Abstract
The study aimed to evaluate extraction efficiency, detection and quantification of phytochemicals, minerals and antioxidative capacity of different parts of Salacia chinensis L. Continuous shaking extraction, steam bath assisted extraction, ultrasonic extraction and microwave assisted extraction with varied time intervals were employed for extraction of phenolics, flavonoids, and antioxidants. Preliminary screening revealed the presence of wide array of metabolites along with carbohydrates and starch. Steam bath assisted extraction for 10 min exposure was found most suitable for extraction phenolics (46.02 ± 2.30 mg of gallic acid equivalent per gram of dry weight and 48.57 ± 2.42 mg of tannic acid equivalent per gram of dry weight) and flavonoids (35.26 ± 1.61 mg of quercetin equivalent per gram of dry weight and 51.60 ± 2.58 mg of ellagic acid equivalent per gram of dry weight). In support, reverse phase-high performance liquid chromatography- diode array detector confirmed the presence of seven pharmaceutically important phenolic acids. Antioxidant capacity was measured by 1, 1- diphenyl-1-picryl hydrazyl (DPPH), ferric reducing antioxidant power (FRAP), 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) scavenging (ABTS) and N, N-dimethyl-p-phenylenediamine (DMPD) assays and represented as trolox equivalent antioxidant capacity (TEAC) and ascorbic acid equivalent antioxidant capacity (AEAC). Antioxidant capacity ranged from 121.02 ± 6.05 to 1567.28 ± 78.36 µM trolox equivalent antioxidant capacity and 56.62 ± 2.83 to 972.48 ± 48.62 µM ascorbic acid equivalent antioxidant capacity. Roots showed higher yields of illustrated biochemical parameters, however fresh fruit pulp was found a chief source of minerals. Gas chromatography-mass spectroscopic analysis revealed the presence of a vast array of phytoconstituents associated with different plant parts. The present study revealed the amounts of minerals and diverse phytoconstituents in various parts of S. chinensis and confirmed its medicinal and nutritional implications.
Abbreviations: ABTS, 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid); AC, Antioxidant capacity; AEAC, ascorbic acid equivalent antioxidant capacity; CSE, continuous shaking extraction; DAD, diode array detector; DMPD, N, N-dimethyl-p-phenylenediamine; DPPH, 1, 1- diphenyl-1-picryl hydrazyl; DW, Dry weight; EAE, ellagic acid equivalent; FRAP, 2, 4, 6-tris (2-pyridyl)-s-triazine; GAE, gallic acid equivalent; GC-MS, gas chromatography-mass spectroscopy; LOD, limit of detection; LOQ, limit of quantification; MAE, microwave assisted extraction; NIST, national institute of standards; QE, quercetin equivalent; RP-HPLC, reversed phase-high performance liquid chromatography; SBAE, steam bath assisted extraction; TAE, tannic acid equivalent; TEAC, trolox equivalent antioxidant capacity; TFC, total flavonoid content; TIC, total ion chromatogram; TPC, total phenolic content; UE, ultrasonic extraction
Keywords: Antioxidative capacity, Extraction optimization, Flavonoids, GC-MS, Minerals, Phenolic acids, Phytoconstituents, RP-HPLC-DAD, Salacia chinensis
Graphical abstract
Highlights
-
•
Comparative illustration of phytochemicals, minerals and antioxidants from various parts of S. chinensis.
-
•
Phytochemical profiling confirmed the presence of different secondary metabolites along with proteins and carbohydrates.
-
•
Chromatographic determination revealed the presence of 7 phenolic acids (RP-HPLC-DAD) and 32 (GC-MS) other compounds.
-
•
Steam bath assisted extraction (10 min) was found effective for the extraction of phenolics, flavonoids and antioxidants.
-
•
Roots are the chief source of secondary metabolites and validated its role in traditional medicine.
1. Introduction
Salacia L. (family - Hippocrateaceae), an important genus having numerous nutritional, medicinal, and pharmaceutical implications is found widely in India, Sri Lanka, China and other Asian countries. Twenty one species are found in India alone [1]. Many species of the genus (including S. chinensis) have been used for thousands of years in traditional medicines, particularly as a food supplement for the prevention of obesity and diabetes [2]. Salacia spp. (including S. chinensis) has been used widely to treat variety of diseases such as arthritis, rheumatism, inflammation, leucorrhoea, fever, venereal and bronchitis [2]. S. chinensis commonly known as Saptarangi or Saptachakra is an important underutilized plant distributed in the Indian subcontinent, including the semi-evergreen forests of the Western Ghats, India. The sweet, translucent and jelly-like pulp surrounding the seeds of the fruit is edible. An anti-obesity effect with dietary intake of S. chinensis fruits was observed in obese human subjects [3], [4].
The presence of certain phytochemicals in roots and leaves of this plant has been reported [5], [6]. Various extracts of S. chinensis have been reported to possess several biological activities including antimicrobial, antidiabetic, and anti-obesity. Pharmacological studies also demonstrated the S. chinensis roots modulate multiple targets such as peroxisome proliferator-activated receptor-alpha-mediated lipogenic gene transcription, angiotensin II/angiotensin II type 1 receptor, alpha-glucosidase, aldose reductase and pancreatic lipase [7]. Moreover, anticancer and anti-HIV activities against resistant strains have also been identified [8]. These pharmacological and medicinal potential of this plant are associated with the presence of certain bioactive molecules like salacinol, mangiferin, kotanalol, betulin-3-caffeate, morolic acid, and oleanolic acid [5], [6]. Enhanced production of some of these compounds was successfully carried out using biotechnological tools especially plant tissue culture [9]. Moreover, molecular markers were also applied for phyto-molecular characterization and association with some important biochemical traits in some medicinal plants [10].
The health-promoting properties has increased interest in studying and quantifying the nutrients and secondary metabolites of this underutilized fruit plant [11], [12]. It is necessary to compare the nutritional and phytochemical values of different parts in order to establish optimal extraction conditions that can be adapted readily in food processing and clinical research programs. In the present study, comparative extraction and quantification of total phenolics, flavonoids, antioxidant properties were determined from different parts of S. chinensis. Moreover, mineral profiling was also carried out for different parts of S. chinensis.
2. Materials and methods
2.1. Collection and processing of plant material
Plant material (root, stem, leaf, fruit pulp and seeds of S. chinensis) were collected from single population at Amboli (15°58′05.6′’N; 73°59′48.7″E; altitude: 739 m) locality of the Northern Western Ghats, India and a specimen was authentically identified and deposited at Herbarium, Department of Botany, Shivaji University, Kolhapur (Voch. No. JC/SC/04). Plants were collected according to their vegetative, flowering and fruiting seasons. The material was cut into small pieces, shade dried (15 days) and ground to fine powder using a Wiley mill.
2.2. Chemicals and reagents
Phenolic acids, standards for antioxidant assays (gallic acid, tannic acid, quercetin, ellagic acid, trolox, and ascorbic acid), 2,2-diphenyl-1-picryl hydrazyl (DPPH), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), 2,2′-azino-bis (3-ethylbenzo-thiazoline-sulphonic acid) (ABTS), N,N-dimethyl-p-phenylenediamine (DMPD) were procured from Sigma Aldrich, Bangalore, India. Methanol, acetone, chloroform, petroleum ether, acetonitrile were of HPLC grade (Qualigens, India). All other chemicals were analytical grade.
2.3. Extract preparation and qualitative screening of phytochemicals
The qualitative phytochemical analysis was carried out for different plant parts viz; root, stem, leaf, fruit pulp and seeds. The extractions were carried out by subjecting 1 g of plant powder into 250 ml conical flask. The flask was subjected with 100 ml of different solvents (methanol, acetone, chloroform, petroleum ether or distilled water individually) and kept for shaking on orbital shaker at 110 ± 2 rpm for 12 h (Rivotek, Riviera, India). Then the extracts were filtered through Whatman No. 1 filter paper and volume was adjusted to 100 ml using respective solvents. The extracts prepared were analyzed for the presence of major phytochemical groups such as proteins, carbohydrates, phenols, tannins, flavonoids, saponins, glycosides, steroids terpenoids and alkaloids by using standard methods [13], [14].
2.4. Comparative extraction of total phenolics, flavonoids and antioxidant capacity
The methods described for extraction of phytoconstituents from S. chinensis [15] and Ancistrocladus heyneanus [16] were employed with minor modifications. Extractions were carried out to evaluate the method of extraction and time period required for the extraction to get the higher yield of total phenolics, flavonoids and antioxidants from different parts of the S. chinensis. Methanol was chosen for these experiments since it was the best solvent for extraction of phenols, flavonoids and antioxidants from fresh fruit pulp of S. chinensis in a previous study [17]. The extraction techniques employed in the present study are as follows.
2.4.1. Continuous shaking extraction (CSE)
One gram powder of plant parts (root, stem, leaf, fruit pulp and seed) were subjected separately to continuous shaking extractions using 100 ml of methanol and placed on orbital shaker (Rivotek, Riviera, India). Extractions were carried out at a controlled temperature (25 ± 2 °C) with constant stirring at 110 ± 2 rpm for 360 or 720 min.
2.4.2. Ultrasonic extraction (UE)
Powdered plant material (root, stem, leaf, fruit pulp and seeds) was subjected separately to ultrasonic extraction at working amplitude of 60 Hz on ultrasonic bath (Revotek, India). One gram of powdered material was transferred to 100 ml of methanol in 250 ml beaker. Samples were exposed to sonication for 10 or 20 min at room temperature.
2.4.3. Microwave assisted extraction (MAE)
One gram powder of root, stem, leaf, fruit pulp and seeds were extracted individually with 100 ml methanol in conical flasks. All flasks were exposed for 5 or 10 min in a microwave oven (Samsung, India) at 180 W. Cooling at regular intervals (2 min) was done to avoid bumping and repeated exposures were allowed to complete the required exposure.
2.4.4. Steam bath assisted extraction (SBAE)
Extractions were carried out on stirred thermal water bath (Equitron, Mumbai, India) and constant temperature (70 °C). One gram of dried plant powder was extracted with 100 ml methanol for 10 or 20 min. The flasks were kept on the thermal water bath for evaluating the efficiency of steam on the extraction.
Extracts prepared using the four different extraction procedures were filtered through Whatman No. 1 filter paper and adjusted to 100 ml and extracts were used for further analysis.
2.5. Determination of total phenolic content (TPC)
The quantification of TPC was carried out using modified Folin–Ciocalteu method [18]. The absorbance of blue color developed was read at 760 nm on double beam spectrophotometer (Thermo Scientific, multiskan Go 1510, USA). The results were compared with standard curves of tannic and gallic acid and were expressed as milligram equivalent per gram dry weight.
2.6. Determination of total flavonoid content (TFC)
Total flavonoid content was quantified by using aluminium chloride calorimetric method [19]. The absorbance of extracts and standard solutions was measured at 367 nm. The results were expressed as milligram of quercetin and ellagic acid equivalent per gram dry weight.
2.7. RP-HPLC-DAD determination of phenolic acids
2.7.1. Sample preparation
One gram powder of plant materials (root, stem, leaf, fruit pulp and seed separately) were extracted using 100 ml of methanol on an orbital shaker with a constant stirring of 110 ± 2 rpm at 28 ± 2 °C, overnight. The extracts were filtered through a 0.45 mm nylon filter (Axiva filters), volume of the extracts were adjusted to 100 ml with methanol and stored in amber vial at 4 °C for less than a week until HPLC analysis.
2.7.2. Instrumentation
Reversed phase HPLC diode array detector (RP-HPLC-DAD) analysis was performed on a Shimadzu chromatographic system (model no. LC-20 CE) consisting of a quaternary pump, manual injector, degasser (DGU-20A5) and dual λ UV absorbance diode array detector (SPD-M20A). LC-Solution software (Version 1.25, Shimadzu Corporation, Japan) was used for data processing. Chromatographic separation was achieved on a Waters, Nova-Pak C18 column (4 µm, 4.6 × 250 mm).
2.7.3. Chromatographic conditions
Mobile phase consisting of water: acetonitrile: glacial acetic acid (90:5:5) was used for separation with an injection volume of 20 µl. A isocratic flow rate of 0.9 ml/min was used with a run time of 60 min. Detection was at 280 nm.
2.7.4. Calculations, calibration curves and linearity
Phenolic compounds were accurately weighed and dissolved in methanol to obtain a standard stock solution (1 mg/ml). The stock solution of phenolic compounds was serially diluted using same solvent to obtain 6 levels (1, 5, 10, 20, 40 and 100 µg/ml) of working concentrations for plotting calibration curves. All solutions and analytes were stored in microfuge tubes at 4 °C for less than a week until further use.
2.7.5. System suitability, limit of detection and limit of quantification
The system suitability test was assessed by three replicates of standard phenolics at a concentration of 40 μg/ml. The peak areas were used to evaluate repeatability of the method and analyzed for resolution and tailing factors. Signal: noise ratios of 3.3 and 10 were used for estimating the limit of detection (LOD) and limit of quantification (LOQ), respectively.
2.8. Antioxidant capacity
2.8.1. DPPH radical scavenging assay
The plant extracts were assessed for their antioxidant activities as a degree of radical scavengers using 1,1-diphenyl-2-picrylhydrazyl (DPPH) in 100 ml of chilled methanol [20]. One hundred microliters (100 µl) of plant extract were reacted with 2.9 ml DPPH solution. The reaction suspension were shaken and kept in dark for 30 min after which the absorbance was measured at 517 nm.
2.8.2. Ferric reducing antioxidant power (FRAP) assay
The assay was performed for different parts using method described by Benzie and Strain [21]. The absorbance was read at 595 nm after 15 min dark incubation at 37 °C.
2.8.3. ABTS radical cation scavenging assay
A modified ABTS [2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)] radical cation decolorization assay [22] was used to analyze the antioxidant activity of the various parts of S. chinensis. The ABTS reagent was diluted in 1:10 ratio using methanol which gives 1.1 ± 0.02 absorbance at 734 nm. Using methanol as a blank, ABTS antioxidant activity was measured at 734 nm.
2.8.4. DMPD radical cation decolorization assay
An improved decolorization assay was performed to evaluate the antioxidant capacity of different plant parts by using N,N-dimethyl-p-phenylenediamine (DMPD) [23]. Plant extract (100 µl) was allowed to react with 2 ml of DMPD reagent in presence of 0.9 ml distilled water. After 15 min dark incubation the absorbance were measured at 505 nm.
For analysis of antioxidant capacity, different concentrations of ascorbic acid and trolox (mg/ml) were used as reference standards. Results were expressed as micromole (µM) of ascorbic acid and trolox equivalent antioxidant capacity (AEAC/TEAC).
2.9. Mineral profile
Different parts of S. chinensis (root, stem, leaves, fruit and seeds) were separated and dried in the shade and ground to a fine powder. The acid digestion process and measurement of contents of inpartic elements was carried out according to methods described previously [24], [25]. The content of the elements were expressed as mg/g DW.
2.10. GC-MS analysis of phytoconstituents
2.10.1. Sample preparation
The extraction was performed using previously described method [26] with little modification. Ten grams dried powder of plant material was subjected for extraction of phytochemicals by using soxhlet extractor. Uniform plant material was placed into a thimble and extracted with 100 ml of ethyl acetate. The polarity of ethyl acetate was elevated by addition of 1 ml of conc. HCl. The process of extraction continued for 3 h with constant temperature (77 °C). Finally, the volume of the filtered extract was adjusted to 100 ml and stored in refrigerator at 4 °C for less than a week until further use.
2.10.2. Chromatographic conditions
GC-MS analysis was carried out using Shimadzu model QP-2010 with a nonpolar 60 M RTX 5MS column. Helium was used as the carrier gas and the temperature programming was set with initial oven temperature at 40 °C and held for 3 min and the final temperature of oven was 480 °C with rate at 10 °C. Two microliters (2 µl) sample was injected using a split less mode. Mass spectra was recorded over 35–650 amu range with electron impact ionization energy 70 eV. The total running time for a sample was 45 min. Relative quantitative determinations were made by relating respective peak areas to total ion chromatogram (TIC).
2.10.3. Identification of compounds
Unknown components compared with known mass spectra of National Institute of Standards (NIST) for molecular identification of compounds. Name, molecular weight, retention time and peak area percentage of the test material was tentatively ascertained.
2.11. Statistical analysis
Statistical analysis was performed using the statistical software GraphPad Prism evaluation version. Data was reported as means ± standard deviation (SD). Significant differences between means were determined using repeated measure one-way ANOVA at P < 0.05 with GraphPad, Software, Ver. 3.06. The chromatographic profiles of all extracts were analyzed using built in Shimadzu LC Solution software (Version 1.25). The principal component analysis (PCA) performed was based on (i) total polyphenols (TPC and TFC); (ii) antioxidant activities (DPPH, FRAP, DMPD and ABTS) and (iii) HPLC analysis.
3. Results and discussion
3.1. Preliminary phytochemical screening
Investigation on the preliminary qualitative phytochemical screening of various extracts of S. chinensis revealed the presence of diverse groups of metabolites such as proteins, carbohydrates, phenols, tannins, flavonoids, saponins, glycosides, steroids, terpenoids and alkaloids (Table 1). It is well documented fact that the presence of these chemicals is associated with various medicinal properties [27]. In this study, methanolic extract of root and leaves of S. chinensis were found to be chief source of major phytochemicals. Terpenoids, a pharmaceutically important group, are present in all the parts of S. chinensis irrespective of solvent system (Table 1). Most interestingly, proteins and carbohydrates were soluble in chloroform and distilled water and associated mainly with fruit pulp and seeds. Their presence might be the possible cause for edible nature of the fruits of S. chinensis. Similarly, carbohydrates are the major ingredients of Cola parchycarpa, C. lepidota fruits [12].
Table 1.
Qualitative phytochemical screening of various extracts of S. chinensis.
| Plant part | Solvent system | Proteins | Carbohydrates |
Phenols | Tannins | Flavonoids | Saponins | Glycosides |
Steroids | Terpenoids | Alkaloids | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fehling Test | Benedicts test | Libermanns Test | Salkowaskis Test | ||||||||||
| Root | M | ++ | – | – | +++ | +++ | ++ | – | ++ | + | + | ++ | – |
| A | + | – | – | +++ | +++ | +++ | – | – | – | + | +++ | ++ | |
| C | – | ++ | – | – | – | – | ++ | + | + | + | ++ | – | |
| P | – | – | – | – | – | – | – | ++ | – | + | + | – | |
| D | + | – | – | +++ | +++ | +++ | + | +++ | ++ | +++ | + | – | |
| Stem | M | + | – | – | + | + | + | – | ++ | + | + | + | – |
| A | – | – | – | – | – | – | – | – | – | + | ++ | ++ | |
| C | – | ++ | – | – | – | – | ++ | + | + | + | + | – | |
| P | – | – | – | – | – | – | – | + | – | + | + | – | |
| D | – | – | – | + | + | + | + | – | ++ | ++ | + | – | |
| Leaf | M | – | – | – | + | + | +++ | – | + | ++ | ++ | + | – |
| A | +++ | – | – | – | – | ++ | – | – | – | – | + | + | |
| C | – | ++ | – | – | – | + | ++ | + | + | – | + | – | |
| P | – | – | – | – | – | – | – | + | – | + | + | – | |
| D | – | – | + | ++ | +++ | +++ | +++ | + | – | + | + | – | |
| Seed | M | – | – | – | + | – | + | – | – | +++ | +++ | ++ | ++ |
| A | +++ | – | – | – | – | – | – | – | – | – | + | + | |
| C | – | +++ | – | + | – | + | + | + | + | + | + | – | |
| P | – | – | – | + | – | – | – | +++ | – | +++ | + | – | |
| D | – | – | – | + | – | + | + | – | – | – | + | – | |
| Fruit Pulp | M | – | – | – | + | – | + | – | – | ++ | +++ | + | + |
| A | +++ | – | – | – | – | – | – | – | – | ++ | + | + | |
| C | – | ++ | – | + | – | + | ++ | + | + | + | + | – | |
| P | – | – | – | + | – | – | – | + | – | + | + | – | |
| D | – | ++ | ++ | + | – | + | + | – | – | – | + | – | |
(M) Methanol, (A) Acetone, (C) Chloroform, (P) Petroleum ether, (D) Distilled water, (+++) Strongly Positive, (++) Moderate Positive, (+) Positive, (-) Negative.
3.2. Total phenolics and flavonoids
Various parts of S. chinensis viz. root, stem, leaf, fruit pulp and seeds were analyzed for their total phenolic and flavonoid contents using different extraction techniques. The extraction conditions certainly affected extraction efficiency of TPC and TFC (Table 2). Among the extraction techniques employed, SBAE with minimum exposure time (10 min) was found with superior extractabilities followed by the MAE (10 min) and CSE (360 min). So, it can be depicted that the extraction conditions could affect the yield of the polyphenols. Similarly, extraction method alters the levels of polyphenols in medicinal plant Clinacanthus nutans [28] and so as in every other species. The phenolic content ranges from 0.03 ± 00 to 46.02 ± 2.30 mg GAE/g and 0.46 ± 0.02 to 48.57 ± 2.42 mg TAE/g DW. The phenolic hierarchy among different plant parts studied was observed to be as root > stem > leaves > fruit pulp > seeds. In the present study, it was observed that the roots are the major source of polyphenols as compared with other plant parts. The maximum yield of phenolics from roots (46.02 ± 2.30 mg GAE/g and 48.57 ± 2.42 mg TAE/g DW) was obtained by SBAE with 10 min of exposure time (Table 2). The stem and leaves also showed the significant contents; however fruit pulp and seeds were found to be poor source of polyphenols.
Table 2.
Total phenolic and flavonoid content among various parts of S. Chinensis with respect to different extraction methods and extraction durations.
| Sr. No. | Plant part | Extraction method | Extraction period (Min.) |
Total phenolic content |
Total flavonoid content |
||
|---|---|---|---|---|---|---|---|
| (mg GAE/g DW)a | (mg TAE/g DW)b | (mg QE/g DW)c | (mg EAE/g DW)d | ||||
| 1 | Root | MAE | 05 | 41.25 ± 2.06 | 43.54 ± 2.18 | 18.41 ± 0.92 | 29.45 ± 1.47 |
| 2 | 10 | 43.37 ± 2.17 | 45.77 ± 2.28 | 24.03 ± 1.20 | 38.44 ± 1.92 | ||
| 3 | UE | 10 | 39.13 ± 2.00 | 41.29 ± 2.06 | 22.76 ± 1.13 | 36.41 ± 1.82 | |
| 4 | 20 | 42.75 ± 2.14 | 45.12 ± 2.25 | 23.97 ± 1.19 | 38.34 ± 1.91 | ||
| 5 | SBAE | 10 | 46.02 ± 2.30 | 48.57 ± 2.42 | 35.26 ± 1.61 | 51.60 ± 2.58 | |
| 6 | 20 | 44.75 ± 2.23 | 47.23 ± 2.36 | 31.71 ± 1.58 | 50.73 ± 2.53 | ||
| 7 | CSE | 360 | 43.37 ± 2.16 | 45.78 ± 2.29 | 28.49 ± 1.42 | 45.58 ± 2.27 | |
| 8 | 720 | 33.62 ± 1.68 | 37.65 ± 3.38 | 32.64 ± 1.78 | 44.13 ± 2.21 | ||
| 9 | Stem | MAE | 05 | 22.41 ± 1.12 | 23.65 ± 1.18 | 05.85 ± 0.29 | 09.35 ± 0.46 |
| 10 | 10 | 17.78 ± 2.17 | 18.76 ± 0.93 | 04.73 ± 0.23 | 07.56 ± 0.37 | ||
| 11 | UE | 10 | 19.74 ± 0.98 | 20.83 ± 1.04 | 05.97 ± 0.29 | 09.55 ± 0.47 | |
| 12 | 20 | 20.25 ± 1.01 | 21.27 ± 1.06 | 05.44 ± 0.27 | 08.69 ± 0.43 | ||
| 13 | SBAE | 10 | 24.80 ± 1.23 | 26.17 ± 1.30 | 07.60 ± 0.38 | 12.15 ± 0.60 | |
| 14 | 20 | 24.60 ± 1.23 | 25.97 ± 1.29 | 15.91 ± 0.79 | 25.45 ± 1.27 | ||
| 15 | CSE | 360 | 22.11 ± 1.10 | 23.34 ± 1.17 | 06.83 ± 0.34 | 10.92 ± 0.54 | |
| 16 | 720 | 14.31 ± 0.72 | 29.03 ± 1.45 | 04.17 ± 0.21 | 04.79 ± 0.24 | ||
| 17 | Leaf | MAE | 05 | 18.07 ± 0.90 | 19.08 ± 0.95 | 13.56 ± 0.67 | 21.68 ± 1.08 |
| 18 | 10 | 17.38 ± 0.86 | 18.34 ± 0.91 | 11.91 ± 0.59 | 19.05 ± 0.95 | ||
| 19 | UE | 10 | 16.42 ± 0.82 | 17.33 ± 0.86 | 12.30 ± 0.61 | 19.67 ± 0.98 | |
| 20 | 20 | 18.26 ± 0.91 | 19.27 ± 0.96 | 14.41 ± 0.72 | 23.05 ± 1.15 | ||
| 21 | SBAE | 10 | 21.44 ± 1.07 | 22.63 ± 1.13 | 17.67 ± 0.88 | 28.27 ± 1.41 | |
| 22 | 20 | 22.97 ± 1.15 | 24.25 ± 1.21 | 17.86 ± 0.89 | 28.56 ± 1.42 | ||
| 23 | CSE | 360 | 17.64 ± 0.88 | 18.62 ± 0.93 | 14.97 ± 0.74 | 23.95 ± 1.19 | |
| 24 | 720 | 12.66 ± 0.63 | 25.72 ± 1.19 | 17.32 ± 0.87 | 21.23 ± 1.06 | ||
| 25 | Fruit Pulp | MAE | 05 | 05.81 ± 0.29 | 06.13 ± 0.30 | 03.24 ± 0.16 | 05.18 ± 0.25 |
| 26 | 10 | 06.18 ± 0.31 | 06.52 ± 0.32 | 03.64 ± 0.18 | 05.81 ± 0.29 | ||
| 27 | UE | 10 | 05.41 ± 0.27 | 05.71 ± 0.28 | 04.49 ± 0.22 | 07.18 ± 0.35 | |
| 28 | 20 | 05.93 ± 0.29 | 06.26 ± 0.31 | 04.32 ± 0.21 | 06.90 ± 0.34 | ||
| 29 | SBAE | 10 | 07.59 ± 0.38 | 08.00 ± 0.40 | 05.92 ± 0.29 | 09.46 ± 0.47 | |
| 30 | 20 | 06.93 ± 0.35 | 07.30 ± 0.36 | 05.52 ± 0.27 | 08.82 ± 0.44 | ||
| 31 | CSE | 360 | 05.53 ± 0.27 | 05.84 ± 0.29 | 06.75 ± 0.33 | 10.79 ± 0.53 | |
| 32 | 720 | 01.37 ± 0.07 | 03.14 ± 0.16 | 02.03 ± 0.10 | 02.12 ± 0.11 | ||
| 33 | Seed | MAE | 05 | 05.14 ± 0.26 | 05.42 ± 0.27 | 03.44 ± 0.17 | 05.49 ± 0.27 |
| 34 | 10 | 04.75 ± 0.24 | 05.01 ± 0.25 | 02.88 ± 0.14 | 04.61 ± 0.23 | ||
| 35 | UE | 10 | 04.19 ± 0.21 | 04.42 ± 0.22 | 02.76 ± 0.13 | 04.41 ± 0.22 | |
| 36 | 20 | 04.23 ± 0.21 | 04.45 ± 0.22 | 03.87 ± 0.19 | 06.18 ± 0.30 | ||
| 37 | SBAE | 10 | 05.01 ± 0.25 | 05.28 ± 0.26 | 06.26 ± 0.31 | 10.01 ± 0.50 | |
| 38 | 20 | 05.17 ± 0.26 | 05.45 ± 0.27 | 03.17 ± 0.15 | 05.07 ± 0.25 | ||
| 39 | CSE | 360 | 04.91 ± 0.24 | 05.18 ± 0.26 | 07.67 ± 0.38 | 12.26 ± 0.61 | |
| 40 | 720 | 00.03 ± 0.00 | 00.46 ± 0.02 | 01.28 ± 0.06 | 01.17 ± 0.06 | ||
Measurements are mean ± SD of three parallel determinations and expressed as
gallic acid.
tannic acid.
quercetin.
ellagic acid equivalent per gram dry weight.
TFC among different parts of S. chinensis is presented in Table 2. The scenario for TFC among different plant parts studied are as root > stem > leaves > fruit pulp > seeds, as observed for TPC. TFC content ranged from 1.28 ± 0.06 to 35.26 ± 1.61 QE/g and 1.17 ± 0.06 to 51.60 ± 2.58 EAE/g DW among different parts of S. chinensis. SBAE yields the higher amount of TFC at minimum exposure (10 min), as observed for TPC as well. Among various plant parts assessed, roots showed the highest values of TFC at 10 min exposure to SBAE (32.26 ± 1.61 mg QE/g and 51.60 ± 2.58 mg EAE/g DW) (Table 2). The plant parts like fruit pulp and seeds were observed with less TFC values compared to the other plant parts.
In the present study, plant part and method of extraction were found to be the most important factors in extraction optimization, however time of extraction least affected variation of yield of TPC as well as TFC. Recently, similar observations were made for Clinacanthus nutans [28] and Achyranthes aspera [29]. It can be noted that, TPC and TFC content in the studied plant parts differ significantly from each other and the contents of TPC, and TFC was higher in roots than other plant parts.
3.3. Identification and quantification of major phenolic acids by RP-HPLC-DAD
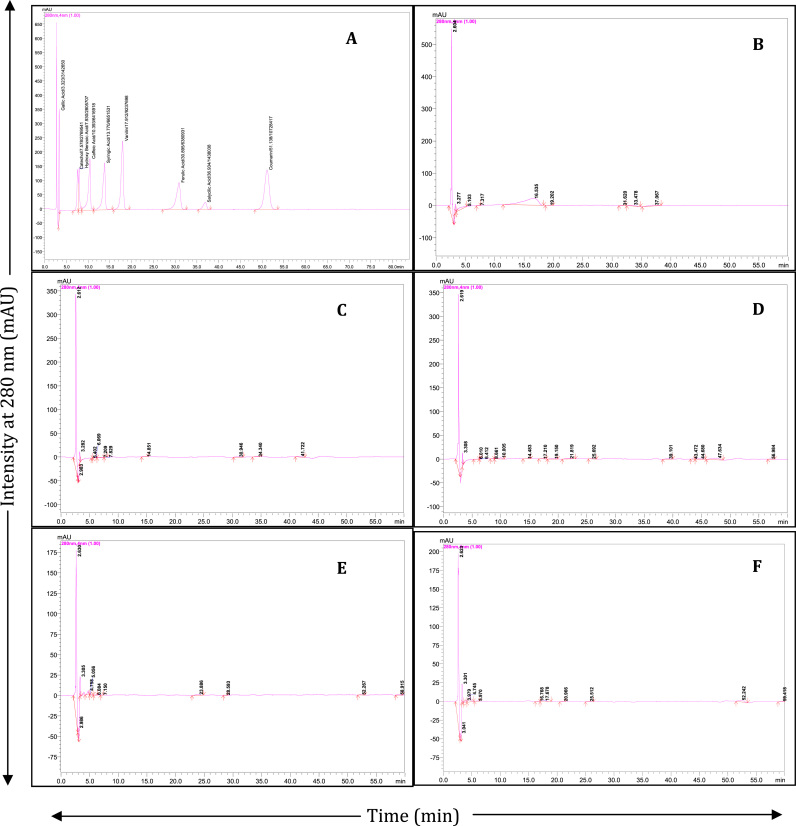
The major phenolic acids in various parts of S. chinensis were identified by RP-HPLC-DAD analysis (Fig. 1). The major peaks identified by comparison with authentic standards. Seven different phenolic acids including gallic acid, catechol, hydroxy benzoic acid, caffeic acid, vanilin, ferulic acid and salicylic acids were detected in various parts of S. chinensis. (Table 3, Fig. 1). In the present study, gallic acid was identified as chief source of polyphenol. It is a naturally abundant plant phenolic compound known for its consumption benefits due to antioxidant and free radical scavenging properties [30]. Root extracts detected with superior phenolic profile with presence of major phenolic compounds like gallic acid (0.683 ± 0.03 mg/g), catechol (0.406 ± 0.02 mg/g), ferulic acid (0.024 ± 0.01 mg/g) and salicylic acid (2.438 ± 0.12 mg/g). Salicylic acid, a key cause of several pharmacological properties [31] was found in the roots of S. chinensis. Catechol is the major source found in the fruit pulp which indicates the nutritional importance of the fruits. The study confirms the presence of four key phenolic acids in the roots which may be responsible for its high antioxidant capacity and proven its medicinal implications. Gallic acid, caffeic acid and vanilin were detected in leaves of S. chinensis. The phenolic profile in leaves of S. chinensis was confirmed recently by LC-ESI-MS/MS analysis [32].
Fig. 1.
RP-HPLC-DAD profiles of phenolic acids and related compounds from various parts of S. chinensis. A: Standards (100 ppm), B: Root, C: Stem, D: Leaf, E: Fruit pulp and F: Seed.
Table 3.
RP-HPLC-DAD profile of phenolic acids (mg/g DW) found in the different parts of S. chinensis.
| Sr. No. | Compound | LOD (µg/ml) | LOQ (µg/ml) |
Plant part |
||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Fruit pulp | Seed | ||||
| 1. | Gallic acid | 0.154 | 0.467 | 0.683 ± 0.034 | 0.498 ± 0.025 | 0.584 ± 0.029 | 0.443 ± 0.022 | 0.451 ± 0.023 |
| 2. | Catechol | 0.139 | 0.420 | 0.406 ± 0.020 | 0.517 ± 0.026 | nd | 0.397 ± 0.020 | nd |
| 3. | Hydroxy Benzoic acid | 0.089 | 0.268 | nd | 0.181 ± 0.009 | nd | nd | nd |
| 4. | Caffeic acid | 0.140 | 0.424 | nd | nd | 0.228 ± 0.011 | nd | nd |
| 5. | Syringic acid | 0.126 | 0.383 | nd | nd | nd | nd | nd |
| 6. | Vanilin | 0.095 | 0.288 | nd | nd | 0.053±0.003 | nd | 0.131 ± 0.007 |
| 7. | Ferulic acid | 0.156 | 0.350 | 0.024 ± 0.001 | 0.079 ± 0.004 | nd | nd | nd |
| 8. | Salicylic acid | 0.152 | 0.462 | 2.438 ± 0.122 | nd | nd | nd | nd |
| 9. | Cumarin | 0.087 | 0.262 | nd | nd | nd | nd | nd |
| 10. | Total Phenolics | – | – | 3.551 ± 0.177 | 1.275 ± 0.064 | 0.865 ± 0.043 | 0.840 ± 0.042 | 0.582 ± 0.030 |
(mean ± standard deviation; n = 3); nd: not detected.
3.4. Antioxidant capacity
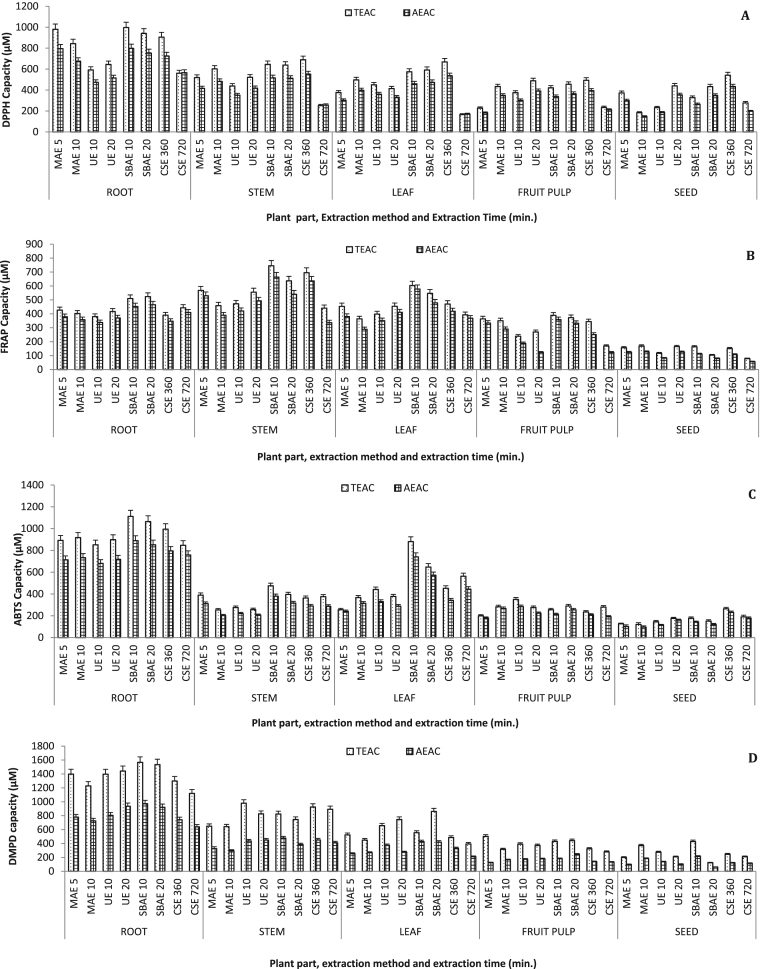
In the present investigation, AC of different parts of S. chinensis assessed by DPPH, FRAP, ABTS and DMPD assays are indicated in Fig. 2. Moreover, each antioxidant assay was evaluated by using two reference standards such as trolox and ascorbic acid. DPPH assay is the most common method to assess the antioxidant capacity of plants. Fig. 2A shows the DPPH radical scavenging capacity of different parts of S. chinensis. The DPPH capacity of the different plant parts ranged from 165.75 ± 8.29 to 997.42 ± 47.05 µM TEAC and 147.23 ± 7.36 to 798.03 ± 37.64 µM AEAC. Among the various extraction methods employed, SBAE, with 10 min exposure, was found most suitable for extracting compounds with DPPH activity. Under these conditions, the roots showed the highest DPPH radical scavenging activity (997.42 ± 47.05 µM TEAC and 798.03 ± 37.64 µM AEAC). Stem and leaves also bear significant DPPH capacity, however dry fruit pulp and seeds possesses poor radical scavenging potential. Kishi et al. [33] isolated various classes of terpenes from S. chinensis and were assessed for its DPPH radical scavenging activity [33]. Some of these compounds showed strong DPPH activity.
Fig. 2.
Antioxidant capacities of various parts of S. chinensis. A: DPPH, B: FRAP, C: ABTS, D: DMPD assay.
As shown in Fig. 2B, there is great variation in the ferric reducing antioxidant power of the different parts of the S. chinensis, ranging from 78.23 ± 3.91 to 745.34 ± 37.27 µM TEAC and from 56.62 ± 2.83 to 664.28 ± 33.21 µM AEAC. Methanolic stem extract possesses the comparatively higher values (745.34 ± 37.27 µM TEAC and 664.28 ± 33.23 µM AEAC) at 10 min exposure to steam bath assisted extraction. Leaves (578.12 ± 28.91 µM AEAC and 547.23 ± 27.36 µM TEAC) and roots (510.21 ± 25.51 µM TEAC and 465.78 ± 23.29 µM AEAC) also showed the fair amount of electron donation to Fe+++.
In ABTS antioxidation capacity, less exposure to SBAE (10 min) and CSE (360 min) had shown the significant antioxidation power (Fig. 2C). The root extract displayed the highest (1112.02 ± 55.60 µM TEAC and 889.64 ± 44.48 µM AEAC) ABTS radical scavenging capacity. Similarly, the roots of Salacia reticulata also possess strong ABTS activity [34]. In contrast, ABTS as well as DPPH activity was recorded highest in leaves of Salacia macrosperma [35]. In present investigation as well, the leaves of S. chinensis showed the fair amount ABTS antioxidative capacity (881.02 ± 44.05 µM TEAC and 740.81 ± 37.04 µM AEAC).
The DMPD assay revealed the variation in extractabilities of the different methods applied. Various methods like SBAE, UE and MAE with variable time intervals have been observed to be competent for DMPD analysis. Overall SBAE was found to be suitable for this antioxidant assay (Fig. 2D). Crude root extracts showed the highest values of the DMPD capacity (1567.28 ± 78.36 µM TEAC and 972.48 ± 48.62 µM AEAC) for SBAE at 10 min exposure time. The fruit pulp and seed extracts were observed with poor DMPD antioxidant capacity.
Overall, antioxidation results demonstrated that the root extracts resemble the highest antioxidation capacity except FRAP assay. Significant activity was also shown by the leaves while dry fruit pulp and seed bears the least capacity of antioxidation. In contrast, it was reported that the fresh fruit pulp of S. chinensis bears good antioxidant capacity [17]. The steam bath assisted extraction procedure with 10 min exposure was found to be suitable in all the antioxidant assays studies.
3.5. Mineral composition
In general, fruits are characterized by a high content of potassium [36]. Similarly, high potassium contents (76.50 mg/g) in S. chinensis fruits were expected. Moreover, the fine nutritional status and consumption value of fruits of S. chinensis were maintained with higher contents of nitrogen, nitrate, and sulphur (Table 4). The roots had the highest ferrous concentration (289.46 mg/g) which was statistically different from the other parts of the S. chinensis (Table 4). The leaves presented the highest amount of phosphorous (1.100 mg/g), sodium (23.50 mg/g), manganese (36.19 mg/g), and molybdenum (0.126 mg/g). These values were significantly different for the other plant parts. Similarly, fair amount of sodium content was previously reported for Salacia senegalensis [25]. Sodium in combination with potassium is involved in maintaining proper acid-base balance and in nerve impulse transmission in the body [25]. The various parts showed significant differences in the zinc and copper content, having highest content in stem. It is noteworthy that zinc is an essential mineral for the metabolism of carbohydrates, lipids and proteins [36]. In case of S. chinensis seeds acquire its regeneration capacity by means of fair contents of macro and microelements.
Table 4.
Mineral composition of various parts of S. chinensis.
| Sr. No. | Parameters |
Mineral content (mg/g DW) |
||||
|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Fruit pulp | Seeds | ||
| 1. | Nitrogen | 16.20 | 17.30 | 17.90 | 21.80 | 11.70 |
| 2. | Nitrate N | 0.560 | 0.570 | 0.550 | 0.570 | 0.460 |
| 3. | Phosphorus | 0.460 | 0.430 | 1.100 | 0.580 | 0.780 |
| 4. | Potassium | 16.00 | 26.00 | 67.00 | 76.50 | 41.00 |
| 5. | Calcium | 34.00 | 16.00 | 29.50 | 29.00 | 21.00 |
| 6. | Magnesium | 0.006 | 0.004 | 0.015 | 0.001 | 0.002 |
| 7. | Sulphur | 1.000 | 1.200 | 1.200 | 1.500 | 1.100 |
| 8. | Sodium | 7.500 | 5.000 | 23.50 | 10.50 | 4.500 |
| 9. | Zinc | 3.784 | 11.30 | pc | pc | pc |
| 10. | Ferrous | 289.46 | 89.17 | 59.25 | 22.26 | 24.16 |
| 11. | Copper | 17.132 | 31.62 | 6.710 | 2.542 | 2.558 |
| 12 | Manganese | 9.342 | 6.818 | 36.19 | Pc | Pc |
| 13. | Molybdenum | 0.120 | 0.124 | 0.126 | 0.082 | 0.100 |
| 14. | Boron | 3.206 | 1.466 | 1.874 | 1.644 | 1.220 |
pc: poor content.
3.6. Identification of phytoconstituents by GC-MS
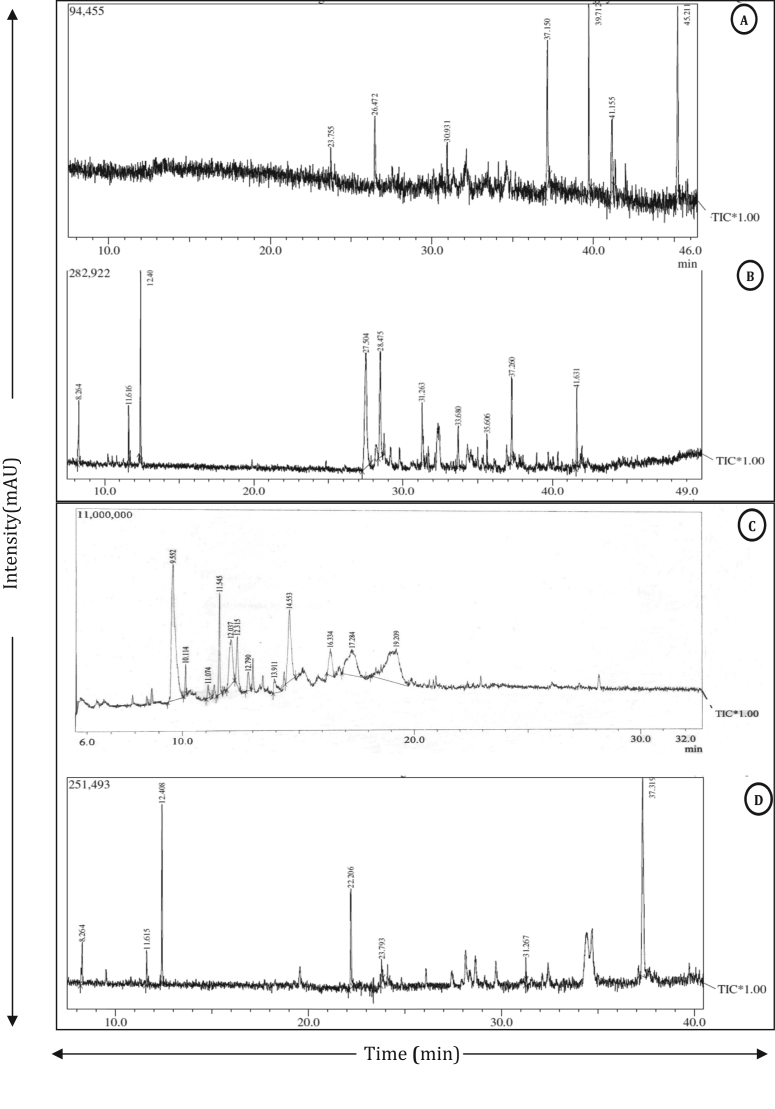
The researchers report successful extraction, detection and tentative identification of a series of compounds extracted from various parts of S. chinensis (Table 5, Fig. 3). In this study the researchers propose the identification of 32 different compounds in S. chinensis. Root and fruit pulp extracts contains maximum number of compounds. Utmost plant parts (except fruit pulp) showed the presence of ethyl.alpha.-d-glucopyranoside which is known to possess antituberculous, antioxidant and anticonvulsant activity [37]. Roots showed the presence of compounds like Ethyl α-d-glucopyranoside (23.11%), tetracosane (13.83%), pentacosane (29.47%). Stem also revealed the presence of compounds like ethyl.beta.-d-riboside (33.72%), benzene, 1,2-dimethyl (12.38%) and ethyl.alpha.-d-glucopyranoside (8.87%) and glycisodic compounds d-Mannitol, 1,4-anhydro (4.07%). Leaf profile revealed the presence of fatty acid compounds like propanol, 2,3-dihydroxy (27.88%), ethyl.alpha.-d-glucopyranoside (22.76%) and 3,7,11,15-tetramethyl-2-hexadecen-1-ol (17.49%). Fruit pulp bears propanol, 2, 3-dihydroxy (26.43%) with sugars like L-glucose (11.78%) and sorbitol (23.65%) which might be responsible for the edible nature of fruits. GC-MS analysis was performed for Salacia oblonga with detection of 16 compounds from roots and aerial parts [26].
Table 5.
Identification of phytochemicals in various parts of S. chinensis by GC–MS.
| Sr. No. | Name of Compound | RT (min.) | Peak Area | Area (%) | Plant part |
|---|---|---|---|---|---|
| 1 | 1,2,3- Propanetriol, monoacetate | 11.545 | 14573547 | 04.88 | FP |
| 2 | 1,2-Benzenedicarboxylic acid, bis | 39.712 | 182718 | 17.86 | R |
| (2-methylpropyl) ester | |||||
| 3 | 1,3- Dioxane, 4-methyl- | 12.790 | 4966087 | 01.66 | FP |
| 4 | 1,3- Dioxolane, 2,4,5- trimethyl-$$ | 13.911 | 6519588 | 02.18 | FP |
| 5 | 1-Butanol, 3-methyl-, acetate | 11.616 | 176549 | 03.74 | ST |
| 11.615 | 81135 | 03.57 | S | ||
| 6 | 2-Furancarboxaldehyde, 5-(hydroxymethyl) | 23.793 | 29399 | 02.62 | S |
| 7 | 2-Furancarboxaldehyde, 5-methyl- | 22.206 | 319252 | 14.06 | S |
| 8 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol $$ | 38.857 | 55258 | 17.49 | L |
| 9 | 3-Deoxy-d-mannoic lactone | 16.334 | 12207552 | 04.09 | FP |
| 10 | 4H-Pyran-4-one, 2,3- dihydro-3,5-dihydroxy -6-methyl | 10.114 | 3622714 | 01.21 | FP |
| 11 | 5-Oxotetrahydrofuran-2- carboxylic acid | 11.074 | 2248963 | 00.75 | FP |
| 12 | Acetic acid, 2-methylpropyl ester | 08.264 | 249978 | 05.30 | ST |
| 08.264 | 142396 | 06.27 | S | ||
| 13 | Benzene, 1,2-dimethyl- | 12.405 | 584006 | 12.38 | ST |
| 12.276 | 38790 | 12.28 | L | ||
| 14 | Benzeneacetic acid,.alpha.,4-dihydroxy-3-methoxy- $$ Mandelic acid, 4-hydroxy-3-methoxy- $$ (4-Hydroxy-3-metho | 35.606 | 122371 | 02.59 | ST |
| 15 | Beta-d-Ribopyranoside, methyl, 3-acetate $$ | 31.263 | 310134 | 06.58 | ST |
| 16 | Butanedioic acid, hydroxy-, diethyl ester, (.+/-.)- $$ Diethyl dl-malate $$ Ethyl dl-malate $$ Butanedioic acid, hydroxy | 23.755 | 22447 | 02.19 | R |
| 17 | Dibutyl phthalate | 41.574 | 27760 | 08.79 | L |
| 18 | Dibutyl phthalate $$ 1,2-Benzenedicarboxylic acid, dibutyl ester $$ Phthalic acid, dibutyl ester $$ n-Butyl phthalate | 41.631 | 249332 | 05.29 | ST |
| 19 | d-Mannitol, 1,4-anhydro- | 33.680 | 191762 | 04.07 | ST |
| 20 | Ethyl.alpha.-d-glucopyranoside $$ | 37.150 | 236450 | 23.11 | R |
| 37.260 | 418505 | 08.87 | ST | ||
| 37.201 | 71904 | 22.76 | L | ||
| 37.319 | 1232724 | 54.27 | S | ||
| 21 | Ethyl.beta.-d-riboside $$ | 27.472 | 108149 | 10.57 | R |
| 27.504 | 1590414 | 33.72 | ST | ||
| 22 | Heptanoic acid, 6-oxo-$$ | 12.315 | 12024118 | 04.03 | FP |
| 23 | L-Glucose | 17.284 | 35174302 | 11.78 | FP |
| 24 | n-Hexadecanoic acid $$ Hexadecanoic acid $$ n-Hexadecoic acid $$ Palmitic acid $$ Pentadecanecarboxylic acid | 41.309 | 88068 | 27.88 | L |
| 25 | Octadecanoic acid, ethyl ester | 41.920 | 34117 | 10.80 | L |
| 26 | Pentacosane | 45.211 | 301486 | 29.47 | R |
| 27 | Propanoic acid, 3- (acetylthio)-2-methyl | 12.037 | 23686766 | 07.93 | FP |
| 28 | Propanol, 2, 3-dihydroxy-$$.alpha.,. beta.- dihydroxypropion-aldehyde $$ DL-GLYC $$ DLG $$ Glyceraldehydes $$ Glyceric Aldehyde | 09.552 | 78956209 | 26.43 | FP |
| 29 | p-Xylene | 12.408 | 422856 | 18.62 | S |
| 30 | Sorbitol | 19.209 | 70642184 | 23.65 | FP |
| 31 | Tetracosane | 41.155 | 141461 | 13.83 | R |
| 32 | Xanthosine $$.beta.-D-Ribofuranoside, Xanthine-9 $$ Xanthine riboside $$ 1H-Purine-2, 6- dione, 3,9- dihydro-9-beta.- D-ribofuranosyl- $$ | 14.533 | 34091613 | 11.41 | FP |
R: Root, ST: Stem, L: Leaf, FP: Fruit Pulp, S: Seed.
Fig. 3.
GC-MS total ion chromatograms (TIC) of various parts of S. chinensis. A: Root, B: Stem, C: Fruit pulp, D: Seed.
3.7. Statistical analysis
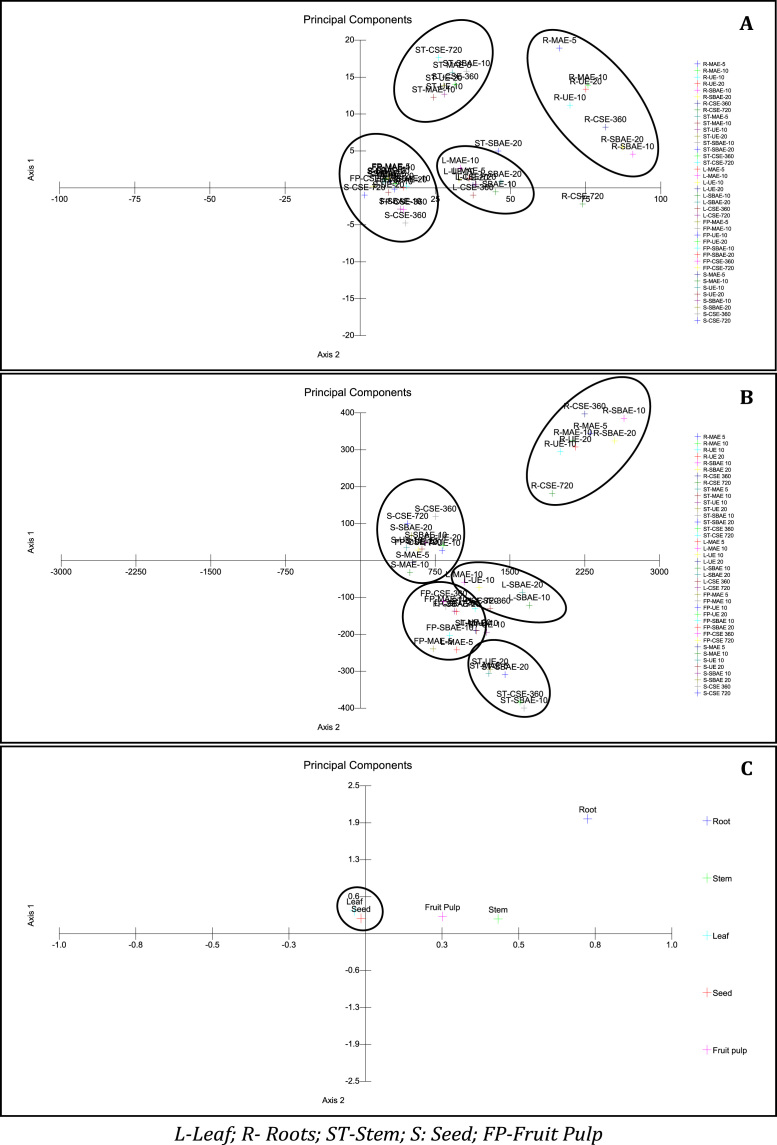
The significance test for the data obtained from the experiments performed were subjected to one way analysis of variance (ANOVA) using Tukey test for TPC, TFC and antioxidant capacity of all samples tested for S. chinensis. The results obtained at p < 0.05 were interpreted as significant. Table 6 represents the result of one way ANOVA using Tukey test. The result also interprets comparison within the group and between the groups studied. The data obtained from the present study was grouped into three different components for statistical analysis (i) total polyphenols (TPC and TFC); (ii) antioxidant activities (DPPH, FRAP, DMPD and ABTS) and (iii) HPLC analysis. Synchrony and equity in the data was maintained by conversion in milligram per gram dried plant tissue (mg/g). In PCA the data is reduced to a set of new latent variables called principal components (PCs). The loadings of the PC define the direction of greatest variability and the score values represent the projection of each object onto PC. Fig. 4A-C depicts dendrogram obtained from data collected from three different phytochemical parameters. Herein, the samples can be divided into four major clades. PCA of results obtained from polyphenol contents showed distribution of species in the positive x and y axis with exception of fruit pulp and seeds (Fig. 4A), might be due to the poor contents of polyphenol (Table 2).
Table 6.
One way analysis of variance (ANOVA) with post test done using Tukey test for the results obtained from each experiment.
| Experiments |
Degree of freedom |
Sum of squares |
Mean square |
F | |||
|---|---|---|---|---|---|---|---|
| Treatments | Residuals | Treatments | Residuals | Treatments | Residuals | ||
| TPC-TA | 25 | 52 | 11693 | 359.09 | 467.72 | 6.91 | 67.73 |
| TPC-GA | 25 | 52 | 11282 | 305.52 | 451.28 | 5.88 | 76.81 |
| TFC-QE | 25 | 52 | 6964.1 | 278.57 | 127.43 | 2.45 | 113.67 |
| TFC-EA | 25 | 52 | 16009 | 301.52 | 640.36 | 5.79 | 110.44 |
| DPPH-AEAC | 25 | 52 | 2233906 | 99288 | 89356 | 1909.40 | 46.80 |
| DPPH-TEAC | 25 | 52 | 3653521 | 150497 | 146141 | 2894.20 | 50.50 |
| FRAP-AEAC | 25 | 52 | 742698 | 74035 | 29708 | 1423.50 | 20.87 |
| FRAP-TEAC | 25 | 52 | 812581 | 93784 | 32503 | 1803.5 | 18.02 |
| ABTS-AEAC | 25 | 52 | 4278771 | 105566 | 171151 | 2030.10 | 84.31 |
| ABTS-TEAC | 25 | 52 | 6517459 | 159602 | 260698 | 3069.30 | 84.94 |
| DMPD-AEAC | 25 | 52 | 4625649 | 117452 | 185026 | 2258.70 | 81.92 |
| DMPD-TEAC | 25 | 52 | 1.077E+07 | 358110 | 430932 | 6886.70 | 62.57 |
Treatment: Within the group; Residual: Between the groups.
Fig. 4.
PCA plots for data obtained from A: Total polyphenols (TPC and TFC); B: Antioxidant activities (DPPH, FRAP, DMPD and ABTS); C: HPLC analysis.
4. Conclusions
In conclusion, different parts of S. chinensis evaluated for the extraction of total phenolics, flavonoids and antioxidants by using various extraction techniques and the results indicated the steam bath assisted extraction technique was most efficient as compared to other extraction techniques. Steam bath assisted extraction for 10 min yielded significant results over other methods for various parts of S. chinensis. The roots were identified as good source for secondary metabolites. Chromatic techniques (RP-HPLC-DAD and GC-MS) confirmed presence of bioactive phenolic acids and thirty-two different phytoconstituents in various parts of S. chinensis. Moreover, mineral analysis validates the rich inpartic status of the plant. The information generated about the mineral minerals, secondary metabolite and antioxidants of S. chinensis fruits may increase the awareness about this underutilized fruit among the users. The present investigation opens an avenue for further research on importance (nutritive, medicinal, etc.) and role (biosynthesis, accumulation etc.) of identified bioactive compounds.
Acknowledgement
The authors are grateful to Science and Engineering Research Board (SERB), DST, Govt. of India for financial assistance (SB/FT/LS-259/2012 dated 02/05/2013). We sincerely thank the Principal and Head, Department of Botany, Yashavantrao Chavan Institute of Science, Satara for encouragement and providing laboratory facilities. We are also indebted to the Director-in-Charge, Regional Medical Research Centre, Indian Council Medical Research, Belagavi.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.08.012.
Appendix A. Transparency document
Supplementary material
References
- 1.Singh N.P., Vohra J.N., Hajra P.K., Singh D.K. Vol. 5. Botanical Survey of India; Calcutta: 2000. Flora of India; pp. 150–162. [Google Scholar]
- 2.Li Y., Huang T.S., Yamahara J. Salacia root, a unique Ayurvedic medicine, meets multiple targets in diabetes and obesity. Life Sci. 2008;82:1045–1049. doi: 10.1016/j.lfs.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Reddy K.N., Pattanaik C., Reddy C.S., Raju V.S. Traditional knowledge on wild food plant in Andhra Pradesh. Ind. J. Trad. Knowl. 2007;6:223–229. [Google Scholar]
- 4.Ramakrishna D., Shashank A.T., Shinomol-George K., Kiran S., Gavishankar G.A. Salacia Sps– a potent source of herbal drug for antidiabetic and antiobesity ailments: a detailed treatise. Int. J. Pharm. Phytochem. Res. 2015;7:374–382. [Google Scholar]
- 5.Yoshikawa M., Nishida N., Shimoda H., Takada M., Kawahara Y., Matsuda H. Polyphenol constituents from Salacia species: quantitative analysis of mangiferin with alpha-glucosidase and aldose reductase inhibitory activities. Yakugaku Zasshi. 2001;121:371–378. doi: 10.1248/yakushi.121.371. [DOI] [PubMed] [Google Scholar]
- 6.Tran T.M., Nguyen T.H.A., Vu D.T., Tran V.S. Study on chemical constituents and cytotoxic activities of Salacia chinensis growing in Vietnam. Z. Nat. 2010;65b:1284–1288. [Google Scholar]
- 7.Tenenbaum A., Fisman E.Z., Motro M. Metabolic syndrome and type 2 diabetes mellitus: focus on peroxisome proliferator activated receptors (PPAR) Cardiovasc. Diabetol. 2001;1:2–4. doi: 10.1186/1475-2840-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guha S., Ghosal S., Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy. 1996;42:443–451. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- 9.Chavan J.J., Ghadage D.M., Bhoite A.S., Umdale S.D. Micropropagation, molecular profiling and RP-HPLC determination of mangiferin across various regeneration stages of Saptarangi (Salacia chinensis L.) Ind. Crop. Prod. 2015;76:1123–1132. [Google Scholar]
- 10.Bhattacharyya P., Kumaria S., Tandon P. Applicability of ISSR and DAMD markers for phyto-molecular characterization and association with some important biochemical traits of Dendrobium nobile, an endangered medicinal orchid. Phytochemistry. 2015;117:306–316. doi: 10.1016/j.phytochem.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Pierson J.T., Monteith G.R., Roberts-Thomson S.J., Dietzgen R.G., Gidley M.J., Shaw P.N. Phytochemical extraction, characterisation and comparative distribution across four mango (Mangifera indica L.) fruit varieties. Food Chem. 2014;149:253–263. doi: 10.1016/j.foodchem.2013.10.108. [DOI] [PubMed] [Google Scholar]
- 12.Ene-Obong H.N., Okudu H.O., Asumugha U.K. Nutrient and phytochemical composition of two varieties of Monkey kola (Cola parchycarpa and Cola lepidota): an underutilised fruit. Food Chem. 2016;193:154–159. doi: 10.1016/j.foodchem.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 13.Harborne J.B. Chapman and Hall, Ltd.; London: 1973. Phytochemical Methods; pp. 49–188. [Google Scholar]
- 14.Yadav R.N.S., Agarwal M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011;3:10–14. [Google Scholar]
- 15.Chavan J.J., Ghadage D.M., Kshirsagar P.R., Kudale S.S. Optimization of extraction techniques and RP-HPLC analysis of antidiabetic and anticancer drug mangiferin from roots of ‘Saptarangi’ (Salacia chinensis L.) J. Liq. Chromatogr. Relat. Technol. 2015;38:963–969. [Google Scholar]
- 16.Pai S.R., Nimbalkar M.S., Pawar N.V., Dixit G.B. Optimization of extraction techniques and quantification of betulinic acid (BA) by RP-HPLC method from Ancistrocladus heyneanus wall. Ex Grah. Ind. Crop. Prod. 2011;34:1458–1464. [Google Scholar]
- 17.Chavan J.J., Jagtap U.B., Gaikwad N.B., Dixit G.B., Bapat V.A. Total phenolics, flavonoids and antioxidant activity of Saptarangi (Salacia chinensis L.) fruit pulp. J. Plant Biochem. Biotechnol. 2013;22:409–413. [Google Scholar]
- 18.Wolfe K., Wu X., Liu R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2013;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 19.Chang C., Yang M., Wen H., Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 20.Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm.-Wiss. Technol. 1995;28:25–30. [Google Scholar]
- 21.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Ann. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radi. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 23.Fogliano V., Verde V., Randazzo G., Rittieni A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999;47:1035–1040. doi: 10.1021/jf980496s. [DOI] [PubMed] [Google Scholar]
- 24.Kadam A.P., Salunkhe N.B., Aparadh V.T., Kadam D.A., Chavan J.J. Discrepancy in polyphenol content and mineral composition from various parts of Solanum xanthocarpum Schrad & Wendl. Nat. Acad. Sci. Lett. 2014;37:187–190. [Google Scholar]
- 25.Adumanya O.C.U., Obiloma A.A., Essien E.B. Proximate, vitamins and mineral composition of Salacia senegalensis Lam (DC) leaves. Open. J. Res. 2015;2:92–105. [Google Scholar]
- 26.Musini A., Jayaram M., Rao P., Giri A. Phytochemical investigations and antibacterial activity of Salacia oblonga wall ethanolic extract. Ann. Phytomed. 2013;2:102–107. [Google Scholar]
- 27.Julsing M.K., Koulman A., Woerdenbag H.J. Combinatorial biosynthesis of medicinal plant secondary metabolites. Biomol. Eng. 2006;23:265–279. doi: 10.1016/j.bioeng.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Mustapa A.N., Martin A., Mato R.B., Cocero K.J. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind. Crop. Prod. 2015;74:83–94. [Google Scholar]
- 29.Upadhya V., Pai S.R., Hegde H.V. Effect of method and time of extraction on total phenolic content in comparison with antioxidant activities in different parts of Achyranthes aspera. J. King Saud. Uni. – Sci. 2015;27:204–208. [Google Scholar]
- 30.Paixao N., Pereira V., Marques J.C., Camara J.S. Quantification of polyphenols with potential antioxidant properties in wines using reverse-phase HPLC. J. Sep. Sci. 2008;31:2189–2198. doi: 10.1002/jssc.200800021. [DOI] [PubMed] [Google Scholar]
- 31.Kontoyannis C.G., Orkoula M. Quantitative non-destructive determination of salicylic acid acetate in aspirin tablets by Raman spectroscopy. Talanta. 1994;41:1981–1984. doi: 10.1016/0039-9140(94)00169-3. [DOI] [PubMed] [Google Scholar]
- 32.Sulaiman C.T., Thushar K.V., George S., Balachandran I. Phenolic characterization of selected Salacia species using LC-ESI-MS/MS analysis. Nat. Prod. Res. 2014;28:1021–1024. doi: 10.1080/14786419.2014.905562. [DOI] [PubMed] [Google Scholar]
- 33.Kishi A., Morikawa T., Matsuda H., Yoshikawa M. Structures of new friedelane- and norfriedelane-type triterpenes and polyacylated eudesmane-type sesquiterpene from Salacia chinensis Linn. (S. prinoides DC, Hippocrateaceae) and radical scavenging activities of principal constituents. Chem. Pharm. Bull. 2003;51:1051–1055. doi: 10.1248/cpb.51.1051. [DOI] [PubMed] [Google Scholar]
- 34.Tupe R.S., Kemse N.G., Khaire A.A. Evaluation of antioxidant potentials and total phenolic contents of selected Indian herbs powder extracts. Int. Food Res. J. 2013;20:1053–1063. [Google Scholar]
- 35.Roopa G., Madhusudhan M.C., Triveni K., Mokaya N.E., Prakash H.S., Geetha N. Evaluation of antioxidant properties of Salacia macrosperma leaf extracts. Int. J. Res. Stud. Sci. Eng. Technol. 2015;2:58–63. [Google Scholar]
- 36.Julian-Loaeza A.P., Santos-Sanchez N.E., Valadez-Blanco R., Sanchez-Guzman B.S., Salas-Coronado R. Chemical composition, color, and antioxidant activity of three varieties of Annona diversifolia Safford fruits. Ind. Crop. Prod. 2011;34:1262–1268. [Google Scholar]
- 37.Rane Z., Anish-Kumar P., Bhaskar A. Phytochemical evaluation by GC-MS and in vitro antioxidant activity of Punica granatum fruit rind extract. J. Chem. Pharm. Res. 2012;4:2869–2873. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material