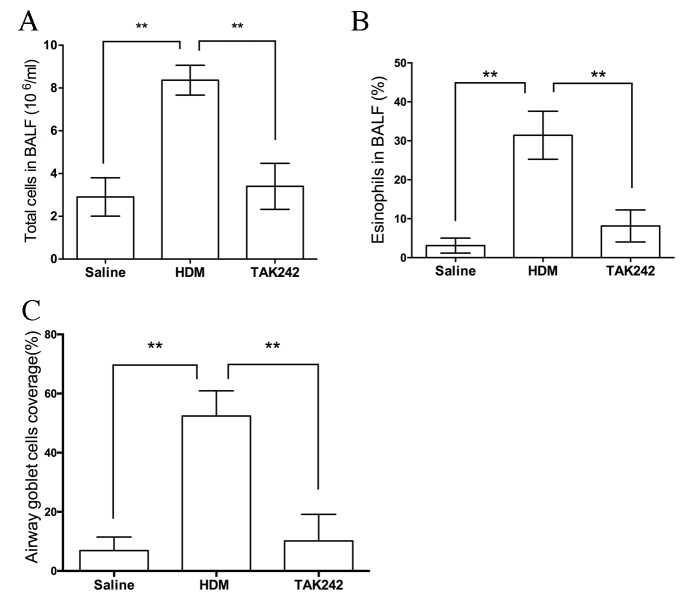
As indicated in Fig. 2, mice exposed to HDM exhibited marked airway structural changes when compared with the saline group (Fig. 2A-F), including inflammatory cell infiltration (Fig. 2D), thickened airway walls, peribronchial collagen deposition, subepithelial fibrosis (Fig. 2E) and goblet cell hyperplasia (Fig. 2F). Notably, TAK242 treatment abolished these airway structural changes induced by HDM treatment (Fig. 2G-I), which indicated that TAK242 may be efficient in the treatment of airway remodeling. Furthermore, the numbers of total cells and eosinophils in the BALF, and the coverage of airway goblet cells, were significantly reduced in TAK242-treated mice compared with HDM-exposed mice (P<0.01; Fig. 3).
Figure 2.
(A-I) TAK242 treatment ameliorated pathological airway structural changes in chronic asthmatic mice. (A, D and G) H&E staining was performed to evaluate the general structural changes of the airways, and (B, E and H) Masson's thichrome and (C, F and I) PAS staining was conducted on the lung sections to visualize collagen deposition and goblet cells, respectively. Magnification, ×20. The white arrows indicate the inflammatory cell infiltration, red frames indicate thickened airway walls, peribronchial collagen deposition and subepithelial fibrosis, and the black arrowheads indicate goblet cell hyperplasia. H&E, hematoxylin and eosin; HDM, house dust mites; PAS, periodic acid-schiff.
Figure 3.
TAK242 treatment reduced pathological cell changes in chronic asthmatic mice. Cell counting of the (A) total cells and (B) eosinophils in the BALF, and (C) the coverage of airway goblet cells in mice. **P<0.01. HDM, house dust mites; BALF, bronchoalveolar lavage fluid.
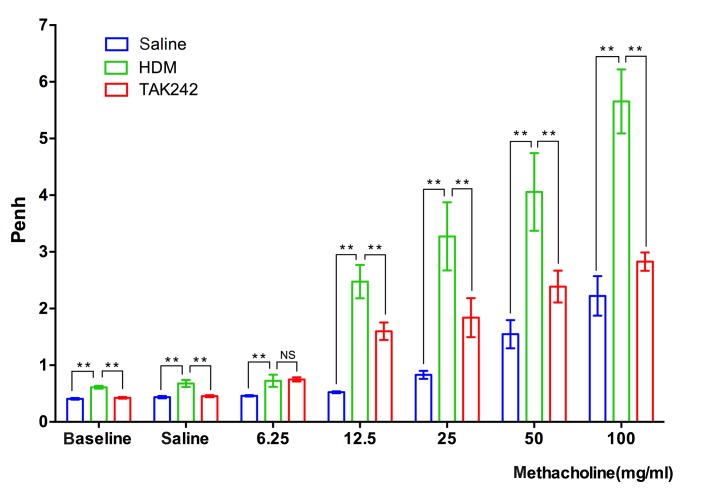
TAK242 pretreatment reduces AHR levels in chronic asthmatic mice
AHR is a clinical sign of chronic asthma, and is characterized by hyperreactivity of the airways in response to relatively low concentrations of bronchial constricting reagents, including methacholine (19). Whole-body plethysmographs demonstrated that the airway reactivity levels of HDM-exposed mice were significantly increased when compared with the saline group at the concentration of 100 µg/ml (P<0.01), which suggested successful establishment of the chronic asthma model. Notably, TAK242 pretreatment significantly reduced the Penh levels in HDM-exposed mice when compared with the HDM group at the concentration of 100 µg/ml (P<0.01; Fig. 4), which further demonstrated that TAK242 may be efficient in treating asthma.
Figure 4.
TAK242 treatment reduced HDM-induced airway hyperreactivity. The Penh of mice was recorded following stimulation with increasing concentrations (0, 6.25, 12.5, 25, 50 and 100 mg/ml) of methacholine. Data are presented as the mean ± standard deviation. **P<0.01 vs. HDM group. HDM, house dust mites.
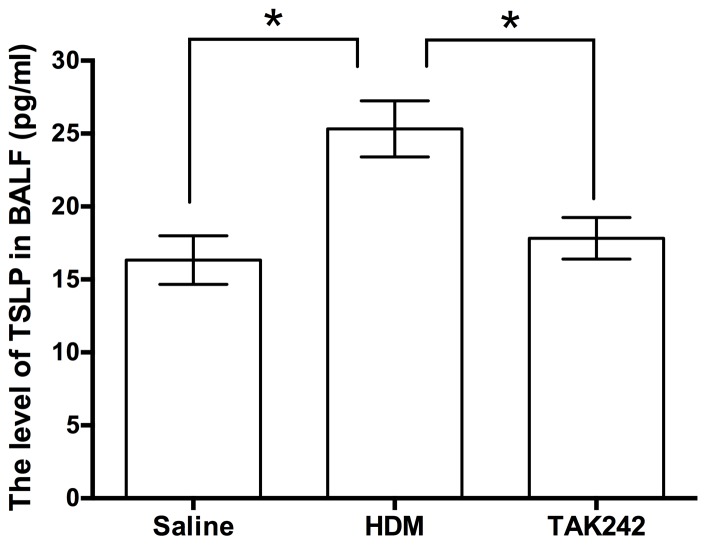
TAK242 inhibits TSLP expression in the chronic asthma model
Our group previously demonstrated that TSLP signaling in airway epithelial cells mediated airway remodeling in asthma (15). To evaluate whether TAK242 blocked airway remodeling through the inhibition of TSLP signaling, TSLP levels in the BALF were measured (Fig. 5). The results indicated that TAK242 significantly reduced the levels of TSLP from 25.32±1.92 pg/ml in HDM-exposed mice to 17.82±1.42 pg/ml in TAK242-treated mice (F=8.391; P<0.05), which was similar to the TSLP levels in the saline group (16.33±1.67 pg/ml).
Figure 5.
The level of TSLP in BALF. Data are presented as the mean ± standard deviation. *P<0.05. HDM, house dust mites; BALF, bronchoalveolar lavage fluid; TSLP, thymic stromal lymphopoietin.
TAK242 reduces Th2 cytokine expression and reverses Th2/Th1 skewing
Th2/Th1 skewing is a central event that aids to trigger pathological changes in asthma, and is initiated by TSLP signaling (20). Therefore, the potential inhibitory effect of TAK242 against the Th2 cell response was determined by measuring levels of the Th2-type cytokines IL-4, IL-13 and Th1-cytokine IFN-γ in the peripheral blood. ELISA results indicated that TAK242 signficantly reduced the levels of all cytokines when compared with those in HDM-exposed mice (P<0.01; Table I). Notably, TAK242 significantly reversed the IFN-γ/IL-4 ratio from 1.10±0.27 to 1.82±0.35 (P=0.0066; Table I), indicating that TAK242 may prevent IFN-γ/IL-4 skewing, potentially through targeting of TLR4 signaling.
Table I.
Effect of TAK242 treatment on the levels of Th1/Th2 cytokines.
| Statistical values | |||||||
|---|---|---|---|---|---|---|---|
| Group (n=5) | HDM vs. Saline | HDM vs. TAK242 | |||||
| Cytokine | Saline | HDM | TAK242 | F-value | P-value | F-value | P-value |
| IFN-γ (pg/ml) | 21.46±4.23 | 52.45±9.86 | 25.87±3.81 | 6.459 | 0.0002 | 5.623 | 0.0005 |
| IL-4 (pg/ml) | 10.23±2.38 | 48.78±7.24 | 14.14±1.62 | 11.31 | 0.0000 | 10.44 | 0.0000 |
| IL-13 (pg/ml) | 31.46±2.96 | 78.75±5.83 | 42.89±5.98 | 16.17 | 0.0000 | 9.601 | 0.0000 |
| IFN-γ/IL-4 | 2.10±0.15 | 1.10±0.27 | 1.82±0.35 | 7.240 | 0.0001 | 3.642 | 0.0066 |
The concentrations of Th1/Th2 cytokines in the saline, HDM and TAK242 groups are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference. Th, T-helper; HDM, house dust mites; F-value, Fisher's post hoc F-statistic; IFN-γ, interferon-γ; IL, interleukin.
Discussion
In the present study, a HDM-induced chronic asthma mouse model was successfully established with high levels of IL-4 and IL-13, which indicated a robust Th2 response (21). This prolonged exposure model exhibited airway inflammation and structural changes characteristic of chronic asthma, including infiltration of inflammatory cells in the airway, thickened basement membranes, upregulated goblet cells within the bronchial epithelia and AHR to methacholine, thus providing a useful tool for the development of therapeutic drugs against chronic asthma.
The specific TLR4 antagonist TAK242, initially developed for sepsis therapy, has been previously demonstrated to reduce lipopolysaccharide-induced inflammation in epithelial cells (16,17). However, to the best of our knowledge, the present study is the first to indicate the potential of TAK242 in the treatment of asthma. Previous studies have suggested that defects in the repair of bronchial epithelial cells may be an initiating factor for subepithelial changes in asthma (20,22,23). Furthermore, the interaction of epithelial cells with allergens, including that with HDM mediated by TLR4, may initiate the Th2 immune response by inducing the secretion of epithelim-derived cytokines, including TSLP, IL-25 and IL-33, via nuclear factor-κB activation (7,24,25). This effect may be sufficient to activate dendritic cells and promote Th2/Th1 skewing (10,26). Notably, our group previously demonstrated in a HDM-mouse model that TSLP signaling was a principle determinant of airway remodeling, by inducing dentritic cell activation and the Th2 immune response (15). Furthermore, blocking of TSLP reduced the levels of IL-13 and transforming growth factor-β, and reversed the pathological airway changes induced by HDM exposure. These findings suggest that TAK242 may be an effective treatment for airway remodeling through its targeting of TLR4-TSLP signaling.
Histological analyses demonstrated that TAK242 markedly alleviated pathological changes in the airways induced by HDM exposure, including thickening of the airway walls, peribronchial collagen deposition and subepithelial fibrosis, which implicates TAK242 as a potent therapeutic agent for airway remodeling in asthma. To investigate the underlying mechanisms, the levels of TSLP and Th1/Th2 cytokines were determined in all experimental groups, and the results suggested that TSLP in the BALF was significantly reduced. This finding was consistent with previous observations that TLR4 activation induced Th2-derived airway inflammation by inducing epithelium-derived cytokines, including TSLP (7,8,23). In addition, the IFN-γ/IL-4 ratio, which reflects the Th1/Th2 immune homeostasis status in vivo (26), was significantly reversed by TAK242 treatment in HDM-exposed mice in the present study.
As neutralizing TSLP may regulate the homeostasis of Th1/Th2, the modulatory effect of TAK242 on the Th1/Th2 balance may be a consequence of TSLP regulation and dendritic cell activation. Further studies are now required to understand the mechanism of action of TAK242 in vitro and in vivo.
In conclusion, the present results indicate that TAK242 exerts potent therapeutic effects against airway remodeling induced by allergens. These findings may aid to identify novel therapeutic strategies for the treatment of asthma and other lung diseases characterized by TLR4-mediated changes in airway structure.
Acknowledgements
The present study was supported by the Science and Technology Planning Project of Guangdong Province (grant nos. 2012B031800096 and 2014A020212120) and the National Natural Science Foundation of China (grant no. 81470219).
References
- 1.Croisant S. Epidemiology of asthma: Prevalence and burden of disease. Adv Exp Med Biol. 2014;795:17–29. doi: 10.1007/978-1-4614-8603-9_2. [DOI] [PubMed] [Google Scholar]
- 2.Vignola AM, Kips J, Bousquet J. Tissue remodeling as a feature of persistent asthma. J Allergy Clin Immunol. 2000;105:1041–1053. doi: 10.1067/mai.2000.107195. [DOI] [PubMed] [Google Scholar]
- 3.Tillie-Leblond I, de Blic J, Jaubert F, Wallaert B, Scheinmann P, Gosset P. Airway remodeling is correlated with obstruction in children with severe asthma. Allergy. 2008;63:533–541. doi: 10.1111/j.1398-9995.2008.01656.x. [DOI] [PubMed] [Google Scholar]
- 4.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144:1026–1032. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 5.Lo CY, Michaeloudes C, Bhavsar PK, Huang CD, Wang CH, Kuo HP, Chung KF. Increased phenotypic differentiation and reduced corticosteroid sensitivity of fibrocytes in severe asthma. J Allergy Clin Immunol. 2015;135:1186–1195. doi: 10.1016/j.jaci.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE. GOAL Investigators Group: Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 7.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, Choi MK, Joo JH, Kim CH, Lee SN, et al. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. 2013;131:549–561. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc. 2009;6:655–659. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 10.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 11.Dust mite allergens and asthma-a worldwide problem. J Allergy Clin Immunol. 1989;83:416–427. doi: 10.1016/0091-6749(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 13.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ZG, Zhang TT, Li HT, Chen FH, Zou XL, Ji JZ, Chen H. Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PLoS One. 2013;8:e51268. doi: 10.1371/journal.pone.0051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 17.Meijvis SC, van de Garde EM, Rijkers GT, Bos WJ. Treatment with anti-inflammatory drugs in community-acquired pneumonia. J Intern Med. 2012;272:25–35. doi: 10.1111/j.1365-2796.2012.02554.x. [DOI] [PubMed] [Google Scholar]
- 18.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, Shelley M, Abbas AR, Austin CD, Jackman J, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–111. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Cockcroft DW. Direct challenge tests: Airway hyperresponsiveness in asthma: Its measurement and clinical significance. Chest. 2010;138:18S–24S. doi: 10.1378/chest.10-0088. (2 Suppl) [DOI] [PubMed] [Google Scholar]
- 20.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130:845–852. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royce SG, Li X, Tortorella S, Goodings L, Chow BS, Giraud AS, Tang ML, Samuel CS. Mechanistic insights into the contribution of epithelial damage to airway remodeling. Novel therapeutic targets for asthma. Am J Respir Cell Mol Biol. 2014;50:180–192. doi: 10.1165/rcmb.2013-0008OC. [DOI] [PubMed] [Google Scholar]
- 23.Lambrecht BN, Hammad H. Asthma: The importance of dysregulated barrier immunity. Eur J Immunol. 2013;43:3125–3137. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- 24.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5:1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li DQ, Zhang L, Pflugfelder SC, De Paiva CS, Zhang X, Zhao G, Zheng X, Su Z, Qu Y. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128:1318–1325. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onoé K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells. Immunol Res. 2007;38:319–332. doi: 10.1007/s12026-007-0011-5. [DOI] [PubMed] [Google Scholar]