Abstract
Wogonoside possesses anti-oxidative, anti-inflammatory, anti-allergy and anti-tumor properties. The aim of the present study was to evaluate whether wogonoside alleviates spinal cord injury (SCI)-induced inflammation via nuclear factor (NF)-κB and nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation. Sprague-Dawley rats were positioned in the jaws of a calibrated aneurysm clip with a closing pressure of 55 g. The jaws were placed on the dorsal and ventral surfaces of the spinal cord and left in place for 1 min. SCI rats were treated with 12, 25 and 50 mg/kg wogonoside. Following this, the locomotor function was assessed using the Basso Beattie Bresnahan scale. The water content of the spinal cord was measured, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 levels were assessed and western blot analysis was performed to evaluate the expressions of NF-κB and NLRP3. Wogonoside was demonstrated to significantly ameliorate the SCI-induced reduction in Basso Beattie Bresnahan score (P<0.01) and significantly reduce the water content of the spinal cord in rats with SCI-induced inflammation (P<0.01). Results also indicated that treatment with wogonoside significantly reduced the levels of IL-1β, TNF-α and IL-6 in rats with SCI-induced inflammation (P<0.01), potentially via the phosphorylation of NF-κB inhibitor α. Furthermore, treatment with wogonoside inhibited the expressions of toll-like receptor 4, NLRP3 and caspase-1 protein in SCI model rats (P<0.01). In conclusion, the results of the present study suggest that wogonoside alleviates SCI-induced inflammation by suppressing NF-κB and NLRP3 inflammasome activation.
Keywords: wogonoside, traumatic spinal cord injury, inflammation, nuclear factor-κB, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3
Introduction
Spinal cord injury (SCI) is a common type of trauma and cause of disability in China (1). The underlying mechanisms that cause secondary neuron injury following primary spinal cord injury are complex (2). It has been reported that experimental pharmacological agents are unable to act on multiple injury mechanisms at the same time, and inhibition of secondary injuries while ignoring their favorable aspects to the body, such as pain reduction and inflammation inhibition, may cause detrimental side-effects (3). Consequently, there is currently a lack of effective and safe clinical treatments for SCI.
SCI typically induces severe pathological nerve damage with limited functional recovery (4). The initial mechanical injury may destroy neurons and neuroglia, while even greater destructive effects are caused by delayed secondary pathological damage (5). Secondary injury may manifest as neuron and/or glial cell apoptosis, increased permeability of the blood-spinal cord barrier and a complicated neuro-inflammatory response, which may endure for months or years after injury (6).
Wogonoside (Fig. 1) has been reported to possess extensive pharmacological activities. In addition to anti-inflammatory, anti-allergy, anti-viral and antipyretic properties, recent studies have demonstrated that wogonoside possesses anti-oxidative and anti-tumor effects, along with cardiovascular protective effects (7,8). Wogonoside is derived from Scutellaria baicalensis, and has exhibited efficacy as an antithrombotic, antihypertensive and antitumor therapeutic, and in the treatment of coronary heart disease (9). The aim of the present study was to determine whether wogonoside alleviated inflammation induced by activation of nuclear factor (NF)-κB and the nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome in an SCI rat model.
Figure 1.
Chemical structure of wogonoside.
Materials and methods
Animals and surgery
Adult male Sprague-Dawley (SD; age, 9–11 weeks; n=50) rats weighing 230–250 g were obtained from the Animal Resource Center of the Yantai Affiliated Hospital of Binzhou Medical University (Yantai, China) for use in the present study. Rats were housed at an ambient temperature of 22±1°C under a 12-h light/dark cycle, and 50–70% relative humidity. Pellet rat chow and tap water were available ad libitum. A rat model of SCI was induced as described previously (10). Briefly, rats were anesthetized intravenous injection (i.v.) with 35 mg/kg pentobarbital (all from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany.) and underwent a laminectomy, during which the T8 and T9 vertebral peduncles were removed. The jaws of a calibrated aneurysm clip with a closing pressure of 55 g were placed on the dorsal and ventral surfaces of the spinal cord and left in place for 1 min to induce SCI. All experimental procedures were approved by the Ethics Committee of the Yantai Affiliated Hospital of Binzhou Medical University.
Experimental groups and procedures
All SD rats were underwent SCI surgery and were randomly divided into four groups: i) Control group (control; n=10), which underwent sham surgery (the operational area was exposed but no trauma was induced) and were administered with physiological saline (1 ml/kg, i.p.); ii) SCI model group (SCI model; n=10), which underwent SCI surgery and were administered with physiological saline (1 ml/kg, i.p.); iii) 12 mg/kg wogonoside group (n=10), administered with 12 mg/kg wogonoside (i.p.) for 10 days; iv) 25 mg/kg wogonoside group (n=10), administered with 25 mg/kg wogonoside (i.p.) for 10 days; and v) 50 mg/kg wogonoside group (n=10), administered with 50 mg/kg wogonoside for 10 days (i.p.; Sigma-Aldrich; Merck KGaA).
Evaluation of locomotor function
Locomotor function was assessed by two researchers, using the Basso Beattie Bresnahan (BBB) locomotor rating scale, as described previously (11). A score of 0 indicates complete paralysis and a score of 21 indicates normal locomotion.
Evaluation of spinal cord contusion volume after SCI
Rats were sacrificed using decapitation following treatment with wogonoside (day 10) under anesthesia (35 mg/kg pentobarbital). Spinal cord contusions were assessed by calculating the water content of the spinal cord. Injured spinal cords were dried at −80°C for 48 h and the volume of spinal cord contusions was calculated using the following formula: Water content of spinal cord (%) = (wet weight - dry weight)/wet weight × 100.
Evaluation of serum oxidative stress and inflammation
Blood (500 µl) was harvested and serum was collected after centrifugation at 2,000 × g for 10 min at 4°C. Serum tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 levels were analyzed using respective commercial immunoassay kits (cat. no. RTA00, RLB00 and R6000B, respectively; R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer's protocol.
Western blot analysis
Following sacrifice, spinal cord tissues were harvested and homogenized in RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen China). The homogenate was centrifuged at 12,000 × g for 20 min at 4°C, and quantification of protein in the supernatant was performed using a bicinchoninic acid assay kit (Beyotime Institute of Biotechnology). A total of 50 µg protein was loaded per lane, separated by 10% SDS-PAGE and electrotransferred onto nitrocellulose membranes. Membranes were blocked with 5%- skim milk powder in TBST for 1 h at 37°C and incubated with anti-TLR4 (sc-10741, 1:200; Santa Cruz Biotechnology, Inc.), anti-NF-κB p65 (cat. no. sc-109; 1:2,000; Santa Cruz Biotechnology, Inc.), anti-phosphorylated (p)-inhibitor of NF-κB (cat. no. 2859; IκB; 1:200; Cell Signaling Technology, Inc.), anti-NLRP3 (cat. no. sc-66846; 1:300; Santa Cruz Biotechnology, Inc.), anti-caspase-1 (cat. no. sc-514; 1:300; Santa Cruz Biotechnology, Inc.) and anti-β-actin (cat. no. sc-7210; 1:500; Sangon Biotech Co., Ltd., Shanghai, China) at 4°C overnight. Membranes were subsequently incubated with secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (cat. no. 14708; 1:5,000; Cell Signaling Technology, Inc.) at 37°C for 1 h. The optical densities of immunopositive bands were visualized with BeyoECL Star (Beyotime Institute of Biotechnology) and determined using GeneTools software, version 4.1 (Synoptics, Ltd., Cambridge, UK).
Statistical analysis
All data are presented as the mean ± standard deviation, assessed using SPSS, version 17.0 (SPSS, Inc., Chicago, IL, UA). Wilcoxon signed-rank tests were performed to evaluate differences in between the injured and control groups. P<0.05 was considered to indicate a statistically significant difference.
Results
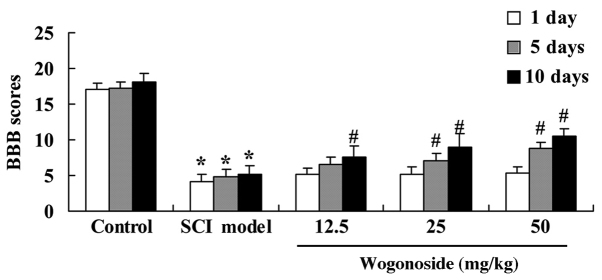
Wogonoside improves BBB score in rats with SCI-induced inflammation
BBB scores were calculated to analyze the effect of wogonoside on SCI-induced inflammation in rats. The BBB scores of rats in the SCI model group were significantly reduced compared with the control group (P<0.01; Fig. 2). Treatment with 12.5 mg/kg wogonoside for 10 days significantly alleviated the SCI-induced reduction in BBB score (P<0.01), as did treatment with 25 or 50 mg/kg wogonoside for 5 days or more (P<0.01; Fig. 2).
Figure 2.
Wogonoside improves BBB score in SCI model rats. *P<0.01 vs. control group; #P<0.01 vs. SCI model group. BBB, Basso Beattie Bresnahan; SCI, spinal cord injury.
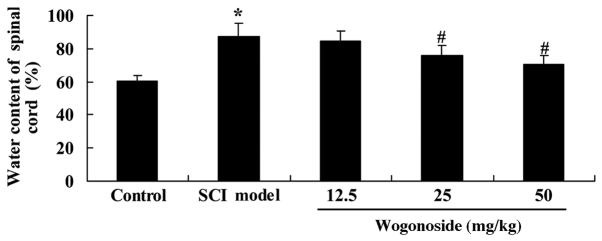
Wogonoside reduces the volume of spinal cord contusions in rats with SCI-induced inflammation
The volume of spinal cord contusions in rats with SCI-induced inflammation was assessed, and was observed to be significantly increased when compared with controls (P<0.01; Fig. 3). In turn, treatment with 25 or 50 mg/kg wogonoside significantly reduced the increased volume of spinal cord contusions in SCI rats (P<0.01; Fig. 3).
Figure 3.
Wogonoside reduces the volume of SCI-induced contusions in rats. *P<0.01 vs. control group; #P<0.01 vs. SCI model group. SCI, spinal cord injury.
Wogonoside suppresses SCI-induced upregulation of IL-1β, TNF-α and IL-6 in rats
To further elucidate the anti-inflammatory effects of wogonoside, ELISA was performed to measure the levels of IL-1β, TNF-α and IL-6 in SCI model rats. IL-1β, TNF-α and IL-6 were significantly upregulated in the SCI model group when compared with the control group (P<0.01; Fig. 4). No significant differences were observed in the levels of inflammatory cytokines between the 12.5 mg/kg wogonoside and model groups; however, treatment with 25 or 50 mg/kg wogonoside significantly reduced the levels of IL-1β, TNF-α and IL-6 compared with the model group (P<0.01; Fig. 4).
Figure 4.
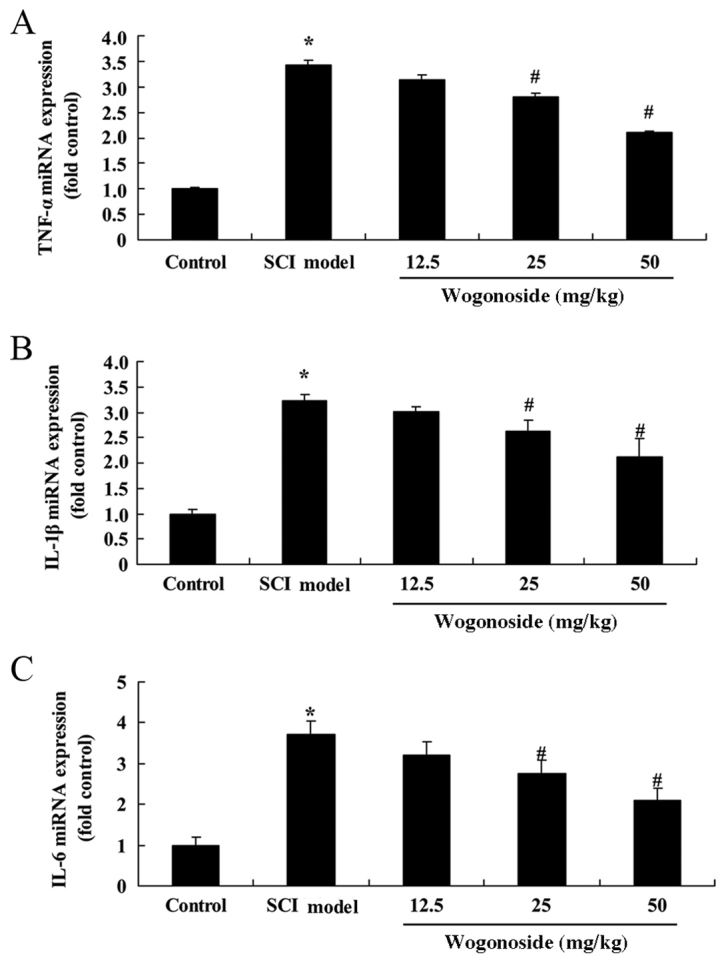
Wogonoside suppresses SCI-induced upregulation of (A) TNF-α, (B) IL-1β and (C) IL-6 in rats. *P<0.01 vs. control group; #P<0.01 vs. SCI model group. SCI, spinal cord injury; IL, interleukin; TNF, tumor necrosis factor.
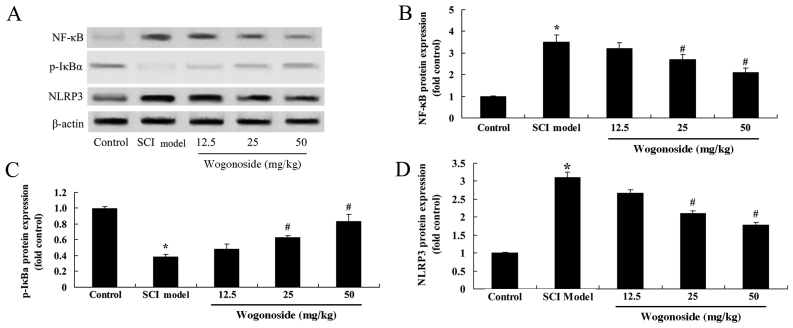
Wogonoside alleviates NF-κB and NLRP3 overexpression, and increases the activation of IκB expression in SCI model rats
Western blotting was used to evaluate the effect of wogonoside on NF-κB, phosphorylated (p)-inhibitor of NF-κB (p-IκB) and NLRP3 expression (Fig. 5A). A significant increase in the protein expression of NF-κB and NLRP3, and inhibition of p-IκB expression were observed in the SCI model group when compared with the control group (P<0.01; Fig. 5B-D). Treatment with 12.5 mg/kg wogonoside had no significant effect, whereas 25 or 50 mg/kg wogonoside significantly reduced NF-κB and NLRP3 expression, and increased p-IκB expression compared with the SCI model group (P<0.01; Fig. 5B-D).
Figure 5.
Wogonoside suppresses SCI-induced upregulation of NF-κB, p-IκB and NLRP3 in rats. (A) Western blot analysis with β-actin as a control. Statistical analysis of (B) NF-κB, (C) p-IκB and (D) NLRP3 protein expression in an SCI rat model. *P<0.01 vs. control group; #P<0.01 vs. SCI model group. SCI, spinal cord injury; NF, nuclear factor; NF-κB, nuclear factor-κB; p-IκB; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3.
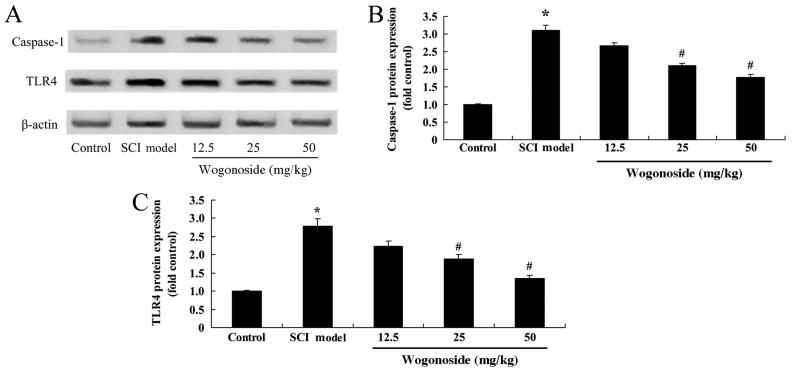
Wogonoside ameliorates caspase-1 and TLR4 overexpression in SCI model rats
The effect of wogonoside on caspase-1 expression in SCI rats was assessed using western blotting (Fig. 6A). SCI was demonstrated to significantly increase the protein expression of caspase-1 and TLR4, relative to the control group (P<0.01; Fig. 6B and C). Treatment with 12.5 mg/kg wogonoside induced no significant difference in caspase-1 or TLR4 expression, whereas 25 or 50 mg/kg wogonoside significantly reduced the levels of caspase-1 in SCI rats (P<0.01; Fig. 6B and C).
Figure 6.
Wogonoside suppresses SCI-induced overexpression of caspase-1 and TLR4 in rats. (A) Western blotting and (B and C) statistical analysis of caspase-1 and TLR4 protein expression in an SCI rat model. *P<0.01 vs. control group; #P<0.01 vs. SCI model group. SCI, spinal cord injury.
Discussion
SCI is a traumatic central nervous system injury, and frequently leads to varying degrees of anesthesia and loss of motor function in the limb below the plane of injury, making it a leading cause of disability in the field of orthopedics (12). Increasing urbanization has led to an increase in injuries caused by traffic accidents, falling accidents and professional accidents for engineers and construction workers (13). Over the last 10 years, the morbidity of SCI in China and other countries has increased (14). SCI-induced functional paralysis causes physiological and psychological trauma to patients, and is a cause of medical and economic stress for families and society (14). In the present study, treatment with wogonoside was demonstrated to significantly increase the BBB score and decrease the volume of spinal cord contusions in rats with SCI-induced inflammation.
Cytokines may be classified into two major types; proinflammatory factors and anti-inflammatory factors. Proinflammatory factors are expressed to a high-level in conditions of oxidative stress, which may further increase the level of oxidative stress (15). During oxidative stress, the upregulated proinflammatory factors include TNF-α, IL-1β, IL-2, IL-6 and IL-12, and NF-κB is also activated (16,17). In the present study, treatment with 25 or 50 mg/kg of wogonoside significantly inhibited the upregulation in IL-1β, TNF-α and IL-6 in SCI model rats. Yang et al (18) suggested that wogonoside induces anti-inflammatory effects in RAW264.7 cells, which indicated that wogonoside possessed anti-inflammation effect in SCI.
Interactions between lipopolysaccharides (LPS) and TLR4 induce the activation of transcription factors, including NF-κB and activator protein-1, which ultimately induces the expression of proinflammatory and anti-inflammatory factors (19). NF-κB is a multi-functional nuclear transcription factor, and is associated with the LPS-induced inflammatory response (19). In the cytoplasm, IκB binds with inactive NF-κB, which covers the nuclear localization sequence of NF-κB and thus prevents the activation and nuclear translocation of NF-κB (20). IκB degradation is therefore required for the activation of NF-κB. IκB is regulated by IκB kinase, which catalyzes phosphorylation of the 42′ serine residue in IκB as a signal for degradation, which induces rapid degradation of IκB (typically within several min) and exposes the nuclear localization sequence of NF-κB (21). The nuclear localization sequence of NF-κB is disclosed and NF-κB is activated and translocated to nucleus (22). Once there, NF-κB binds with the κB locus in the NF-κB reactive gene to regulate gene transcription and downstream cellular processes, including cell growth, differentiation, adhesion, apoptosis and the inflammatory response (22). Excessive phosphorylation of IκB results in a loss of NF-κB inhibition (23). In the present study, it was demonstrated that treatment with 25 or 50 mg/kg of wogonoside significantly inhibited inflammation through the suppression of NF-κB/IκB in an SCI rat model. Similarly, Zhang et al (7) reported that wogonoside ameliorates LPS-induced acute lung injury in mice through the suppression of TLR4-mediated NF-κB signaling.
The NLRP3 inflammasome promotes the secretion of IL-1β, IL-18 and IL-33, and the production of these factors is critical in the control of pathological infection (24). However, excessive cytokine production is harmful to the body (24), and therefore activation of the NLRP3 inflammasome must be strictly regulated to maintain hemostasis (25). Similarly, Sun et al (26) demonstrated that wogonoside protects against dextran sulfate sodium-induced colitis by suppressing NF-κB and NLRP3 inflammasome activation in mice.
In conclusion, the present study demonstrated that wogonoside alleviated the reduced BBB scores and increased spinal cord contusion volume of SCI model rats. Furthermore, it was observed that wogonoside ameliorated inflammation via the NF-κB/IκB and NLRP3/caspase-1/TLR4 pathways. The present study was limited as it only assessed the in vivo aspect, therefore in vitro or clinical models should be assessed in future studies. The results of the present study suggest that wogonoside is a potential novel treatment for SCI trauma, and may have clinical applications in ameliorating the associated inflammation.
Acknowledgements
This study was supported by the Pharmaceutical Health Science and Technology Development Program of Shandong Province (grant no. 2015WS0477).
References
- 1.Yalçın S, Ersöz M. Urodynamic findings, bladder emptying methods and therapeutic approaches in patients with upper lumbar and lower lumbar-sacral spinal cord injury. Neurol Sci. 2015;36:2061–2065. doi: 10.1007/s10072-015-2311-1. [DOI] [PubMed] [Google Scholar]
- 2.Pirouzmand F. Epidemiological trends of spine and spinal cord injuries in the largest Canadian adult trauma center from 1986 to 2006. J Neurosurg Spine. 2010;12:131–140. doi: 10.3171/2009.9.SPINE0943. [DOI] [PubMed] [Google Scholar]
- 3.Werndle MC, Zoumprouli A, Sedgwick P, Papadopoulos MC. Variability in the treatment of acute spinal cord injury in the United Kingdom: Results of a national survey. J Neurotrauma. 2012;29:880–888. doi: 10.1089/neu.2011.2038. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian J Med Res. 2012;135:287–296. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosety-Rodriguez M, Camacho A, Rosety I, Fornieles G, Rosety MA, Diaz AJ, Bernardi M, Rosety M, Ordonez FJ. Low-grade systemic inflammation and leptin levels were improved by arm cranking exercise in adults with chronic spinal cord injury. Arch Phys Med Rehabil. 2014;95:297–302. doi: 10.1016/j.apmr.2013.08.246. [DOI] [PubMed] [Google Scholar]
- 6.Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29:421–435. doi: 10.1007/s12264-013-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Ren Y, Yang C, Guo Y, Zhang X, Hou G, Guo X, Sun N, Liu Y. Wogonoside ameliorates lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2014;37:2006–2012. doi: 10.1007/s10753-014-9932-z. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Wen R, Lin Q, Wang N, Lu P, Zhu X. Wogonoside shows antifibrotic effects in an experimental regression model of hepatic fibrosis. Dig Dis Sci. 2015;60:3329–3339. doi: 10.1007/s10620-015-3751-4. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Hui H, Xu J, Yang H, Zhang X, Liu X, Zhou Y, Li Z, Guo Q, Lu N. Wogonoside induces growth inhibition and cell cycle arrest via promoting the expression and binding activity of GATA-1 in chronic myelogenous leukemia cells. Arch Toxicol. 2016;90:1507–1522. doi: 10.1007/s00204-015-1552-3. [DOI] [PubMed] [Google Scholar]
- 10.Ravikumar R, Fugaccia I, Scheff SW, Geddes JW, Srinivasan C, Toborek M. Nicotine attenuates morphological deficits in a contusion model of spinal cord injury. J Neurotrauma. 2005;22:240–251. doi: 10.1089/neu.2005.22.240. [DOI] [PubMed] [Google Scholar]
- 11.Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, et al. MASCIS evaluation of open field locomotor scores: Effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Liu H, Liu Z, Wang L. Acupuncture for neurogenic bladder due to spinal cord injury: A systematic review protocol. BMJ Open. 2014;4:e006249. doi: 10.1136/bmjopen-2014-006249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neirinckx V, Cantinieaux D, Coste C, Rogister B, Franzen R, Wislet-Gendebien S. Concise review: Spinal cord injuries: How could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells. 2014;32:829–843. doi: 10.1002/stem.1579. [DOI] [PubMed] [Google Scholar]
- 14.Curt A, Ellaway PH. Clinical neurophysiology in the prognosis and monitoring of traumatic spinal cord injury. Handb Clin Neurol. 2012;109:63–75. doi: 10.1016/B978-0-444-52137-8.00004-8. [DOI] [PubMed] [Google Scholar]
- 15.Evans CT, Hershow RC, Chin A, Foulis PR, Burns SP, Weaver FM. Bloodstream infections and setting of onset in persons with spinal cord injury and disorder. Spinal Cord. 2009;47:610–615. doi: 10.1038/sc.2009.2. [DOI] [PubMed] [Google Scholar]
- 16.Heyninck K, Lahtela-Kakkonen M, Van der Veken P, Haegeman G, Vanden Berghe W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem Pharmacol. 2014;91:501–509. doi: 10.1016/j.bcp.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Sakthivel KM, Guruvayoorappan C. Acacia ferruginea inhibits tumor progression by regulating inflammatory mediators-(TNF-a, iNOS, COX-2, IL-1β, IL-6, IFN-γ, IL-2, GM-CSF) and pro-angiogenic growth factor-VEGF. Asian Pac J Cancer Prev. 2013;14:3909–3919. doi: 10.7314/APJCP.2013.14.6.3909. [DOI] [PubMed] [Google Scholar]
- 18.Yang YZ, Tang YZ, Liu YH. Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264.7 cells. J Ethnopharmacol. 2013;148:271–276. doi: 10.1016/j.jep.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Yunusova T, Akhtar M, Poltoratsky V. Analysis of LPS-induced, NFκB-dependent interleukin-8 transcription in kidney embryonic cell line expressing TLR4 using luciferase assay. Methods Mol Biol. 2014;1172:305–314. doi: 10.1007/978-1-4939-0928-5_28. [DOI] [PubMed] [Google Scholar]
- 20.Shifera AS. Protein-protein interactions involving IKKgamma (NEMO) that promote the activation of NF-kappaB. J Cell Physiol. 2010;223:558–561. doi: 10.1002/jcp.22105. [DOI] [PubMed] [Google Scholar]
- 21.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5:1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie YF, Shu R, Jiang SY, Song ZC, Guo QM, Dong JC, Lin ZK. miRNA-146 negatively regulates the production of proinflammatory cytokines via NF-κB signalling in human gingival fibroblasts. J Inflamm (Lond) 2014;11:38. doi: 10.1186/s12950-014-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassel SL, Joly S, Sutterwala FS. The NLRP3 inflammasome: A sensor of immune danger signals. Semin Immunol. 2009;21:194–198. doi: 10.1016/j.smim.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butts B, Gary RA, Dunbar SB, Butler J. The importance of NLRP3 inflammasome in heart failure. J Card Fail. 2015;21:586–593. doi: 10.1016/j.cardfail.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Zhao Y, Yao J, Zhao L, Wu Z, Wang YD, Miao H, Guo Q, Lu N. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-kappaB and NLRP3 inflammasome activation. Biochem Pharmacol. 2015;94:142–154. doi: 10.1016/j.bcp.2015.02.002. [DOI] [PubMed] [Google Scholar]