Abstract
The present study aimed to investigate the expression of microRNA (miRNA or miR)-186 in tumor tissue, blood and urine from patients with bladder cancer. The mechanism by which miR-186 regulates the invasion and metastasis of bladder cancer was also assessed. A total of 76 patients who underwent surgical resection of bladder cancer tissues between August 2012 and January 2016 were included in the present study. Blood and urine samples were also collected from the 76 patients and another 66 healthy subjects. Expression of vascular endothelial growth factor C (VEGF-C) mRNA and miR-186 was measured using reverse transcription-quantitative polymerase chain reaction. Western blot analysis was performed to assess VEGF-C protein expression in tumor tissues. The content of VEGF-C protein in blood and urine samples was measured using an enzyme-linked immunosorbent assay. To identify the direct interaction between miR-186 and VEGF-C mRNA, a dual luciferase reporter assay was performed. The present findings demonstrated that VEGF-C mRNA expression in tumor tissues, blood and urine of bladder cancer patients was upregulated. VEGF-C protein expression in bladder cancer tissues was also enhanced. VEGF-C protein content in blood and urine from bladder cancer patients was elevated, consistent with the results for VEGF-C mRNA. Expression of miR-186 was reduced in tumor tissues, blood and urine. Dual luciferase reporter assay demonstrated that miR-186 regulated the expression of VEGF-C by binding with its 3′-untranslated region. Therefore, the results of the present study indicate that the expression of VEGF-C mRNA and protein is upregulated in tumor tissues, blood and urine from patients with bladder cancer, while that of miR-186 is downregulated in these samples. miR-186 potentially regulates the invasion and metastasis of bladder cancer via VEGF-C, and may become a gene marker for bladder cancer in the future.
Keywords: bladder cancer, microRNA-186, vascular endothelial growth factor C
Introduction
Bladder cancer is the fourth most common solid tumor in males and the seventh most common solid tumor in females, globally (1). According to statistics published in 2012, the number of new bladder cancer cases in the world reached 430,000, and the number of mortalities caused by bladder cancer reached 165,000 (2). Bladder cancer is one of the most common malignant tumors that affects the urinary system and its incidence and mortality rate is increasing (3). Bladder cancer typically exhibits atypical clinical symptoms, lacks specificity and is not easily diagnosed in the early stages (2). The majority of patients are identified to have bladder cancer during the treatment of other diseases, and have therefore missed the opportunity for optimal treatment. Therefore, more accurate indices for clinical staging, treatment and prognosis of bladder cancer are urgently required (4,5). The occurrence and development of bladder cancer is the result of the combined action of several types of pathways, which involve various mRNA and microRNA (miRNA or miR) molecules (6,7). The activation of the vascular endothelial growth factor C (VEGF-C) signaling pathway is closely associated with bladder cancer (8). It is reported that silencing of VEGF-C gene expression by RNA interference markedly inhibits the proliferation, migration and invasion of bladder cancer cells (9). However, the upstream regulators of VEGF-C and their mechanisms of action are rarely studied.
miR-186 is one of the most studied miRNA molecules, and it regulates the apoptosis, proliferation and autophagy of various types of tumors (10–13). However, few reports have been published regarding the effect and mechanism of action of miR-186 on bladder cancer (14,15). In addition, to the best of our knowledge, the regulatory effect of miR-186 on VEGF-C has not yet been reported. The present study determined the expression of VEGF-C in tumor tissues, blood and urine of patients with bladder cancer, and aimed to identify an association between miR-186 and VEGF-C in bladder cancer.
Materials and methods
Patients
A total of 76 patients who underwent surgical resection of bladder cancer tissues in Department of Urology, The First Affiliated Hospital of Soochow University (Suzhou, China) between August 2012 and January 2016 were included in the present study. Bladder cancer tissues and tumor-adjacent normal tissues were collected from each patient. According to 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer (16), all patients were diagnosed by experienced pathologists to have bladder cancer, including 61 cases of transitional cell carcinoma, 10 cases of squamous cell carcinoma and 5 cases of adenocarcinoma. Blood and urine samples were also collected from the 76 patients and another 66 healthy subjects (control group). Among the 76 patients with bladder cancer, 51 were male and 25 were female, with an age rage of 33–81 years and a median age of 61 years. In the control group, 48 were male and 18 were female, with an age range of 30–79 years and a median age of 59 years. No patients in the control group had previously been diagnosed with bladder cancer. Prior to surgery, none of the patients received treatments, including hormones, traditional Chinese medicine, radiotherapy or chemotherapy. All procedures were approved by the Ethics Committee of Soochow University (Suzhou, China). Written informed consent was obtained from all participants or their families.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Prior to total RNA extraction, tissues (100 mg) were ground into powder using liquid nitrogen and 1 ml TRIzol (10606ES60; Yeasen, Shanghai, China) was added for lysis, and liquid samples (100 µl) were mixed with 1 ml TRIzol. Following lysis, total RNA was extracted using the phenol chloroform method. The purity of RNA was determined at A260/A280 using ultraviolet spectrophotometry (Nanodrop ND 1000; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Subsequently, cDNA was obtained by RT using a TIANScript II cDNA First Chain Synthesis kit (KR107; Tiangen Biotech Co., Ltd., Beijing, China), according to the manufacturer's instructions, from 1 µg RNA and stored at −20°C. Primers for VEGF-C were as follows: 5′-AGCTACCTCAGCAAGACGTTA-3′ (upstream) and 5′-GCAGGAAGTGTGATTGGCAAA-3′ (downstream), and the primers for GAP DH (reference) were as follows: 5′-CCTCAAGATCATCAGCAAT-3′ (upstream) and 5′-CCATCCACAGTCTTCTGGGT-3′ (downstream). The PCR reaction system (20 µl) contained 10 µl SuperReal PreMix with SYBR-Green (FP204; Tiangen Biotech Co., Ltd.), 0.5 µl upstream primer, 0.5 µl downstream primer, 2 µl cDNA and 7 µl ddH2O. The reaction protocol was as follows: Initial denaturation for 2 min at 95°C, followed by 45 cycles of denaturation for 20 sec at 95°C, annealing for 30 sec at 54°C, and elongation for 40 sec at 72°C (iQ5; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCq method (17) was used to calculate the relative expression of VEGF-C mRNA against GAPDH. All samples were tested in triplicate.
Primers for miR-186 were as follows: 5′-CCCGATAAAGCTAGATAACC3-3′ (upstream) and 5′-CAGTGCGTGTCGTGGAGT-3′ (downstream), and the primers for U6 were as follows: 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (upstream) and 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (downstream). The reaction protocol was as follows: Initial denaturation for 5 min at 95°C, followed by 40 cycles of denaturation for 10 sec at 95°C, annealing for 20 sec at 60°C, and elongation for 20 sec at 72°C (iQ5; Bio-Rad Laboratories, Inc.). The 2−ΔΔCq method (17) was used to calculate the relative expression of miR-186 against internal reference U6. All samples were tested in triplicate.
Western blot analysis
Total protein was extracted from samples using radioimmunoprecipitation assay lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% TritonX-100 and 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's protocol. Following lysis in ice for 50 min, the mixture was centrifuged at 12,000 × g/min at 4°C for 5 min. The supernatant was used to determine the protein concentration using a bicinchoninic acid protein concentration determination kit (RTP7102; Real-Times Biotechnology Co., Ltd., Beijing, China), according to the manufacturer's instructions. Protein samples (20 µg) were mixed with sodium dodecyl sulfate loading buffer prior to denaturation in a boiling water bath for 5 min. Samples were then separated by 10% SDS-PAGE. Resolved proteins were transferred to polyvinylidene difluoride membranes on ice (100 V; 2 h) and blocked with 5% skimmed milk at room temperature for 1 h. Subsequently, the membranes were incubated with rabbit anti-human VEGF-C polyclonal primary antibody (1:1,000; ab9546; Abcam, Cambridge, UK) and rabbit anti-human β-actin primary antibody (1:5,000; ab129348; Abcam) at 4°C overnight. Following extensive washing with phosphate-buffered saline with Tween-20 (PBST) three times for 15 min, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3,000; ab6721; Abcam) for 1 h at room temperature. A further three washes with PBST for 15 min were completed and the membrane was then developed with enhanced chemiluminescence detection kit (ab65623; Abcam) for imaging. Image Lab v3.0 software (Bio-Rad Laboratories, Inc.) was used to acquire and analyze imaging signals. The relative content of VEGF-C protein was expressed as VEGF-C/β-actin.
Enzyme-linked immunosorbent assay (ELISA)
Blood and urine samples were centrifuged at 1,200 × g for 10 min at 4°C, and supernatants were assessed using a human VEGF-C ELISA kit (ab100664; Abcam), according to the manufacturer's instructions. In microplates, standards (50 µl), samples (10 µl sample liquid and 40 µl diluent) and blanks were added into predefined wells. In the wells for standards and samples, horseradish peroxidase-labelled conjugates (100 µl) were added prior to sealing the plates for incubation at 37°C for 1 h. Plates were then washed five times with wash buffer (provided by the kit), and substrates A (50 µl) and B (50 µl) were added into each well. Following incubation at 37°C for 15 min, stop solution (50 µl) was added into each well and the absorbance of each well was measured at 450 nm within 15 min using a microplate reader (Multiskan FC; Thermo Fisher Scientific, Inc.).
Dual luciferase reporter assay
Bioinformatics prediction is a powerful tool used for studying the functions of miRNAs. To understand the regulatory mechanism of VEGF-C in bladder cancer, the present study used miRanda (microrna.org/microrna/home.do), TargetScan (targetscan.org), PiTa (genie.weizmann.ac.il/pubs/mir07/mir07_data.html), RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid/) and PICTA (pictar.mdc-berlin.de/) to predict miRNA molecules that may regulate VEGF-C. This revealed that miR-186 was able to potentially regulate VEGF-C (Fig. 1). According to the bioinformatic results, wild-type (WT) and mutant seed regions of miR-186 in the 3′-untranslated region (UTR) of the VEGF-C gene were chemically synthesized in vitro using ClonExpress II One Step Cloning kit (C112-01/02; Vazyme Biotech Co., Ltd., Nanjing, China) according to the manufacturer's manual, added to Spe-1 and HindIII restriction sites, and then cloned into pMIR-REPORT luciferase reporter plasmids for the present study. Plasmids (0.8 µg) with WT or mutant 3′-UTR DNA sequences were co-transfected with agomiR-186 (100 nM; Sangon Biotech Co., Ltd., Shanghai, China) into HEK293T cells. Following cultivation for 24 h, the cells were lysed using a dual luciferase reporter assay kit (Promega Corp., Madison, WI, USA) according to the manufacturer's protocol. Fluorescence intensity was measured using a GloMax 20/20 luminometer (Promega Corp.). Using Renilla fluorescence activity as internal reference, the fluorescence values of each group of cells were measured.
Figure 1.
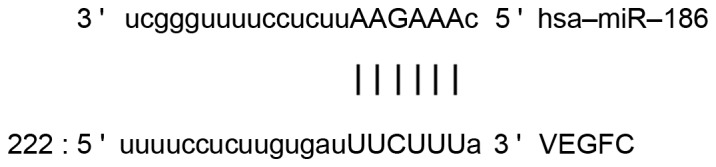
Bioinformatics prediction of direct interactions between miR-186 and VEGF-C were used to predict miRNA molecules that may regulate VEGF-C. The findings demonstrated that miR-186 was able to potentially regulate VEGF-C. miR, micro RNA; VEGF-C, vascular endothelial growth factor C.
Statistical analysis
Results were analyzed using SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation. Data were assessed for normality. Multigroup measurement data were analyzed using one-way analysis of variance. In case of homogeneity of variance, Least Significant Difference and Student-Newman-Keuls methods were used; in case of heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method was used. P<0.05 was considered to indicate a statistically significant difference.
Results
VEGF-C mRNA expression is upregulated in tumor tissues, blood and urine of bladder cancer patients
To measure the expression of VEGF-C mRNA in various samples, RT-qPCR was performed. The findings indicated that the level of VEGF-C mRNA in tumor tissues (P<0.01), blood (P<0.05) and urine (P<0.01) was significantly increased compared with controls (Fig. 2). These results suggest that VEGF-C has a regulatory role in bladder cancer at the transcription level.
Figure 2.
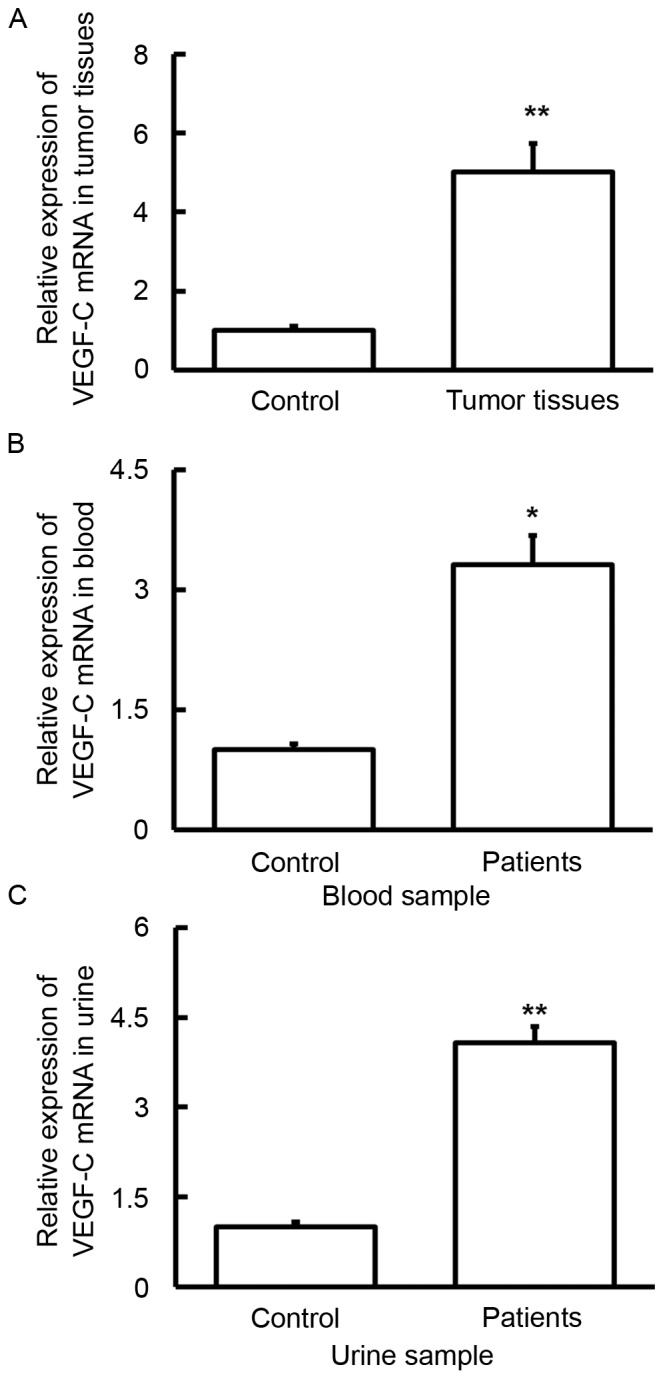
Expression of VEGF-C mRNA in (A) tumor, (B) blood and (C) urine samples from patients with bladder cancer. Reverse transcription-quantitative polymerase chain reaction was used to determine the expression of mRNA. VEGF-C mRNA levels in tumor tissues, blood and urine were significantly increased compared with controls. *P<0.05 and **P<0.01 vs. controls. Data are presented as the mean + standard deviation. VEGF-C, vascular endothelial growth factor C.
VEGF-C protein expression in bladder cancer tissues is enhanced
To determine the protein expression of VEGF-C in tumor tissues, western blot analysis was performed. Compared with the control, VEGF-C protein expression in tumor tissues was significantly increased (P<0.05; Fig. 3), which is consistent with the results of VEGF-C mRNA (Fig. 2). These results indicate that VEGF-C also exerts its regulatory effect on bladder cancer progression at the protein level.
Figure 3.
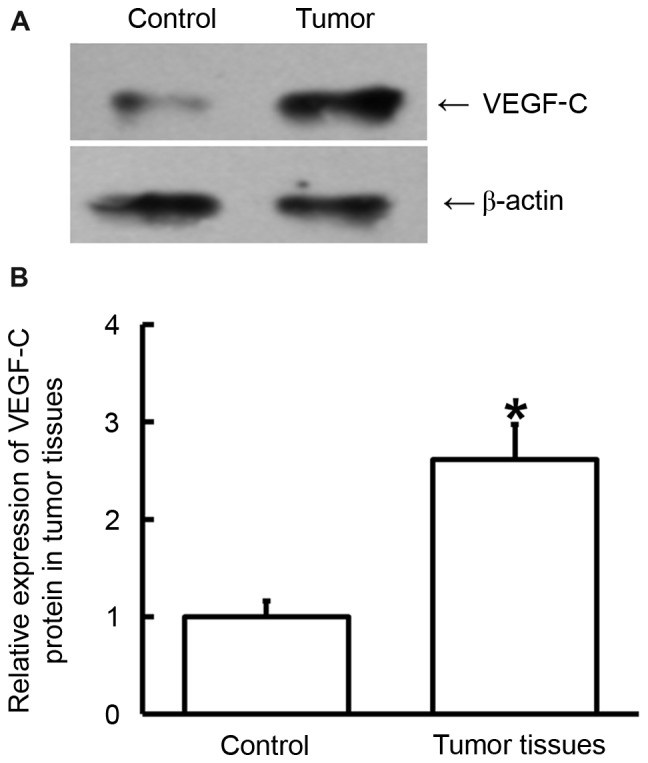
Expression of VEGF-C protein in tumor samples from patients with bladder cancer. (A) Western blot analysis was used to measure protein expression and this was (B) subsequently quantified. Compared with the control, VEGF-C protein expression in tumor tissues was significantly increased. *P<0.05 vs. control. Data are presented as the mean ± standard deviation. VEGF-C, vascular endothelial growth factor C.
VEGF-C protein content in blood and urine from patients with bladder cancer are elevated, consistent with VEGF-C mRNA results
To examine the concentration of VEGF-C protein in blood and urine samples from bladder cancer patients, ELISA was performed. The data indicated that the protein level of VEGF-C in blood and urine samples from patients with bladder cancer was significantly higher than those in the control (P<0.01; Fig. 4). These results suggest that VEGF-C protein contents in blood and urine from bladder cancer patients are elevated, which is consistent with VEGF-C mRNA results (Fig. 2).
Figure 4.
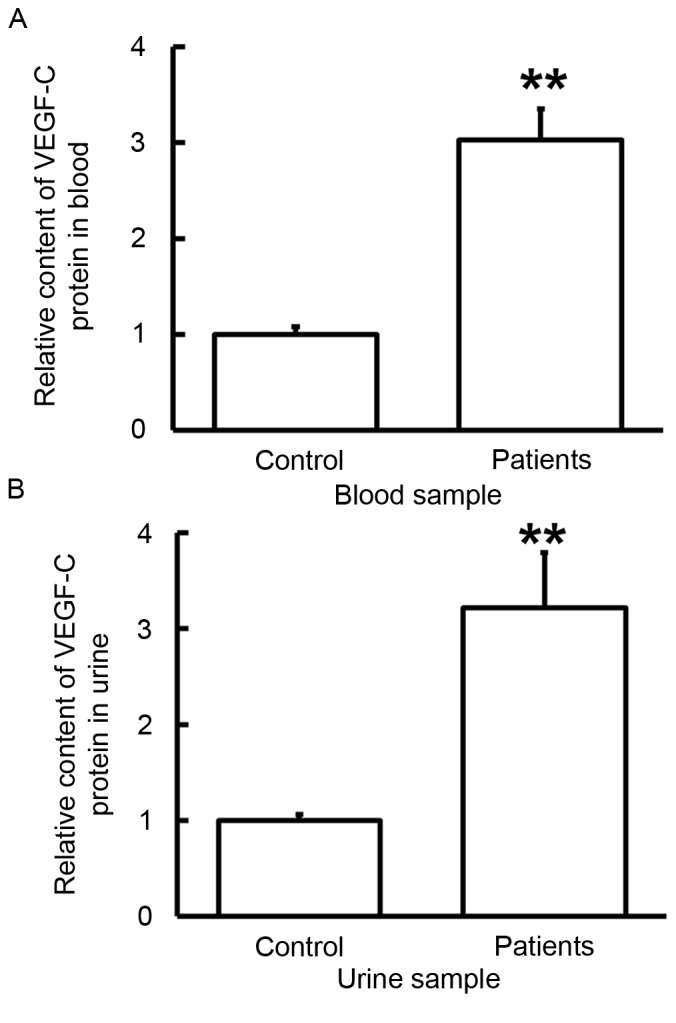
Content of VEGF-C protein in (A) blood and (B) urine samples from patients with bladder cancer. ELISA was used to determine the content of VEGF-C and the protein levels of VEGF-C in serum and urine samples from patients were significantly higher than those in control. **P<0.01 vs. controls. Data are presented as the mean ± standard deviation. VEGF-C, vascular endothelial growth factor C.
Altered VEGF-C expression may be induced by the level of miR-186
To evaluate the level of miR-186, RT-qPCR was used. Compared with the control groups, the expression of miR-186 in tumor tissue (P<0.05), blood (P<0.05) and urine (P<0.01) samples was significantly downregulated (Fig. 5). These results indicate that altered VEGF-C expression may be induced by different miR-186 levels.
Figure 5.
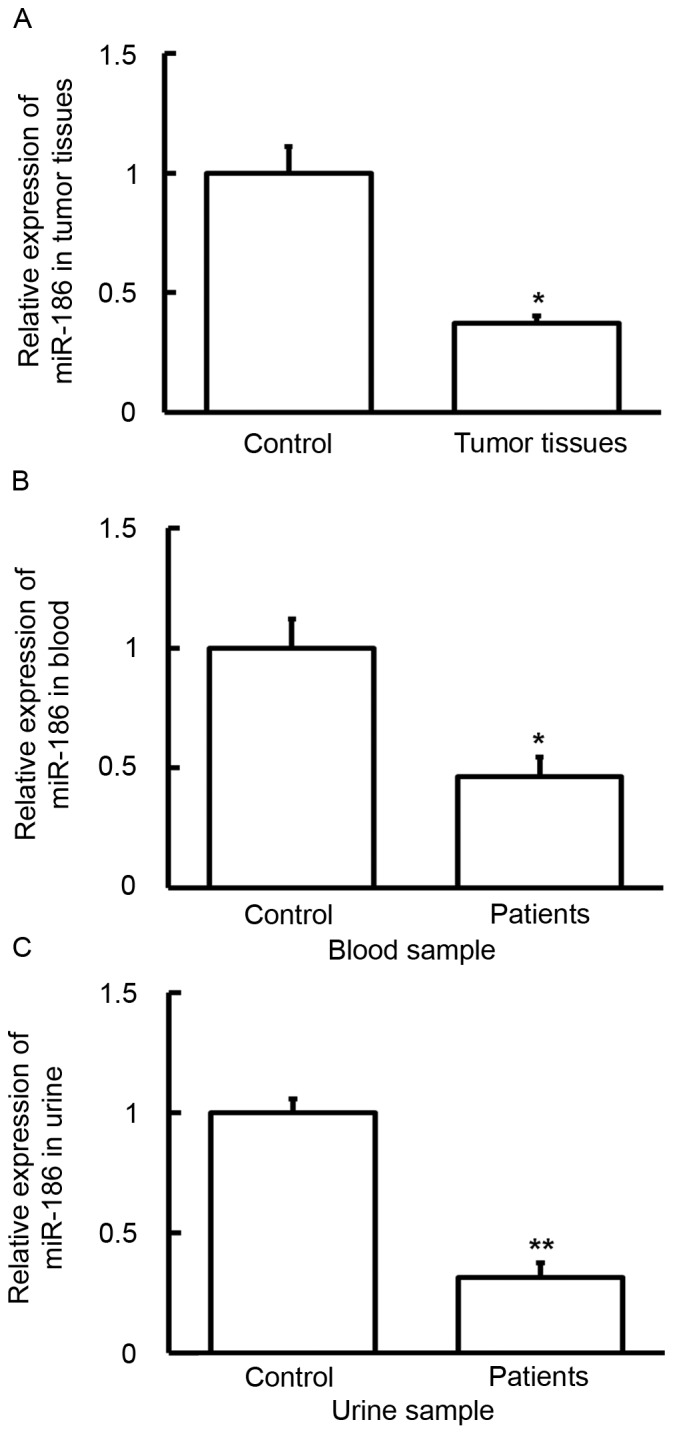
Expression of miR-186 in (A) tumors, (B) blood and (C) urine samples from patients with bladder cancer. Reverse transcription-quantitative polymerase chain reaction was used to determine the expression of miR-186. Compared with control groups, the expression of miR-186 in tumor tissue, blood and urine samples was significantly downregulated. *P<0.05 and **P<0.01 vs. controls. Data are presented as the mean ± standard deviation. miR, micro RNA.
miR-186 regulates the expression of VEGF-C by binding with its 3′-UTR
To identify a direct interaction between miR-186 and VEGF-C, a dual luciferase reporter assay was performed. The data demonstrated that the fluorescence value in the WT group was significantly lower than the control (P<0.05; Fig. 6), whereas the mutant group was not significantly different from control. These results suggest that miR-186 regulates the expression of VEGF-C by binding with its 3′-UTR.
Figure 6.
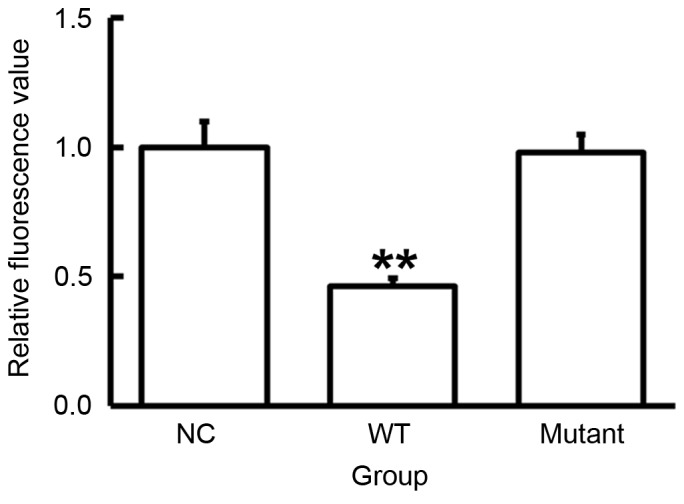
Fluorescence values of HEK293T cells transfected with WT or mutant 3′-UTR DNA sequences of VEGF-C and agomiR-186. A dual luciferase reporter assay was used to evaluate the interaction between miR-186 and VEGF-C. Fluorescence values in the WT group were significantly lower than the control, while the values in the mutant group were not significantly different from the control values. **P<0.01 vs. NC. Data are presented as the mean ± standard deviation. VEGF-C, vascular endothelial growth factor C; WT, wild type; UTR, untranslated region; NC, negative control.
Discussion
The occurrence and development of bladder cancer is a complex process involving multiple genes accompanied by epigenetic changes. With the broadening of the prospects of biological treatments, it is important to elucidate the pathogenesis of bladder cancer and identify novel treatment targets. The VEGF-C signaling pathway is often associated with the course and development of bladder cancer. The binding of VEGF-C and its specific receptor VEGFR-3 activates downstream molecules, induces the formation and expansion of lymphatic vessels inside or surrounding the tumors, and promotes lymphatic metastasis of bladder cancer (18). It is reported that the expression of VEGF-C promotes the hyperplasia of lymphatic vessels within tumors, the degree of which is closely associated with the local lymphatic and lung metastasis of breast cancer (19). Another study indicated that lymphatic hyperplasia within tumors was more important than those near tumors in local lymph node metastasis of the tumor (20). In addition, it was revealed that the expression of VEGF-C and VEGF-D within tumors is closely associated with lymphatic hyperplasia and lymphatic vessel density (21). By contrast, another study demonstrated that VEGF-C expression was not associated with lymphatic vessel hyperplasia within tumors, but those surrounding tumors (22). Padera et al (23) demonstrated that a high expression of VEGF-C facilitates lymphatic vessel hyperplasia surrounding tumors and lymphatic metastasis, but there are no functional lymphatic vessels within tumors, suggesting that functional lymphatic vessels that surround tumors are sufficient for lymphatic metastasis. In the present study, the expression of VEGF-C mRNA and protein in the bladder cancer tissues is significantly higher than normal tissues, being consistent with previous studies (24,25). Similar results were obtained from blood and urine samples from patients with bladder cancer, suggesting that changes in VEGF-C expression are associated with the occurrence and development of bladder cancer. Furthermore, an abnormal expression of VEGF-C may be a key point in the pathogenesis and metastasis of bladder cancer.
Using bioinformatics, the present study demonstrated that miR-186 is potentially an upstream miRNA that regulates VEGF-C. A number of studies indicated that miR-186 may become a novel target in the prevention, diagnosis and treatment of tumors. Zhang et al (13) revealed that miR-186 may be a diagnostic and predictive factor for pancreatic ductal adenocarcinoma, and it affects the proliferation and invasion of tumor cells (13). Lee et al (12) demonstrated that upregulated miR-186 is associated with the proliferation of human lung fibroblasts and Sun et al (11) indicated that miR-186 regulates the formation of tumor-related fibroblasts. In addition, Cui et al (10) revealed that miR-186 inhibits the proliferation and metastasis of non-small-cell lung carcinoma cells by targeting Rock1. The present study demonstrated that VEGF-C is upregulated and miR-186 is downregulated in tumor tissues, and blood and urine samples. This implies that miR-186 affects the transcription and translation of VEGF-C potentially by targeting VEGF-C gene. The dual luciferase reporter assay demonstrated that miR-186 is able to directly bind with the 3′-UTR seed region of VEGF-C mRNA and regulate its expression.
Notably, the sample types used in the present study include blood and urine that were obtained non-invasively and easily. The results of the current study indicate that blood and urine samples possess good sensitivity, specificity and potential for the diagnosis of bladder cancer (26,27). In conclusion, the present study suggests that miR-186 regulates the invasion and metastasis of bladder cancer, potentially via VEGF-C. However, the direct associated between bladder cancer and the regulation of VEGF-C by miR-186 requires further study.
Acknowledgements
The authors of the current study are particularly grateful to Professor Jun Ouyang from the Department of Urology, The First Affiliated Hospital of Soochow University (Jiangsu, China) for his instructions on experimental design and financial support, and to Dr Qiaocheng Qiu from Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University (Jiangsu, China) for his valuable suggestions on experimental operation.
References
- 1.Li F, An SL, Zhou Y, Liang ZK, Jiao ZJ, Jing YM, Wan P, Shi XJ, Tan WL. Milk and dairy consumption and risk of bladder cancer: A meta-analysis. Urology. 2011;78:1298–1305. doi: 10.1016/j.urology.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Li F, Huang X, Hua Q, Huang T, Liu Z, Liu Z, Zhang Z, Liao C, Chen Y, et al. The association of tea consumption with bladder cancer risk: A meta-analysis. Asia Pac J Clin Nutr. 2013;22:128–137. doi: 10.6133/apjcn.2013.22.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Ji Z, Wang Q, Shi B, Shou C, Liu C, Fan H, Li H, Davidson KT, Wakefield MR, et al. Expression of gamma-synuclein in bladder carcinoma: A possible marker for prognosis. Anticancer Res. 2016;36:951–956. [PubMed] [Google Scholar]
- 5.Li CE, Chien CS, Chuang YC, Chang YI, Tang HP, Kang CH. Chronic kidney disease as an important risk factor for tumor recurrences, progression and overall survival in primary non-muscle-invasive bladder cancer. Int Urol Nephrol. 2016;48:993–999. doi: 10.1007/s11255-016-1264-5. [DOI] [PubMed] [Google Scholar]
- 6.Xiao S, Wang J, Xiao N. MicroRNAs as noninvasive biomarkers in bladder cancer detection: A diagnostic meta-analysis based on qRT-PCR data. Int J Biol Markers. 2016;31:e276–e285. doi: 10.5301/jbm.5000199. [DOI] [PubMed] [Google Scholar]
- 7.Tan ST, Liu SY, Wu B. TRIM29 overexpression promotes proliferation and survival of bladder cancer cells through NF-κB signaling. Cancer Res Treat. 2016;48:1302–1312. doi: 10.4143/crt.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zu X, Tang Z, Li Y, Gao N, Ding J, Qi L. Vascular endothelial growth factor-C expression in bladder transitional cell cancer and its relationship to lymph node metastasis. BJU Int. 2006;98:1090–1093. doi: 10.1111/j.1464-410X.2006.06446.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HH, Qi F, Shi YR, Miao JG, Zhou M, He W, Chen MF, Li Y, Zu XB, Qi L. RNA interference-mediated vascular endothelial growth factor-C reduction suppresses malignant progression and enhances mitomycin C sensitivity of bladder cancer T24 cells. Cancer Biother Radiopharm. 2012;27:291–298. doi: 10.1089/cbr.2010.0919. [DOI] [PubMed] [Google Scholar]
- 10.Cui G, Cui M, Li Y, Liang Y, Li W, Guo H, Zhao S. MiR-186 targets ROCK1 to suppress the growth and metastasis of NSCLC cells. Tumour Biol. 2014;35:8933–8937. doi: 10.1007/s13277-014-2168-6. [DOI] [PubMed] [Google Scholar]
- 11.Sun P, Hu JW, Xiong WJ, Mi J. miR-186 regulates glycolysis through Glut1 during the formation of cancer-associated fibroblasts. Asian Pac J Cancer Prev. 2014;15:4245–4250. doi: 10.7314/APJCP.2014.15.10.4245. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Kim SY, Bae YS. Upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells. Mol Cells. 2014;37:620–627. doi: 10.14348/molcells.2014.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZL, Bai ZH, Wang XB, Bai L, Miao F, Pei HH. miR-186 and 326 predict the prognosis of pancreatic ductal adenocarcinoma and affect the proliferation and migration of cancer cells. PLoS One. 2015;10:e0118814. doi: 10.1371/journal.pone.0118814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao K, He L, Gan Y, Zeng Q, Dai Y, Tan J. miR-186 suppresses the growth and metastasis of bladder cancer by targeting NSBP1. Diagn Pathol. 2015;10:146. doi: 10.1186/s13000-015-0372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Yuan D, Li J, Zheng S, Wang B. miR-186 downregulates protein phosphatase PPM1B in bladder cancer and mediates G1-S phase transition. Tumour Biol. 2016;37:4331–4341. doi: 10.1007/s13277-015-4117-4. [DOI] [PubMed] [Google Scholar]
- 16.Alfred Witjes J, Lebret T, Compérat EM, Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J, Rouanne M, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Kim C, Kim MJ, Schwendener RA, Alitalo K, Heston W, Kim I, Kim WJ, Koh GY. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer. 2011;10:36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 20.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. [PubMed] [Google Scholar]
- 21.Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–1926. [PubMed] [Google Scholar]
- 22.Franchi A, Gallo O, Massi D, Baroni G, Santucci M. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: A morphometric study with clinical correlations. Cancer. 2004;101:973–978. doi: 10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 23.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HH, Qi F, Cao YH, Zu XB, Chen MF. Expression and clinical significance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol Lett. 2015;10:2610–2616. doi: 10.3892/ol.2015.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou XU, Qi L, Tong S, Cui YU, Chen J, Huang T, Chen Z, Zu XB. miR-128 downregulation promotes growth and metastasis of bladder cancer cells and involves VEGF-C upregulation. Oncol Lett. 2015;10:3183–3190. doi: 10.3892/ol.2015.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata M, Muto S, Horie S. Molecular biomarkers in bladder cancer: Novel potential indicators of prognosis and treatment outcomes. Dis Markers. 2016;2016:8205836. doi: 10.1155/2016/8205836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VAN Bruwaene S, Costello AJ, VAN Poppel H. Prognosis of node-positive bladder cancer in 2016. Minerva Urol Nefrol. 2016;68:125–137. [PubMed] [Google Scholar]


