Abstract
An appropriate loading control is critical for Western blot analysis. Housekeeping proteins (HKPs), such as β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-tubulin, are commonly used to normalize protein expression. But HKP expression can be impacted by certain experimental conditions, such as ischemic myocardial infarction. This study was undertaken to look for an appropriate loading control for western blot analysis of ischemic myocardium. Myocardial ischemic infarction was induced by left anterior descending coronary artery (LAD) ligation in Rhesus monkeys and C57BL/6 mice. The heart tissue samples from different areas and time points after surgery were subjected to western blot or gel staining. The level of β-actin, GAPDH, β-tubulin, and total protein were tested. The total protein level was consistent in all groups, whereas the protein level of β-tubulin and β-actin were different in all groups. However, the protein level of GAPDH was stable in the Rhesus monkey model. We concluded that total protein was the most appropriate internal control in different stages of myocardial ischemic disease of various animal models. GAPDH is a reliable internal control only for ischemic myocardium of Rhesus monkey.
Abbreviations: HKP, housekeeping protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LAD, left anterior descending coronary artery; H&E, hematoxylin and eosin; EDTA, ethylene diamine tetraacetic acid; SDS, sodium dodecyl sulfate; PAGE, polyacrylamide gel electrophoresis; TBS, Tris-buffered saline; TBST, TBS with Tween; PVDF, polyvinylidene fluoride; CBB, Coomassie brilliant blue
Keywords: Loading control, Western blot, Housekeeping proteins, Total protein, Myocardial ischemia
Highlights
-
•
Loading control for WB has been studied in animal models of myocardial ischimia.
-
•
GAPDH is a reliable loading control in Rhesus monkeys model of myocardial ischemia.
-
•
Total protein level is the best loading control for western blot in animal models.
1. Introduction
Western blot has been an essential technique for detecting the relative expression of proteins in different samples (such as cells, tissues, etc.) since the first publication in 1979. In order to quantify the expression levels of the target proteins in various samples, loading controls are commonly used as internal standards [1]. The loading controls should have a constant expression level regardless of experimental conditions. Housekeeping proteins (HKPs), such as β-actin [2], glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [3], and β-tubulin [4] have been extensively used as loading controls on the account of their expression levels are generally assumed to be insensitive to the influence of various physiological conditions and treatments. However, studies have shown that expression levels of these HKPs were varied in some experimental conditions or diverse samples [2], [4], [5], [6]. Under these circumstances, loading controls should be cautiously selected.
Ischemic heart disease is typically caused by blockage of coronary artery, which leads to the loss of vital components in the heart, such as cardiomyocytes, resulting in myocardial infarction and eventual cardiac dysfunction or heart failure [7]. The cell type and proteins expressed in the ischemic heart are usually different among various stages after the onset of disease. This makes the selection of loading control for western blot analysis of the ischemic tissue a challenge. In the present study, a mouse model was used for its common employment in the study, and a Rhesus monkey model was used for its similarity to human disease [8], [9], [10].
In our study, we found that the appropriate loading control for western blot analysis of ischemic myocardium in Rhesus monkey and mouse models of ischemic heart disease is total protein level. And it can be applied practically in other studies related to ischemic heart disease.
2. Materials and methods
2.1. Animals
Male Rhesus monkeys, aged 2–3 years and weighed 4.5–6.0 kg, were acclimatized to the laboratory conditions at least one month in the Association for Assessment and Accreditation of Laboratory Animal Care accredited facility.
Male C57BL/6 mice, aged 7–12 weeks and weighed 20–30 g, were obtained from Beijing Vital River Experimental Animal Breeding and Research Center, a Chinese government-accredited experiment animal breeding and research center.
All animal procedures according with the Institutional Animal Care and Use Committee (IACUC) of Sichuan University West China Hospital follows the guidelines of the US National Institutes of Health.
2.2. Induction of myocardial infarction
As described in our previous studies [11], [12], all Rhesus monkeys were divided into three groups: early stage of myocardial ischemia (2 h after surgery, n = 2, 24 h after surgery, n = 2), myocardial infarction (4 months after surgery, n = 4), and sham-operated controls (n = 4). Rhesus monkeys in the former two groups were subjected to left anterior descending coronary artery (LAD) ligation to induce myocardial ischemic infarction. The sham-operated controls were subjected to the same surgical procedure except for the LAD occlusion and ligation. The number of Rhesus monkey sample was very limited.
As described in our previous studies [13], [14], [15], C57BL/6 mice (n = 18) were subjected to LAD ligation to induce myocardial ischemia. The sham-operated controls (n = 8) were subjected to the same surgical procedure except for the LAD occlusion and ligation.
2.3. Tissue preparation
For early stage of myocardial ischemia group of Rhesus monkeys, 2 h (n = 2) or 24 h (n = 2) after surgery, the animals were sacrificed to obtain heart tissue respectively. For myocardial infarction group and sham-operated controls of Rhesus monkeys, 4 months after surgery, the animals were sacrificed to harvest heart tissue. As for mice, 1 d (n = 6), 4 d (n = 6), 7 d (n = 6) after surgery, they were sacrificed to harvest the heart tissues. Ischemia or infarct area was distinguished from remote area by its pale appearance.
All heart tissues were stored in liquid nitrogen for western blot. Another portion was fixed with 4% paraformaldehyde and embedded in paraffin as previously described [16], and then sectioned at 4 mm intervals. The sections were stained with hematoxylin and eosin (H&E) to observe the structure, and with Sirius red to observe collagen deposition [17].
2.4. Western blot analysis
Heart tissues were lysed in RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40%, and 0.1% SDS), supplemented with 1% complete EDTA-free protease inhibitor cocktail (Roche Diagnostics, Mannhein, Germany). The supernatant was separated by centrifugation at 12,000 g for 15 min at 4 °C and total protein concentration was detected by BCA protein assay kit (Thermo Pierce, USA) using bovine serum albumin as standard. Protein samples were mixed with 5× loading buffer, denatured in boiling water for 10 min, then cooled and stored at −20 °C for further study. Equal amounts of protein from each sample (30 μg) and pre-stained protein marker (26616, SM0671; 26619, SM1811, Thermo, USA) were separated by 10% SDS-PAGE gel, and transferred to a polyvinylidene fluoride membrane (PVDF, Bio-Rad Laboratories) under electrophoretical conditions (300 mA, 2 h). Membranes were blocked in Tris-buffered saline with Tween solution (TBST, 50 mM Tris-HCl, PH 8.0, 150 mM NaCl, and 0.1% Tween-20) containing 5% nonfat dry milk for 1 h at room temperature. The blots were then incubated with primary antibodies anti-β-actin, GAPDH, and β-tubulin, (at 1:1000 dilution, ZSGB-BIO, China) overnight at 4 °C. The following day, blots were rinsed in TBST for 6 times, 5 min each, and incubated for 1 h at 37 °C with horseradish peroxidase conjugated rabbit anti mice secondary antibodies (at 1:2000 dilution, Sigma A9044, USA). After being rinsed in TBST for 6 times, 5 min each and in Tris-buffered saline (TBS) for 5 min, target proteins were visualized as black bands by using chemiluminescenece horseradish peroxidase substrate (Millipore Corporation, USA), and analyzed by densitometry using Fusion-Capt Software (VILBER LOURMAT, France).
2.5. Total protein staining of gel
Right after electrophoresis, gels were incubated in Coomassie brilliant blue solution (CBB, 0.1% R250, 25% isopropanol, 10% acetic acid) for 45 min and de-stained in de-stain solution (10% acetic acid, 7% ethanol) overnight, and finally washed with double distilled water. Stained gels were imaged on the Fusion scanner and analyzed by Fusion-Capt Software (VILBER LOURMAT, France).
2.6. PVDF membrane staining
After immunodetection, PVDF membrane was washed by parsing solution (0.19% Glycine, 1% SDS, pH 2.0) for 2 h, and stained by CBB solution for 5 min and de-stained in 50% methanol solution for 20 min. At last, they were washed with double distilled water. Stained PVDF membrane were imaged directly on Fusion scanner and analyzed by Fusion-Capt Software (VILBER LOURMAT, France).
2.7. Statistical analysis
All data are presented as mean ± SD. SPSS for Windows (USA) was applied to perform the statistical processing. One-way ANOVA was used for statistical evaluation for sham and different areas of the myocardial ischemic group, P < 0.05 was considered as statistically significant.
3. Results
3.1. Animal models of ischemic heart disease
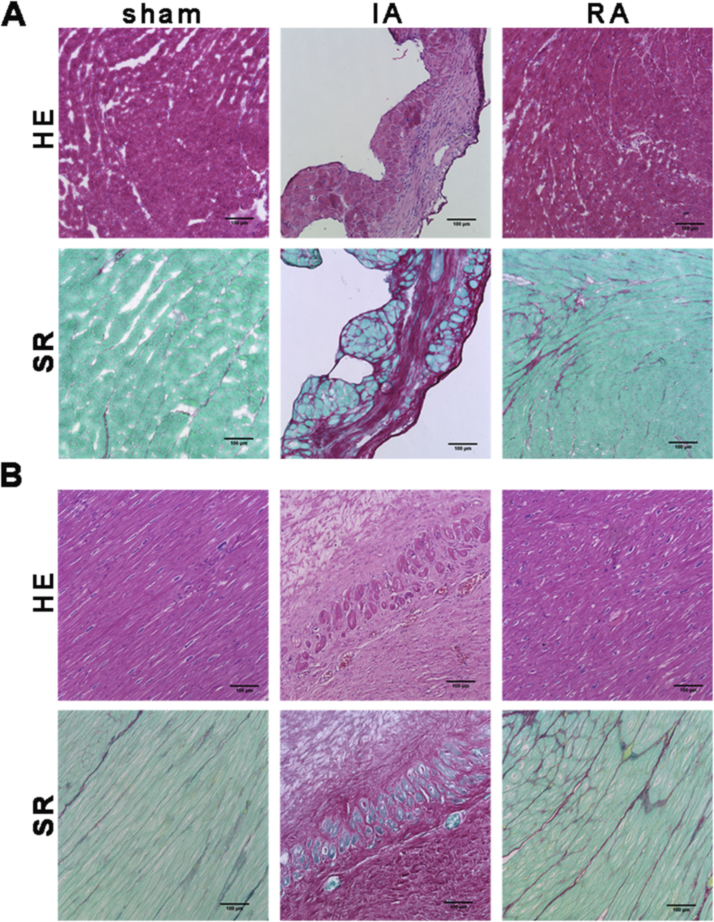
H&E staining and Sirius red staining of transversely sectioned heart tissues were used to define the successful establishment of ischemic heart disease animal model (Fig. 1A and B). Infarct area was full of thick and wave shaped collagens, which were stained red by Sirius red.
Fig. 1.
H&E and Sirius red staining of heart tissue. (A) H&E and Sirius red staining of transversely sectioned mouse hearts. (B) H&E and Sirius red staining of transversely sectioned Rhesus monkey hearts. The infarct area was full of collagens. Bar = 100 µm. Sham: sham operated control; IA: infarct area; RA: remote area.
3.2. Expression of β-tubulin, β-actin and GAPDH in heart tissues of animal models of myocardial ischemia/ infarction
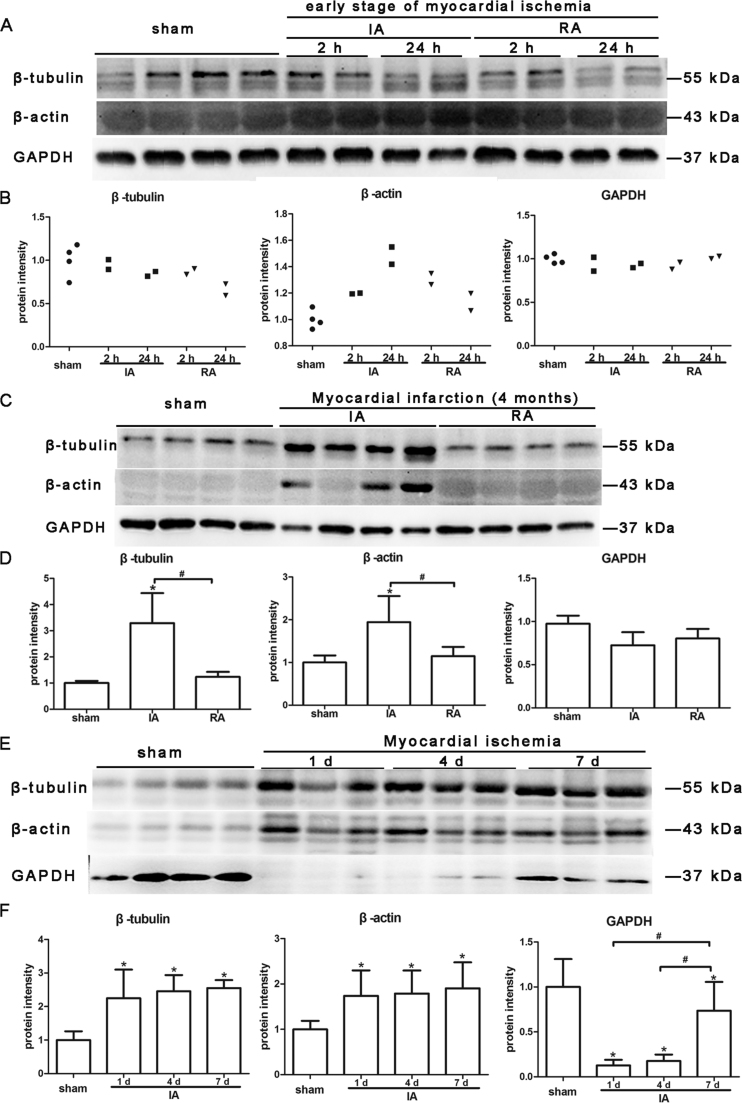
We detected the protein level of β-tubulin, β-actin and GAPDH in heart tissues by western blot. Protein level of β-tubulin was various in the heart tissue during different stages of myocardial ischemia/ infarction Rhesus monkey model. In the early stage of ischemia, protein level of β-tubulin decreased in remote area compared with sham control (Fig. 2A and B). On the contrary, β-tubulin was increased in infarct area of 4-month myocardial infarction (Fig. 2C and D). In mice model, β-tubulin was increased in ischemic area of every group compared with sham-operated control (Fig. 2E and F).
Fig. 2.
Protein levels of β-tubulin, β-actin and GAPDH in the heart tissue from animal model of myocardial ischemia. (A, B) Western blot and quantitative analysis of β-tubulin, β-actin and GAPDH protein levels in heart tissue from Rhesus monkey model of early stages of myocardial ischemia (2 h after surgery, n = 2; 24 h after surgery, n = 2). (C, D) Western blot and quantitative analysis of β-tubulin, β-actin and GAPDH protein levels in heart tissue from Rhesus monkey model of myocardial infarction (4 months after surgery, n = 4). (E, F) Western blot and quantitative analysis of β-tubulin, β-actin and GAPDH protein levels in heart tissue from mouse model of different time point (1 d after surgery, n = 6; 4 d after surgery, n = 6; 7 d after surgery, n = 6). Sham: sham operated control (n = 8); IA: ischemic/ infarct area; RA: remote area. Blots were analyzed by densitometry using Fusion-Capt Software. Results are presented as means ± SD. Three independent experiments were run for each result. *: P < 0.05 versus sham operated control; #: P < 0.05.
Protein level of β-actin was elevated in ischemic monkey heart tissue of early stage (Fig. 2A and B) and 4 months after surgery compared with remote area and sham control (Fig. 2C and D). Moreover, it was also raised in ischemic myocardium of mice of different time points post LAD ligation (Fig. 2E and F).
GAPDH levels were unchanged in the ischemic monkey heart tissue compared with sham control (Fig. 2A - D). But in the mouse model, GAPDH was decreased in the ischemic myocardium from all three time points (1 d, 4 d, and 7 d) post-surgery (Fig. 2E and F).
3.3. Total protein level in heart tissues of animal models of myocardial ischemia/ infarction
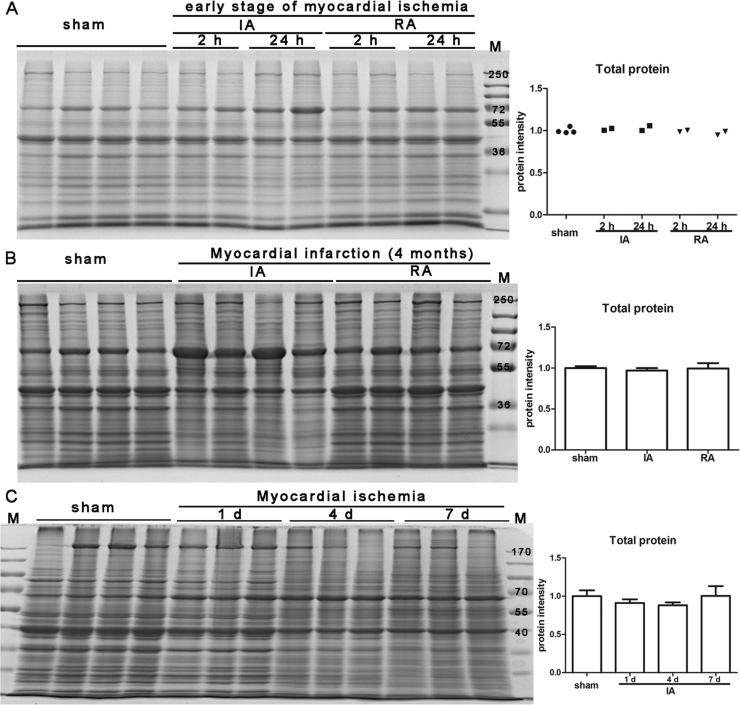
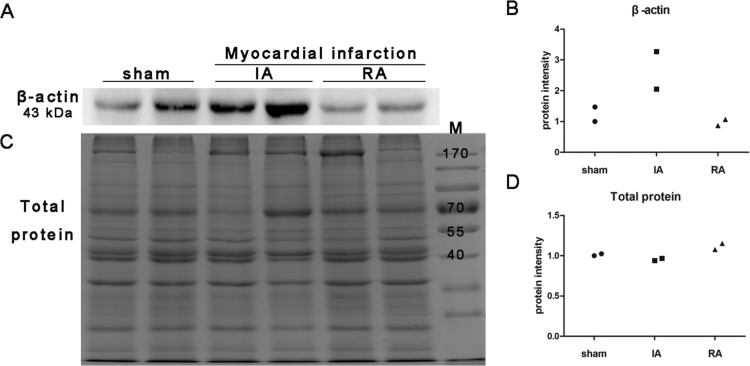
In order to test the level of total protein, we stained the gels with CBB directly after electrophoresis. Total protein level was constant in heart tissues of different animal models of myocardial ischemia/ infarction (Fig. 3), which indicates that total protein is a good loading control for western blot in our model. Next, we stained PVDF membrane by CBB after immunoblotting instead of gel to reduce the possible influence on the immunodetection of target proteins. Total protein level was also constant on PVDF membrane, and was not influenced by transferring (Fig. 4).
Fig. 3.
Gel staining image of total protein in the heart tissue from animal model of myocardial ischemia. (A, B, C) Gel staining image of total protein in the heart tissue from Rhesus monkey model of early stages of myocardial ischemia (A), Rhesus monkey model of myocardial infarction (B), and mouse model of different time point (C). Quantitative analysis of total protein was shown in the right panel. Sham: sham operated control (n = 4); IA: ischemic/infarct area; RA: remote area; M: pre-stained protein marker. Gels were analyzed by Fusion-Capt Software. Results are presented as means ± SD. Three independent experiments were run for each result. *: P < 0.05 versus sham operated control; #: P < 0.05.
Fig. 4.
Staining of total protein level on blots as a validated loading control for Western blot in the heart tissue from Rhesus monkey model. (A, B) Western blot and quantitative analysis of β-actin in the heart tissue from Rhesus monkey model of myocardial ischemia (4 months after surgery, n = 2). (C, D) Total protein level shown by blot staining, which was washed with parsing solution after β-actin detecting. Sham: sham operated control (n = 2); IA: ischemic area (n = 2); RA: remote area (n = 2); M: pre-stained protein marker. Blots were analyzed by densitometry using Fusion-Capt Software. Three independent experiments were run for each result.
4. Discussion
Since the first use of western blot method as a semi-quantitative analysis of protein in 1979, loading controls were used to normalize the possible errors from the whole process of western blot. An ideal loading control should be abundant in numerous samples and stable to various physiological conditions or treatments. Some HKPs, such as β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-tubulin have been used as loading controls for a long period.
However, it has been reported that HKP expression levels were varied in some experimental conditions or diverse samples. Dittmer et al. showed the expression of β-actin was related to the incubation time and it was not stable in MDA-MB-231 cells [2]. Liu and his colleagues demonstrated that the β-actin level was increased after spinal cord injury [4]. Another example of varied HKP expression levels is β-actin and GAPDH were shown that their levels were decreased with aging [6]. Martina B reported that the expression of GAPDH was altered after isoprenaline treatment [5]. Therefore, an appropriate loading control is critical for specific study model. Our present study showed that β-tubulin and β-actin were differentially expressed in heart tissues from different stages both in Rhesus monkeys model and mice model of myocardial ischemia. The expression level of GAPDH was consistent in Rhesus monkey model, but inconsistent in mice, indicating that GAPDH could be considered as loading control in western blot analysis of Rhesus monkey heart tissues.
Besides HKPs, we also tested the total protein level in our models because it has been reported to be a reliable loading control. Total protein staining was first published in 1995 [18], which makes use of the signal intensity of the entire loading lane as a loading control [19]. Total protein normalization is more accurate because the normalization is done against the whole lane (multiple proteins), not a single protein. And there is no need to strip and re-immunoprobe blots, reducing the possible error caused by handling the blot. The methods to stain total protein involves staining of gel or blot by Ponceau [20] or CBB [21]. Gel staining has been employed as loading control in some studies [19] however, gel staining could not eliminate errors produced by the transferring procedure. Moreover, gel staining involves incubating and washing gels before detection of target proteins, which increases the risk of protein degradation and losing. Therefore, blot staining is a good choice of loading control. In our present study, we found that the level of total protein is consistent in different heart tissues by staining gel with CBB. Then we stained blots with CBB after detection of target proteins, and showed that the blot staining is feasible and reliable as loading control in our model, as discussed in other experimental conditions [18], [19], [21]. A technology called Stain-Free was developed in 2013 [22], [23], [24] in which a trihalo compound responds to tryptophan residues when exposed to ultraviolet irradiation of certain wave length, thus creating no need of incubating or washing steps of gel. But the accuracy of this method is dependent on the amount of tryptophan in samples and requires a special instrument.
In summary, our data illustrated that β-tubulin and β-actin were not stable in animal models of myocardial ischemic infarction. GAPDH can work as a reliable loading control only in Rhesus monkeys model of myocardial ischemia. Total protein level is the best loading control for western blot in animal models of myocardial ischemic infarction.
Acknowledgement
This work was supported by National Science Foundation of China (81230004 and 81600214). The authors thank Pengfei Han and Qin Sheng for providing the animal models. We also thank Ying Xiao and Ning Wang for pathological technical support.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.09.001.
Appendix A. Transparency document
Supplementary material
References
- 1.Dennis-Sykes C.A., Miller W.J., Mcaleer W.J. A quantitative Western Blot method for protein measurement. J. Biol. Stand. 1985;13:309. doi: 10.1016/s0092-1157(85)80044-5. [DOI] [PubMed] [Google Scholar]
- 2.Dittmer A., Dittmer J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27:2844–2845. doi: 10.1002/elps.200500785. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Wu M., He G., Zhang X., Li W., Gao Y., Li Z., Wang Z., Zhang C. Glyceraldehyde-3-phosphate dehydrogenase: a universal internal control for Western blots in prokaryotic and eukaryotic cells. Anal. Biochem. 2012;423:15–22. doi: 10.1016/j.ab.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Liu N.-K., Xu X.-M. beta-tubulin is a more suitable internal control than beta-actin in western blot analysis of spinal cord tissues after traumatic injury. J. Neurotrauma. 2006;23:1794–1801. doi: 10.1089/neu.2006.23.1794. [DOI] [PubMed] [Google Scholar]
- 5.Michel-Reher M.B., Michel M.C. Regulation of GAPDH expression by treatment with the β-adrenoceptor agonist isoprenaline—is GADPH a suitable loading control in immunoblot experiments? Naunyn-Schmiedeberg's Arch. Pharmacol. 2015;388:1119–1120. doi: 10.1007/s00210-015-1166-6. [DOI] [PubMed] [Google Scholar]
- 6.Vigelsø A., Dybboe R., Hansen C.N., Dela F., Helge J.W., Grau A. Guadalupe. GAPDH and β-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J. Appl. Physiol. 2015;118:386. doi: 10.1152/japplphysiol.00840.2014. [DOI] [PubMed] [Google Scholar]
- 7.He W., Kang Y. James. Ischemia-induced copper loss and suppression of angiogenesis in the pathogenesis of myocardial infarction. Cardiovasc. Toxicol. 2013;13:1–8. doi: 10.1007/s12012-012-9174-y. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson T.B., Prichard R.W., Morgan T.M., Petrick G.S., Klein K.P. Remodeling of coronary arteries in human and nonhuman primates. Jama. 1994;271:289. [PubMed] [Google Scholar]
- 9.Teofilovski-Parapid G., Kreclović G. Coronary artery distribution in Macaca fascicularis (Cynomolgus) Lab. Anim. 1998;32:200. doi: 10.1258/002367798780600007. [DOI] [PubMed] [Google Scholar]
- 10.Tohno Y., Tohno S., Laleva L., Ongkana N., Minami T., Satoh H., Oishi T., Hayashi M., Sinthubua A., Suwannahoy P., Mahakkanukrauh P. Age-related changes of elements in the coronary arteries of monkeys in comparison with those of humans. Biol. Trace Elem. Res. 2008;125:141–153. doi: 10.1007/s12011-008-8167-y. [DOI] [PubMed] [Google Scholar]
- 11.Yang P., Han P., Hou J., Zhang L., Song H., Xie Y., Chen Y., Xie H., Gao F., Kang Y.J. Electrocardiographic characterization of rhesus monkey model of ischemic myocardial infarction induced by left anterior descending artery ligation. Cardiovasc. Toxicol. 2011;11:365. doi: 10.1007/s12012-011-9129-8. [DOI] [PubMed] [Google Scholar]
- 12.Sun X., Cai J., Fan X., Han P., Xie Y., Chen J., Xiao Y., Kang Y.J. Decreases in electrocardiographic R-wave amplitude and QT interval predict myocardial ischemic infarction in Rhesus monkeys with left anterior descending artery ligation. PloS One. 2013:e71876. doi: 10.1371/journal.pone.0071876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikonomidis J.S., Hendrick J.W., Parkhurst A.M., Herron A.R., Escobar P.G., Dowdy K.B., Stroud R.E., Hapke E., Zile M.R., Spinale F.G. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am. J. Physiol. - Heart Circ. Physiol. 2004;288:H149. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 14.D. Angoulvant, S. Fazel, R.D. Weisel, T.Y.Y. Lai, P.W. Fedak, L. Chen, S. Rafati, C.K. Seneviratne, N. Degousee, R.-.K. Li, Cell-based gene therapy modifies matrix remodeling after a myocardial infarction in tissue inhibitor of matrix metalloproteinase-3–deficient mice, The Journal of Thoracic and Cardiovascular Surgery, 137, 471-480, e472. [DOI] [PubMed]
- 15.Cheng X., Hou J., Liu J., Sun X., Sheng Q., Han P., Kang Y.J. Safety evaluation of sevoflurane as anesthetic agent in mouse model of myocardial ischemic infarction. Cardiovasc. Toxicol. 2016:1–7. doi: 10.1007/s12012-016-9368-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W., Zhao X., Xiao Y., Chen J., Han P., Zhang J., Fu H., James Kang Y. The association of depressed angiogenic factors with reduced capillary density in the Rhesus monkey model of myocardial ischemia. Metallomics. 2016;8:654–662. doi: 10.1039/c5mt00332f. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y., Nie X., Han P., Fu H., Kang Y. James. Decreased copper concentrations but increased lysyl oxidase activity in ischemic hearts of rhesus monkeys. Metallomics. 2016;8:973–980. doi: 10.1039/c6mt00037a. [DOI] [PubMed] [Google Scholar]
- 18.Klein D., Kern R.M., Sokol R.Z. A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem. Mol. Biol. Int. 1995;36:59–66. [PubMed] [Google Scholar]
- 19.Aldridge G.M., Podrebarac D.M., Greenough W.T., Weiler I.J. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romerocalvo I., Ocón B., Martínezmoya P., Suárez M.D., Zarzuelo A., Martínezaugustin O., de Medina F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Welinder C., Ekblad L. Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 2011;10:1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 22.Gilda J.E., Gomes A.V. Western blotting using in-gel protein labeling as a normalization control: stain-free technology. Methods Mol. Biol. 2015;1295:381. doi: 10.1007/978-1-4939-2550-6_27. [DOI] [PubMed] [Google Scholar]
- 23.Gürtler A., Kunz N., Gomolka M., Hornhardt S., Friedl A.A., Mcdonald K., Kohn J.E., Posch A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013;433:105–111. doi: 10.1016/j.ab.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Rivero-Gutiérrez B., Anzola A., Martínez-Augustin O., de Medina F.S. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal. Biochem. 2014;467:1–3. doi: 10.1016/j.ab.2014.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material