Highlights
-
•
A focused on the erectogenic potentials of two tropical pumpkin seeds was established.
-
•
The antioxidant properties of these seed varieties were also discussed.
-
•
The inhibitory effects of the seeds on key enzymes relevant to ED were critically explored.
-
•
The potentials of the seeds to manage ED were recommended.
1. Introduction
Erectile dysfunction (ED) is defined as the consistence inability or difficulty to maintain erections sufficient for satisfactory sexual intercourse [34]. It is a condition that has been estimated to have affected about 150 million individuals worldwide [2] and data from the ENIGMA study in 2004 suggested that the condition is prevalent in approximately 17% of all men [14]. Studies have found that ED may be present in up to half of the male population between 40 and 70 years old [17]. In the Cologne study of men aged 30–80 years, the prevalence of ED was 19.2%, with a steep age-related increase from 2.3% to 53.4% [30], [31]. However, basic science research on erectile physiology has been devoted to investigating the pathogenesis of ED and that has led to the conclusion that erectile dysfunction is predominately a disease of neurovascular origin. Its incidence dramatically increases in men with diabetes mellitus, hypercholesterolemia, and cardiovascular disease.
Loss of the functional integrity of the endothelium and subsequent endothelial dysfunction plays an integral role in the occurrence of ED. The decrease in nitric oxide (NO) bioavailability in endothelial dysfunction may be caused by reductions in the enzyme endothelial NO synthase (eNOS), a lack of substrate or cofactors for eNOS, alterations in intracellular signaling such that eNOS is not appropriately activated or uncoupled, or accelerated degradation of NO by reactive oxygen species (ROS), such as superoxide anion. Nevertheless, it is feasible to regulate NO biosynthesis in both endothelial and smooth muscle cells of the penile tissues by controlling the availability of arginine for endothelial nitric oxide synthase (eNOS), while neuronal nitric oxide synthase could as well be enhanced by the content of aspartic acid which stimulate the synthesis and release of luteinizing and testosterone secretion for penile erection. However, inhibition of arginase is associated with enhanced non-adrenergic non-cholinergic activities (NANC) and endothelium-dependent vasorelaxation of human corpus cavernosum (CC) smooth muscle thereby, suggesting that arginase inhibition could increase NO biosynthesis through a NOS-dependent manner [11].
Furthermore, synthetic phosphodiesterase type-5 inhibitors (iPDE5) enhance NO activity and are currently the most effective oral drugs for the treatment of male ED. Moreover, these drugs sometimes induce myriads of unwanted adverse effects coupled with their associated expensive cost. Therefore, a search for cheap and potential natural dietary inhibitors devoid of side effects is highly desirable. On the other hand, fluted pumpkin seeds are one of the most naturally abundant and cheapest sources of amino acids and polyphenols while; amino acid supplements have been reported as a potential component in the management of erectile dysfunction [12]. This evince is strongest for arginine and aspartate while compared with other amino acids. This could be the basic framework for their employment in folkloric medicine to treat male erectile dysfunction [19], [45]. However, the dearth of information observed on the mechanisms of action involved in their therapeutic effects has necessitated these findings. Hence, this study sought to characterize the bioactive constituents of some fluted pumpkin seeds Cucumeropsis mannii naudin (white melon seed) and Citrullus lanatus (kalahari) and their interactions with some enzyme systems implicated in male erectile dysfunction; in order to provide the biochemical rationale for the use of these seeds in the treatment of male erectile dysfunction.
2. Materials and methods
Fresh samples of fluted pumpkins seeds white melon and Kalahari were purchased from an ancient Oja Oba market in Owo Kingdom, Ondo State Nigeria. Authentication of the seeds was carried out by Omotayo F. O of the Plant Science and Biotechnology Department, Ekiti State University, Ado - Ekiti (EKSU) Nigeria (Herbarium numbers: UHAE. 2016/087 and UHAE. 2016/088) for white melon seed and Kalahari seeds respectively. The seeds were washed under running tap, shelled and sun-dried before being powdered for aqueous extraction. Gas Chromatography (GC-MS) (Bidlingmeyer et al., 1984) and High Performance Liquid Chromatography-diode array detector (HPLC-DAD) (Shimadzu, Kyoto, Japan) were used for the amino acids and phenolics characterization of the seeds respectively. All chemicals were of analytical grade purchased from Fisher Scientific (Oakville, ON, Canada).
2.1. Determination of total phenolic content
The total phenolic content was determined according to the method of [41]. Briefly, appropriate dilutions of the extracts were oxidized with 2.5 ml 10% Folin-Ciocalteau's reagent (v/v) and neutralized by 2.0 ml of 7.5% sodium carbonate. The reaction mixture was incubated for 40 min at 45 °C and the absorbance was measured at 765 nm in the spectrophotometer. The total phenolic content was subsequently calculated as gallic acid equivalent.
2.2. Determination of total flavonoid content
The total flavonoid content was determined using a slightly modified method reported by [32]. Briefly, 0.5 ml of appropriately diluted sample was mixed with 0.5 ml methanol, 50 μL of 10% AlCl3, 50 μL of 1 M potassium acetate, and 1.4 ml water and allowed to incubate at room temperature for 30 min. The absorbance of the reaction mixture was subsequently measured at 415 nm in the JENWAY UV–visible spectrophotometer; the total flavonoid content was subsequently calculated.
2.3. In vitro antioxidant studies
2.3.1. 1, 1-diphenyl–2 picrylhydrazyl free radical scavenging ability
The free radical scavenging ability of the seed extracts against DPPH (1,1-diphenyl–2 picrylhydrazyl) free radical was evaluated as described by Gyamfi et al. [21]. Briefly, appropriate dilution of the extracts (1 ml) was mixed with 1 ml, 0.4 mM methanolic solution containing DPPH radicals, the mixture was left in the dark for 30 min and the absorbance was taken at 516 nm. The DPPH free radical scavenging ability was subsequently calculated.
2.4. Fenton reaction (degradation of deoxyribose)
The method of Halliwell and Gutterdge [22] was used to determine the ability of the extract to prevent Fe2+/ H2O2 induced decomposition of deoxyribose. The extracts 0–100 μL was added to a reaction mixture containing 120 μL of 20 mm deoxyribose, 400 μL of 0.1 m phosphate buffer, 40 μL of 500 µm of FeSO4, and the volume were made up to 800 μL with distilled water. The reaction mixture was incubated at 37 °C for 30 min and the reaction was then stopped by the addition of 0.5 ml of 28% trichloro acetic acid. This was followed by addition of 0.4 ml of 0.6% thiobarbituric acid solution. The tubes were subsequently incubated in boiling water for 20 min. The absorbance was measured at 532 nm in a spectrophotometer.
2.5. Copper (Cu2+) chelation assay
Copper chelating ability activity was measured by the methods as described [44]. To 0.5 ml of CuSO4 (1 mg/ml) was added 0.4 ml of 0.1 M acetate buffer, pH 5.6 and 100 μL of the sample. After 2 min, 250 μL of 4 mM pyrocatechol solution was added. After 10 min the absorbance of the sample was measured at a wavelength 632 nm against a control sample containing 0.5 ml CuSO4 (1 mg/ml) and 250 μL of 4 mM pyrocatechol solution. Blank determination was an acetate buffer at pH 5.6. Copper ion chelating abilities of the seeds were calculated according to the formular:
Where AS– absorbance of sample; AC – absorbance of control.
2.6. Fe2+ chelation assay
The Fe2+ chelating ability of the extracts were determined using a modified method of Minnoti and Aust [33] with a slight modification by Puntel et al. [38]. Freshly prepared 500 µM FeSO4 (150 μL) was added to a reaction mixture containing 168 μL 0.1 M Tris-HCl (pH 7.4), 218 μL saline and the extracts (0 − 25 μL). The reaction mixture was incubated for 5 min, before the addition of 13 μL 0.25% 1, 10-phenanthroline (w/v). The absorbance was subsequently measured at 510 nm in a spectrophotometer. The Fe (II) chelating abilities of the seeds were subsequently calculated.
2.7. Arginase activity assay
The rats were scarified by cervical dislocation under mild diethyl ether anaesthesia and rapidly dissected. Penile tissues were removed and homogenized in cold phosphate buffer with pH 7.2. The supernatant which was used as source of the enzymes was prepared by centrifuging the suspensions for 20 min at 4000 r.p.m in a Kenxin refrigerated centrifuge Model KX3400 C. Arginase activity was determined by the measurement of urea produced by the reaction of Ehrlich's reagent. The reaction mixture contained in final concentration 1.0 mM Tris-HCl buffer, pH 9.5 containing 1.0 mM MnCl 0.1 M arginine solution and 50 μL of the enzyme preparation in a final volume of 1.0 ml. The mixture was incubated for 10 min at 37 °C. The reaction was terminated by the addition of 2.5 ml Erhlich reagent (2.0 g of p-dimethylaminobenzaldelyde in 20.0 ml of concentrated hydrochloric acid and made up to 100 ml with distilled water). The optical density reading was taken after 20 min at 450 nm. The control experiment was performed without the test extracts and the arginase inhibitory activity was expressed as percentage inhibition [25]. % Inhibition = [(AbsControl – AbsSamples)/AbsControl] × 100
2.8. Lipid Peroxidation Assay
2.8.1. Preparation of Tissue Homogenates
Rats were decapitated under mild diethyl ether anaesthesia and the penis was rapidly isolated and placed on ice and weighed. This tissue was subsequently homogenized in cold saline (1/10 w/v) with about 10-up-and-down strokes at approximately 1200 rev/min in a Teflon glass homogenizer. The homogenate was centrifuged for 10 min at 3000 × g to yield a pellet that was discarded, and a low-speed supernatant (S1) was kept for lipid peroxidation assay [5].
2.9. Phosphodiesterase type 5 (PDE-5) assay
The phosphodiesterase 5 inhibitory activity of the pumpkin seeds were tested using the method of Kelly and Butler [26] with slight modification. The substrate, p-nitrophenyl phenylephosphate (PNPPP Sigma Aldrich, USA), 55.84 mg were dissolved in 20 ml of Tris buffer pH 7.4 to produce 5 mM working solution. The substrate, enzyme, and the extract solution were immersed separately in a water bath at 37 °C for 10 min to equilibrate. Different concentration of the extracts were mixed with 100 μL of the enzyme and incubated in a water bath maintained at 37 °C to allow the extract of the active site of the enzyme. 1 ml of the substrate was subsequently added to the mixture (enzyme with different concentration of the extracts was left to stand for 30 s, change in absorbance of each concentration for 5 min was read using spectrophotometer (Jenway 6305 uv/vis spectrophotometer)) at 400 nm.
2.10. Quantification of phenolic compounds by HPLC-DAD
Sample extracts were separated at a concentration of 10 mg/ml using a reversed-phase Phenomenex C18 analytical column (4.6 mm × 250 mm, 5 µm particle size). The mobile phase, consisting phosphoric acid (0.5%) in Milli-Q water deionized water (A) and methanol (B), was pumped at 0.5 ml/min into the HPLC system and with injection volume of 40 μL with the following gradient elution program: 0–2 min, 1–5% B; 2–10 min, 5–20% B; 10–32 min, 20–45% B; 32–45 min, 70% B; and 45–50 min, 100% B. Subsequently, the B content was decreased to the initial conditions and for 10 min the column was re-equilibrated (total run time 50 min) [7]. The extracts and mobile phase were filtered through 0.45 µm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use. Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.025 – 0.300 mg/ml for gallic acid, caffeic acid and p-coumaric acid, and 0.030–0.200 mg/ml for quercitrin, quercetin, rutin, luteolin and kaempferol. Quantifications were carried out by integration of the peaks using the external standard method, at 271 nm for gallic acid; 325 nm for p-coumaric and caffeic acids; and 366 for quercetin, quercitrin, rutin, luteolin and kaempferol. The chromatography peaks were confirmed by comparing its retention time with those of reference standards and by DAD spectra (200–500 nm). All chromatography operations were carried out at ambient temperature and in triplicate. LOD and LOQ were calculated based on the standard deviation of the responses and the slope using three independent analytical curves, as defined by Boligon et al. [7]. LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
3. Amino acids characterization
Modified AOAC Method 982.30, 2006 was followed in the extraction of the sample for the amino acid analysis. The dried and pulverized sample was made to be free of water by ensuring constant weight for a period of time in the laboratory. The sample of 10.0 g was weighed into the 250 ml conical flask capacity. The sample was defatted by extracting the fat content of the sample with 30 ml of the petroleum spirit three times with soxhlet that was equipped with thimble. The sample was hydrolyzed three times for complete hydrolysis to be achieved. The amino acid content of the sample was recovered by extracting with 30 ml of the dichloro methane three times before concentrating to 1.0 ml. The concentrated extract was derivatised for volatility that is suitable for gas chromatography analysis
3.1. Data analysis data analysis
The mean values from triplicate experiments were pooled and expressed as the mean standard deviation (STD). One way analysis of variance was used to analyze the results. Tukey's test was used for the post hoc analysis, and the least significance difference (LSD) was carried out. GraphPad Prism 6 software was used for the analysis followed by the use of Statistical Package for Social Science (SPSS) 16.0 for windows. The significance level was taken at P<0.05 [47] and the IC50 (extract concentration causing 50% enzyme inhibition/antioxidant activity) was performed using non-linear regression analysis.
4. Results
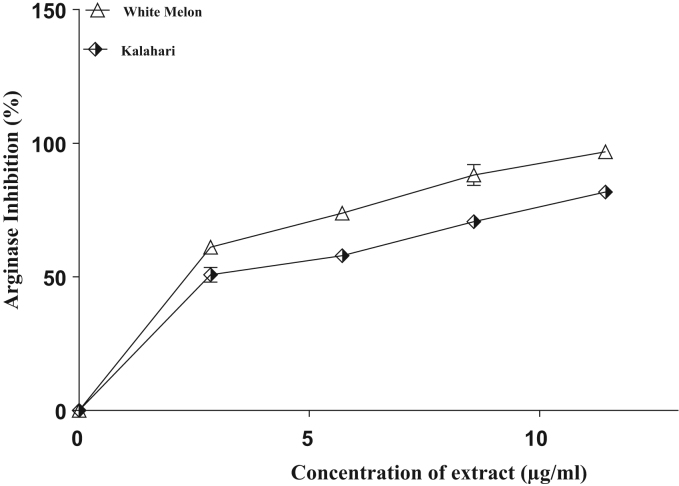
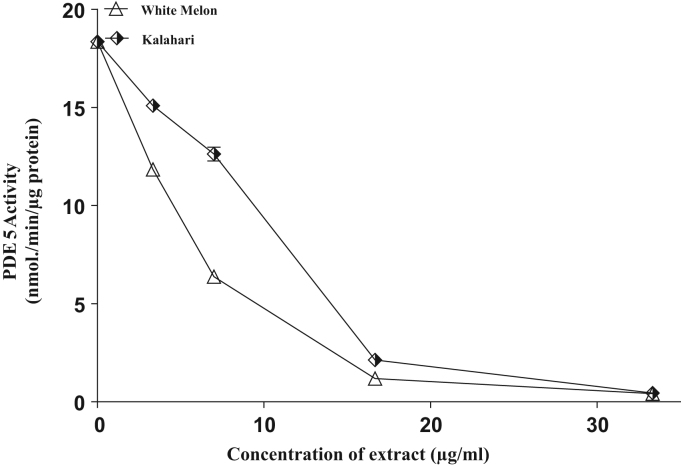
Seed extracts from White melon and Kalahari inhibited arginase and PDE-5 activities in a concentration dependent manner (Fig. 1, Fig. 2). The IC50 values (Table 2) revealed that white melon (IC50 = 4.9 µg/ml) had higher inhibitory ability than kalahari (6.1 µg/ml) and more so, the phosphdiesterase-5 inhibitory ability in (Fig. 2) and the IC50 in (Table 2) revealed a significant (p˂0.05) difference with (52.3 µg/ml) kalahari having lower inhibitory ability than (50.6 µg/ml) white melon.
Fig. 1.
Interaction of extracts from some pumpkin seeds with arginase activity on corpus cavernosum.
Fig. 2.
Interaction of extracts from some pumpkin seeds with phosphodiesterase 5′ activity on corpus cavernosum.
Table 2.
IC50 values for the inhibitions of Arginase and Phosphodiesterase 5̕ activities of white melon and kalahari on corpus cavernosum (μg/ml).
| Sample | Arginase | PDE 5′ |
|---|---|---|
| White melon | 4.9 ± 1.1c | 50.6 ± 0.1a |
| Kalahari | 6.1 ± 2.2b | 52.3 ± 0.2b |
Values represent means of triplicate; values with same letters along the same row are not significantly (P>0.05) different. PDE-5̕- Phosphodiesterase-5̕.
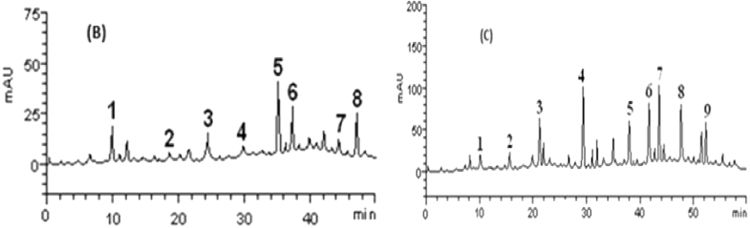
Furthermore, Table 1 and Fig. 9 represent the HPLC-DAD analysis of the phenolic composition of white melon and kalahari. The results showed the presence of gallic acid (tR = 9.87 min, peak 1), caffeic acid (tR = 18.36 min, peak 2), p-coumaric acid (tR = 24.05 min, peak 3), rutin (tR = 29.78 min, peak 4), quercitrin (tR = 44.31 minpeak 5), quercetin (tR = 37.19 min, peak 6), luteolin (tR = 47.68 min, peak 7) kaempferol (tR 35.62 min, peak 8), and catechin (tR = 16.59 min, peak 9). Moreover, Table 4 represents the amino acids composition of the seeds as revealed by GC/MS where aspartate and arginine had the highest amino acids content (Table 2).
Table 1.
Phenolics composition of some tropical pumpkin seeds (mg/ml) as revealed by HPLC-DAD Analysis.
| White melon Kalahari | Gallic acid | |
|---|---|---|
| Gallic acid | 2.37 ± 0.02a | 0.85 ± 0.02a |
| Caffeic acid | 0.45 ± 0.01b | 2.43 ± 0.01b |
| p-Coumaric acid | 2.29 ± 0.01a | 3.76 ± 0.03c |
| Rutin | 0.46 ± 0.03b | 2.41 ± 0.01b |
| Quercitrin | 10.81 ± 0.02c | 3.05 ± 0.02d |
| Quercetin | 2.38 ± 0.01d | 3.79 ± 0.03c |
| Luteolin | 7.19 ± 0.02d | 3.89 ± 0.02b |
| Kaempferol | 0.67 ± 0.03e | 3.12 ± 0.01d |
| Catechin | – | 0.89 ± 0.01a |
Results are expressed as mean ± standard deviations (SD). Averages followed by different letters differ by Tukey test at p < 0.05.
Fig. 9.
Representative HPLC–DAD chromatography profile of phenolic constituents of (B) Cucumeropsis mannii naudin and (C) Citrullus lanatus. Peak assignment: (1) gallic acid, (2) caffeic acid, (3) p-coumaric acid, (4) rutin, (5) luteolin, (6) quercetin, (7) quercitrin, (8) kaempferol and (9) catechin.
Table 4.
Amino acid composition of some pumpkin seed extracts as revealed by GC/MS.
| Amino Acids |
Samples amounts (g/100 g) |
|
|---|---|---|
| White melon | Kalahari | |
| Glycine | 2.38254 | 3.27133 |
| Alanine | 5.16324 | 2.35199 |
| Serine | 2.54555 | 2.54158 |
| Proline | 2.86644 | 2.34655 |
| Valine | 1.43196 | 2.85824 |
| Threonine | 3.47532 | 1.84888 |
| Isoleucine | 4.38583 | 2.26368 |
| Leucine | 4.26095 | 3.85557 |
| Aspartate | 14.23419 | 7.08283 |
| Lysine | 9.20556 | 1.86174 |
| Methionine | 5.33269 | 1.04466 |
| Glutamate | 2.26334 | 4.62755 |
| Phenylalanine | 3.57870 | 3.27697 |
| Histidine | 2.21366 | 1.28078 |
| Arginine | 9.05577 | 6.86265 |
| Tyrosine | 2.40959 | 1.99547 |
| Cysteine | 1.14744 | 1.23942 |
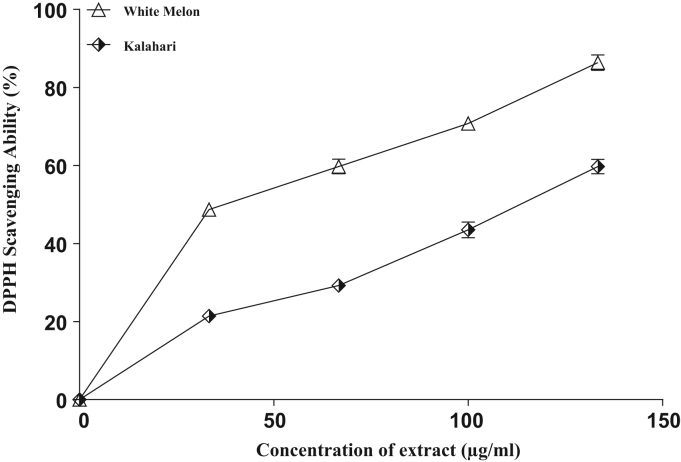
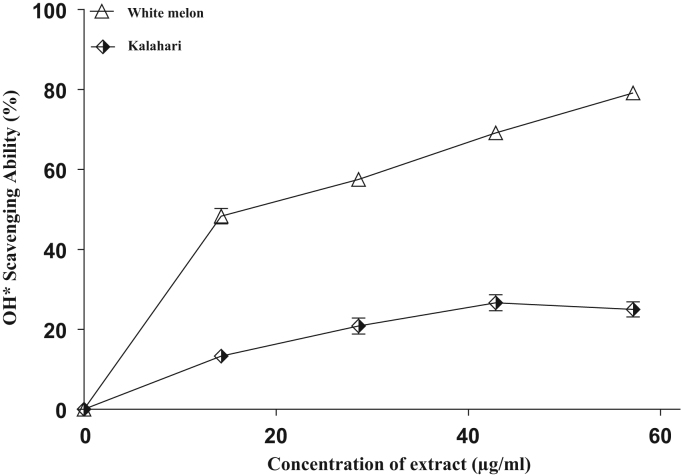
The DPPH free radical scavenging ability of the extracts is presented in (Fig. 3) and their IC50 in (Table 3). The results revealed that the seed extracts scavenged DPPH* in a concentration-dependent manner. However, extracts from white melon had the highest scavenging ability (68.9 µg/ml) compared with the extracts of kalahari with the scavenging abilities of (111.4 µg/ml) at the highest concentration tested. More so, the hydroxyl radical (OH*) scavenging abilities of the seeds extracts are presented in (Fig. 4) and their IC50 in Table 3. The results revealed that the extracts scavenged OH* produced from the decomposition of deoxyribose in Fenton reaction. Nonetheless, the OH* scavenging ability (31.2 µg/ml) of white melon extract was significantly higher (p<0.05) than that of (91.2 µg/ml) kalahari.
Fig. 3.
DPPH free radical scavenging ability of extracts from some pumpkin seeds.
Table 3.
IC50 values for the DPPH and OH* Scavenging abilities, FeSO4 and SNP induced MDA production in rat's corpus cavernosum in vitro. CU2+ and FeSO4 chelating abilities of white melon and kalahari (μg/ml).
| White melon | kalahari | |
|---|---|---|
| DPPH | 68.9 ± 0.9c | 111.4 ± 0.9a |
| FENTON | 31.2 ± 0.1c | 91.2 ± 2.4a |
| MDA inhibiting ability | ||
| FeSO4 | 110.9 ± 3.7c | 189.4 ± 1.9a |
| SNP | 109.2 ± 1.0b | 126.9 ± 1.0b |
| Metal chelating ability | ||
| CU2+ | 19.1 ± 2.2a | 20.2 ± 1.1b |
| FeSO4 | 18.3 ± 0.1c | 38.5 ± 1.3a |
Values represent means ± standard deviation of triplicate readings. Values with the same superscript letter on the same row are not significantly different (p > 0.05).
Fig. 4.
OH* radical scavenging abilities of extracts from some pumpkin seeds.
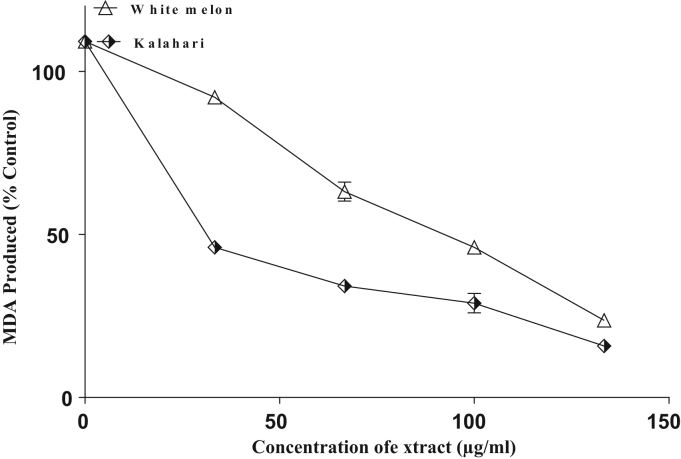
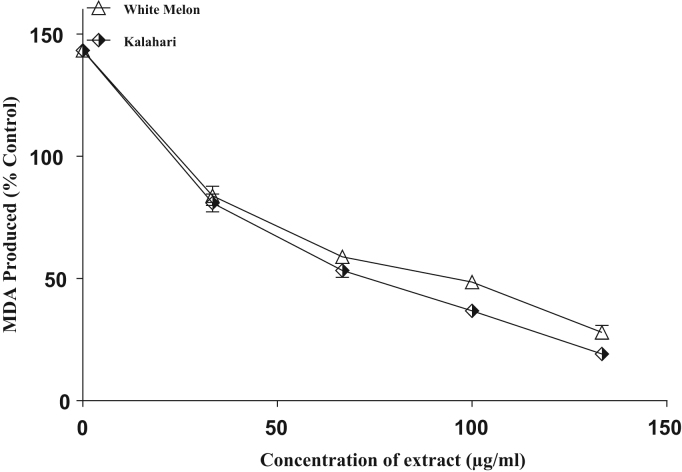
The incubation of rat's corpus cavernosum in the presence of Fe2+ caused a significant increased in the MDA content (Fig. 5). Nevertheless, the samples inhibited malondialdehyde production in a concentration dependent manner, with (IC50 = 110.9 µg/ml) white melon having significantly (p < 0.05) higher inhibitory ability than (IC50 = 189.4 µg/ml) kalahari. Similarly, the incubation of rat's corpus cavernosum in the presence sodium nitroprusside (SNP) caused a significant increase in the MDA content (Fig. 6) with (IC50 = 109.2 µg/ml) white melon having the highest inhibitory effect on the sodium nitroprusside-induced lipid peroxidation.
Fig. 5.
Inhibition of Fe2+ induced lipid peroxidation in rat's corpus cavernosum by extracts of some pumpkin seeds.
Fig. 6.
Inhibition of SNP induced lipid peroxidation in rat's corpus cavernosum by extracts of some pumpkin seeds.
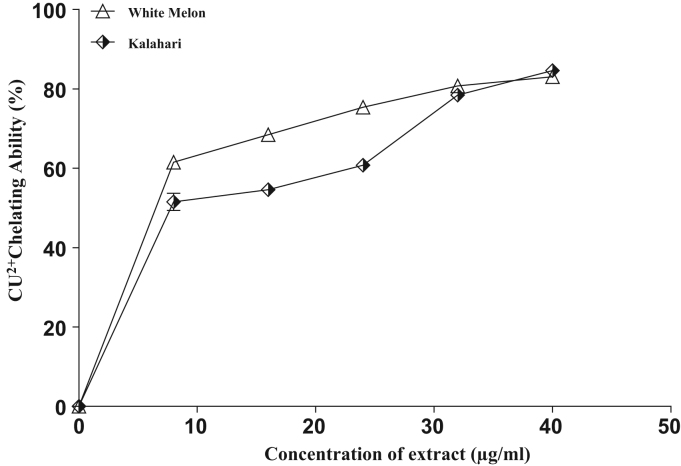
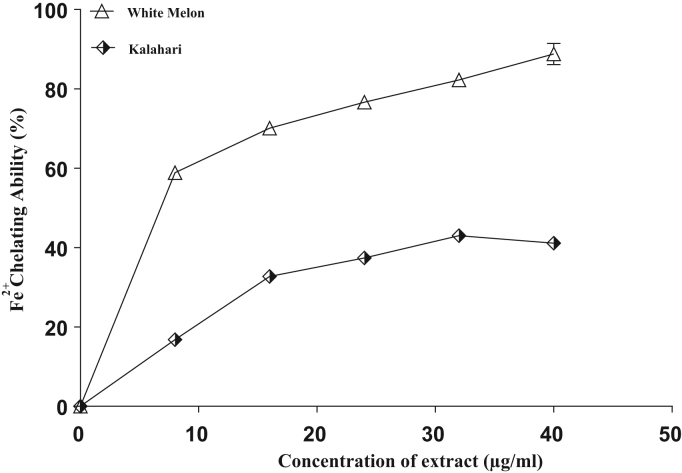
Furthermore, as shown in Fig. 7 and Table 3, (IC50 = 19.1 µg/ml) white melon had significantly (p < 0.05) higher CU2+ chelating ability than (IC50 = 20.2 µg/ml) kalahari. Similarly, Fig. 8 and Table 3 represent Fe2+ chelating ability of extracts from the tested seeds. The result revealed a significant (P < 0.05) difference in the chelating ability of the extracts. However, (38.5 µg/ml) kalahari had lower Fe2+ chelating abilities than (18.3 µg/ml) white melon as revealed by the IC50 (Table 3).
Fig. 7.
Cu2+ Chelating ability of extracts from some pumpkin seeds in-vitro.
Fig. 8.
Fe2+ Chelating ability of extracts from some pumpkin seeds in-vitro.
5. Discussion
The recent identification of some physiologically relevant amino acids in the management of erectile dysfunction [27] is worthy of exploration. L-arginine is important in the expression of endothelial nitric oxide synthase (eNOS) of the corpus cavernosum which promotes nitric oxide generation [8]. Moreover, previous studies have reported that dietary L-arginine supplementation as well as acute infusion of L-arginine results in improved nitric oxide (NO) release and increased endothelium-dependent vasodilatation in the corpus cavernosum [20]; this could probably be by augumenting the arginine pool needed by eNOS as substrate. Therefore, the high arginine content of the selected tropical pumpkin seeds identifies them as dietary arginine sources; and this may be responsible in part to the erectile function promoting effect of the pumpkins as reported in folklore (Fig. 9)
More so, the observed high L-aspartate content of the selected pumpkin seeds as compared to other amino acids (Table 4) also suggests possible erectogenic potential. Previous findings have established the role of D-aspartic acid in the promotion of erectile function [43]. D-aspartic acid (D-Asp) is an endogenous amino acid found in the neuroendocrine tissues (pituitary gland and testes) of vertebrates where it has a role in the regulation of the release and synthesis of luteinizing hormone (LH) and testosterone [13]; key factors modulating erectile function. D-asp is synthesized by a D-aspartate racemase which convert L-aspartic acid into D-Asp [13]. Hence, dietary source of L-aspartate may help in enriching the D-aspartate pool, as its high concentration stimulates LH and testosterone. Compelling evidence suggests that testosterone is involved in the regulation of corporeal expression/activity of NOS isoforms (eNOS and nNOS), thus maintaining an adequate NO supply. Studies performed in orchidectomized animals demonstrated that administration of testosterone or its metabolite 5α-dihydrotestosterone restored erectile responses and NOS bioactivity, increasing NO production in corpus cavernosum and penile arteries [29], [40].
Several vasodilators have been implicated in erectile process, however, nitric oxide (NO) still remain the major vasodilator involved [28], [42]. Nitric oxide (NO) production absolutely depends on the availability of L-arginine to endothelial nitric oxide synthase (eNOS), since eNOS shares L-arginine as a common substrate with arginase [8]; thus establishing a critical competition for the available substrates between arginase and eNOS. Consequenlty, inhibition of arginase activity has been suggested as a practical therapeutic means for boosting L-arginine content as substrate for eNOS and ensuring its availability for eNOS. Hence, the observed arginase inhibitory effect of the selected tropical pumpkin seeds (Fig. 1 and Table 2) suggests an anti-erectile dysfunction property.
Over the years, accumulating evidences has established the role of arginase in erectile pathology [9]. Previous study has revealed an increase in the arginase activity in the corpus cavernosum induced by hyperglycemia and aging [39]. As sexual dysfunction is related to endothelial dysfunction and decreased availability of L-arginine, inhibition of arginase in the corpus cavernosum could be one of the mechanisms through which enhanced penile eNOS activity and increase cyclic guanosine monophosphate (cGMP) levels can be achieved, thus restoring endothelial-derived nitric oxide vasodilation and erectile function [6]. Furthermore, the inhibitory effect of the tropical pumpkin seeds might be related to their phenolic constituents (Table 1); as white melon with the highest phenolic content had the highest arginase inhibitory effect. Meanwhile, previous findings have revealed the arginase inhibitory properties of phenolic compounds and suggest plant foods as potential sources of arginase inhibitor [3].
In the corpus cavernosum and other penile tissues, the actions of cGMP are curtailed primarily by phosphodiesterase-5 (PDE-5). PDE-5 is a critical enzyme of the NO/cGMP signaling which terminate NO-induced cGMP-mediated vasorelaxation, restoring basal smooth muscle tone and penile flaccidity. Hence, the level of cGMP in the corpus cavernosum is a function of the rate of its synthesis by cyclases and degradation by PDEs. To this end, oral PDE-5 inhibitors (sildenafil citrate, tadalafil and vardenafil) have been developed and their therapeutic effect has been widely acclaimed as a practical approach to the management of ED. PDE-5 inhibitors prolongs the action of cGMP, thereby amplifying the NO signal [1]. However, these synthetic PDE-5 inhibitors come with their known adverse effect which necessitated the search for more natural alternative with little or no side effects.
As observed from this study, both tropical pumpkin seeds tested exhibited PDE-5 inhibitory effect (Fig. 2); this is a clear indication that, their diet therapeutic potential as PDE-5 inhibitors is promising. More so, previous studies have identified some novel PDE-5 inhibitors such as phenolics in plant foods [16], and the vasodilating effect of wine-derived phenolic compounds was associated with the inhibition of PDE-5 [15]. Nonetheless, strong associations exist between the PDE-5 inhibitory effect of the tropical pumpkin seeds and their phenolic contents/constituents, suggesting that phenolics compounds might be responsible for their PDE-5 inhibitory action. Inhibition of PDE-5 will enhance the accumulation of cGMP/cAMP which triggers reduction in cytosolic Ca2+ levels inducing vasodilating effect and stimulating erection.
A correlation between ED and endothelial function has been established [46], therefore, alteration of the normal functioning of the endothelial layer of the corpus cavernosum could result in ED. Studies have shown that endothelial cells are vulnerable to free radical damage [23], [24]; hence, improved antioxidant level could help counteract this free radical mediated damage to the endothelial cells. Moreover, previous findings have revealed the role of oxidative stress in the ethiology of ED and improved antioxidant system would be desirable in the management of ED. Meanwhile, one practical way to improve the antioxidant status of the body is through the consumption of plant foods rich in phenolic compounds [36]. This is owing to the fact that experiments have shown that the antioxidant properties of plant foods are a function of their phenolic contents [10]. And phenolic-rich plant foods have demonstrated potent antioxidant properties in both in vitro and in vivo experimental models [35].
Therefore, the strong antioxidant properties of the tropical pumpkin seeds observed in this study as exemplified by their reducing power, radical (ABTS˙+, DPPH, and OH) scavenging abilities and their metal (Cu2+ and Fe2+) chelating properties suggests that they might be good dietary sources of antioxidant. Quercitrin and luteolin (common phenolics in the pumpkin seeds) have been reported to possess strong antioxidant potentials [37]. Hence, it is probable that quercitrin and luteolin may contribute to the antioxidant activities observed from the tropical pumpkin seeds. In addition, the observed inhibitory effect of the pumpkin seeds on some pro-oxidants (Fe2+ and sodium nitroprusside, SNP) induced lipid peroxidation in isolated corpus cavernosum tissue homogenates suggests their protective capability against oxidative stress to the endothelial layer of this tissue, thus preventing the initiation of ED. Peroxidation of lipid is associated with a loss of membrane fluidity and an increase of membrane permeability, causing a decrease in physiological performance [4].
The chemical structure of iron and its capacity to drive a one electron reaction makes it a key factor in the formation of radicals [18]. Fe2+ takes part in the Fenton reaction to generate OH radical which could react with important macromolecule or damage critical cell membrane leading to the pathogenesis of ED. Furthermore, several reactive oxygen species (ROS) could induce oxidative cell damage and reduce bioavailability of nitric oxide (NO), culminating in decreased cGMP levels and impaired cavernosal smooth muscle relaxation. This could be observed in SNP being an NO releasing drug could rapidly disintegrate to form NO, which when in excess could rapidly react with superoxide to produce peroxynitrite, though short-lived but very reactive radical that could damage several important biologically relevant macromolecules, thus promoting ED. This, it is suggested that the ability of the pumpkin seeds to inhibit lipid peroxidation induced by these pro-oxidants could be through Fe2+ chelation, scavenging of radicals such as OH radical and peroxynitrite; and this is consistent with earlier studies where inhibitory effects of plant extracts/constituents against pro-oxidant induced lipid peroxidation in selected animal tissues was established [36]. Furthermore, Fe2+ chelating ability of some phenolic compounds have been substantiated in previous studies [36].
Nevertheless, it is worth noting that white melon with the highest phenolic content/constituents exhibited the highest antioxidant properties while kalahari with the least phenolic content/constituents had the least. This is consistent with previous studies that have established strong correlation between the phenolic contents and antioxidant properties of plant foods [10]. Furthermore, the metal chelating (Fe2+ and Cu2+) ability of the tropical pumpkin seeds (Fig. 7, Fig. 8) could be one of the mechanisms underlying the inhibitory effect of these seeds on key enzymes relevant to ED management. Since most of these enzymes use these biologically relevant transition metals in their active sites as cofactor for catalysis, chelation of these metals by other compounds would elicit an inhibitory effect on the enzyme activity. Therefore, with phenolics as known metal chelators, it would not be a coincidence to realize that white melon with the highest phenolic content/constituents exhibited the highest inhibitory effect on critical enzymes considered under this study.
6. Conclusion
The inhibitory abilities of these pumpkin seeds on phosphodiesterase – 5 and arginase present their erectogenic potentials. This could however, be as a result of the high content of some physiologically relevant amino acids in addition to their phenolic contents. Therefore, their erectile promoting benefit could be proposed to be a function of their phytoconstituents.
Conflicts of Interests
The authors declare no conflict of interests on this paper
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.08.001.
Appendix A. Transparency document
Supplementary material
References
- 1.Andersson K.E. Pharmacology of erectile function and dysfunction. Am. J. Clin. Urol. 2001;28:233–247. doi: 10.1016/s0094-0143(05)70134-8. [DOI] [PubMed] [Google Scholar]
- 2.Aytac I.A., McKinlay J.B., Krane R.J. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. Br. J. Urol. Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Bagnost T., Berthelot A., Bouhaddi M., Laurant P., André C., Guillaume Y. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-L-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. J. Hypertens. 2008;26(6):1110–1118. doi: 10.1097/HJH.0b013e3282fcc357. [DOI] [PubMed] [Google Scholar]
- 4.Balu M., Sangeetha P., Haripriya D., Panneerselvam C. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. J. Neurosci. 2005;383:295–300. doi: 10.1016/j.neulet.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 5.Belle N.A.V., Dalmolin G.D., Fonini G., Rubim M.A., Rocha J.B.T. Polyamines reduces lipid peroxidation induced by different prooxidant agents. Brain Res. 2004;1008:245–251. doi: 10.1016/j.brainres.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Bivalacqua T.J., Burnett A.L., Hellstrom W.J., Champion H.C. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am. J. Physiology. 2007;292(H13):40–51. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- 7.Boligon A.A., Freitas R.B., Brum T.F., Waczuk E.P., Klimaczewski C.V., Ávila D.S., Athayde M.L., Bauermann L.F. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm. Sin. B. 2014;4(5):358–367. doi: 10.1016/j.apsb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher J.L., Moali C., Tenu J.P. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol. Life Sci. 1999;55:1015–1028. doi: 10.1007/s000180050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christ G.J. Gap junctions and ion channels: relevance to erectile dysfunction. Int. J. Impot. Res. 2000;12:S15. doi: 10.1038/sj.ijir.3900573. [DOI] [PubMed] [Google Scholar]
- 10.Chu Y., Sun J., Wu X., Liu R.H. Antioxidant and anti-proliferative activity of common vegetables. J. Agric. Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 11.Cox J.D., Kim N.N., Traish A.M., Christianson D.W. Arginase-boronic acid complex highlights a physiological role in erectile function. Nat. Struct. Biol. 1999;6:1043–1047. doi: 10.1038/14929. [DOI] [PubMed] [Google Scholar]
- 12.D'Aniello A., Di Cosmo A., Di Cristo C., Annunziato L., Petrucelli L., Fisher G.H. Involvement of D-aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996;59:97–104. doi: 10.1016/0024-3205(96)00266-4. [DOI] [PubMed] [Google Scholar]
- 13.D'Aniello A. D-Aspartic acid: an endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007;53:215–234. doi: 10.1016/j.brainresrev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.DeBoer B.J., Bots M.L., Lycklama A.A.B., Nijeholt A., Moors J.P.C., Pieters H.M., Verheij T.J.M. Erectile dysfunction in primary care: prevalence and patientcharacteristics. the enigma study. Int. J. Impot. Res. 2004;16(4):358–364. doi: 10.1038/sj.ijir.3901155. [DOI] [PubMed] [Google Scholar]
- 15.Dell’Agli M., Busciala A., Bosisio E. Vascular effects of wine polyphenols. J. Cardiovasc. Res. 2005;63:593–602. doi: 10.1016/j.cardiores.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Dell’Agli M., Bush P.A., Dorey F.J. Polyphenols in orange peels (citrus specie) enhances relaxation corpus cavernosum tissue: implications to erectile physiology and dysfunction. Can. J. Pharmacol. 2006;73(12):1714–1726. [Google Scholar]
- 17.Feldman H.A.I., Goldstein D.G., Hatzichristou R.J., Krane McKinlay J.B. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J. Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 18.Fraga C.G., Oteiza P.I. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180:23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- 19.Artés-Hernández Francisco, Pedro A. Robles low UV-C illumination for keeping overall quality of fresh-cut watermelon post harvest. Biol. Technol. 2010;55:114–120. [Google Scholar]
- 20.Gur S., Ozturk B., Karahan S.T. Impaired endothelium-dependent and neurogenic relaxation of corpus cavernosum from diabetic rats: improvement with L-arginine. Urol. Res. 2000;28:14–19. doi: 10.1007/s002400050003. [DOI] [PubMed] [Google Scholar]
- 21.Gyamfi M.A., Yonamine M., Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. General. Pharmacol. 1999;32:661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B., Gutterdge J.M.C. Formation of a thiobarbituric-acid- reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981;128:347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- 23.Jeremy J.Y., Angelini G.D., Khan M. Platelets, oxidant stress and erectile dysfunction: an hypothesis. J. Cardiovasc. Res. 2000;46:50–54. doi: 10.1016/s0008-6363(00)00009-2. [DOI] [PubMed] [Google Scholar]
- 24.Jones R.W., Rees R.W., Minhas S., Ralph D., Persad R.A., Jeremy J.Y. Oxygen free radicals and the penis. Expert Opin. Pharmacother. 2002;3:889–897. doi: 10.1517/14656566.3.7.889. [DOI] [PubMed] [Google Scholar]
- 25.Kaysen, Strecker Increased arginase activity levels caused by nitric oxide synthase dysfunction. N. Engl. J. Med. 1973;323:1234–1238. [Google Scholar]
- 26.Kelly S.J., Butler L.G. Enzymatic hydrolysis of phosphonate esters. Reaction mechanism of intestinal 5′- nucleotide phophodiesteras. Biochemistry. 1977;16(6):1102–1104. doi: 10.1021/bi00625a011. [DOI] [PubMed] [Google Scholar]
- 27.Lamm S., Couzens G.S. Prelox® for sexual satisfaction, a clinical trial study report. Eur. Bull. Drug Res. 2002;11:6–9. [Google Scholar]
- 28.Leite R., Giachini F.R., Carneiro F.S., Nunes K.P., Tostes R.C., Webb R.C. Targets for the treatment of erectile dysfunction: is NO/cGMP still the answer? Recent Pat. Cardiovasc. Drug Disco. 2007;2:119–132. doi: 10.2174/157489007780832579. [DOI] [PubMed] [Google Scholar]
- 29.Lugg J.A., ajfer J.R., Gonzalez-Cadavid N.F. Dihydrotestosterone is the active androgen in the maintenance of nitric oxide-mediated penile erection in the rat. J. Endocrinol. 1995;136(4):1495–1501. doi: 10.1210/endo.136.4.7534702. [DOI] [PubMed] [Google Scholar]
- 30.McMahon C.G. Dapoxetine: a new option in the medical management of premature ejaculation. Ther. Adv. Urol. 2012;4(5):233–251. doi: 10.1177/1756287212453866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon C.G. Dapoxetine: a new option in the medical management of premature ejaculation. Ther. Adv. Urol. 2012;4(5):233–251. doi: 10.1177/1756287212453866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Faso honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–587. [Google Scholar]
- 33.Minnoti G., Aust S.D. An investigation into the mechanism of citrate Fe2+ dependent lipid peroxidation. Free Radic. Biol. Med. 1987;3:379–387. doi: 10.1016/0891-5849(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 34.NIH Consensus Conference Impotence. NIH consensus development panel on impotence. J. Am. Med. Assoc. 1993;270:83–89. [PubMed] [Google Scholar]
- 35.Oboh G., Akindahunsi A.A. Change in the ascorbic acid, total phenol and antioxidant activity of some sun-dried green leafy vegetables in Nigeria. Nutr. Health. 2004;18:29–36. doi: 10.1177/026010600401800103. [DOI] [PubMed] [Google Scholar]
- 36.Oboh G., Rocha J.B.T. Antioxidant in foods: a new challenge for food processors. In: Panglossi H.V., editor. Leading Edge Antioxidants Research. Nova Science Publishers Inc; New York US: 2007. pp. 35–64. [Google Scholar]
- 37.Petersen R.W., Simmonds H.K. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinnensis. Afr. J. Food, Agric. Nutr. Dev. 2002;16:10214–10226. doi: 10.3390/molecules161210214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puntel R.L., Nogueira C.W., Rocha J.B.T. Krebs cycle intermediates modulate thiobarbituric reactive species (TBARS) production in rat brain in vitro. Neurochem. Res. 2005;30:225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 39.Sakai Y., Masuda H., Kihara K., Kurosaki E., Yamauchi Y., Azuma H. Involvement of increased arginase activity in impaired cavernous relaxation with aging in the rabbit. J. Urol. 2004;172:369–373. doi: 10.1097/01.ju.0000121691.06417.40. [DOI] [PubMed] [Google Scholar]
- 40.Schirar A., Onnefond C.B., Meusnier C., Devinoy E. Androgens modulate nitric oxide synthase messenger ribonucleic acid expression in neurons of the major pelvic ganglion in the rat. J. Endocrinol. 1997;138(8):3093–3102. doi: 10.1210/endo.138.8.5310. [DOI] [PubMed] [Google Scholar]
- 41.Singleton V.L., Orthofor R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Method Enzymol. 1999;299:152–178. [Google Scholar]
- 42.Toda N., Ayajiki K., Okamura T. Nitric oxide and penile erectile function. Pharmacol. Ther. J. 2005;106:233–266. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Topo E., Soricelli A., D'Aniello A., Ronsini S., D'Aniello G. The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. J. Reprod. Biol. Endocrinology. 2009;7(120):2–11. doi: 10.1186/1477-7827-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres-Fuentes W.P., Benson G.S., McConnell J. Role of copper and oxidative stress in cardiovascular diseases. Ann. Biol. Res. 2011;1(3):158–173. [Google Scholar]
- 45.Yativ M., Harary I., Wolf S. Sucrose accumulation in watermelon fruits: genetic variation and biochemical analysis. J. Plant Physiol. 2010;167:589–596. doi: 10.1016/j.jplph.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Yavuzgil O., Kaya C., Borgquist R. Endothelial function in patients with vasculogenic erectile dysfunction. Int. J. Cardiol. 2005;34:235–238. doi: 10.1016/j.ijcard.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Zar J.H. Prentice-Hall Inc.; USA: 1984. Biostatistical Analysis; p. 620. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material