Abstract
Pattern recognition receptors (PRRs) may contribute to inflammatory bowel diseases (IBD) development due to their microbial-sensing ability and the unique microenvironment in the inflamed gut. In this study, the PRR mRNA expression profile together with T cell-associated factors in the colon was examined using a chronic colitis mice model. 8–12 week old C57BL/6 mice were exposed to multiple dextran sodium sulfate (DSS) treatments interspersed with a rest period to mimic the course of chronic colitis. The clinical features and histological data were collected. The mRNA expressions of colonic PRRs, T cell-associated components were measured. Finally, the colons were scored for Foxp3+ cells. During chronic colitis, the histological data, but not the clinical manifestations demonstrated characteristic inflammatory symptoms in the distal colon. In contrast to acute colitis, the expression of all Toll-like receptors (Tlrs), except Tlr5 and Tlr9, was unaffected after repeated DSS treatments. The expression of Nod1 was decreased, while Nod2 increased. After third DSS treatment, only the expressions of Tlr3 and Tlr4 were significantly enhanced. Unlike other PRRs, decreased Tlr5 and increased Tlr9 mRNA expression persisted during the chronic colitis period. As the colitis progress, only the mRNA expression of Ifnγ and Il17 staid increased during chronic colitis, while the acute colitis-associated increase of Il23, and Il10 and Il12 was abolished. Finally, increased histological score of Foxp3+ cell in colon was found during the chronic colitis period. This study provides an expression pattern of PRRs during chronic colitis that is accompanied by a Th1- and Th17 cell-mediated immune response.
Keywords: Inflammatory bowel disease, Toll like receptors, Th cell responses
Highlights
-
•
This study provides an extensive survey of PRRs in the colon during chronic DSS-induced colitis.
-
•
Chronic DSS colitis upregulates the mRNA expression of Tlr3, Tlr4, Tlr9 and Nod2.
-
•
As the DSS colitis progresses to a chronic status the expression of Tlr5 decreases.
-
•
The chronic DSS colitis results in a (progressive) increase of Th1 and Th17 cells.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disorder and is characterized by a relapse-remitting course that is caused by recurrent intestinal inflammation [1]. The two major forms of IBD, Crohn's disease (CD) and ulcerative colitis (UC), often have an onset during early adulthood and significantly affect the quality of the life [2]. The established high prevalence of IBD population in US and Europa indicate the requirement for an efficient treatment for IBD [2]. However, the existing treatments are mainly focus on relieving of symptoms and are often accompanied with unwanted side effects [3]. To elucidate the disease pathogenesis and develop more efficient treatment, there is growing interest for targeting pattern recognition receptors (PRRs). The unique microenvironment in the gut, where abundant microorganisms co-exist and the microbial-sensing ability of PRRs suggest that PRRs could contribute to both maintaining and breakdown of the intestinal homeostasis [4], [5]. The development of gut dysbiosis and imbalances in host-microbiome interaction has been demonstrated to contribute to the extent, severity and chronicity of intestinal inflammation [2]. Furthermore, an aberrant immune response towards gut bacteria has been suggested to be the major contributor in the inflammatory response of IBD [6].
Toll-Like receptors (TLRs) are possibly the best studied PRRs that sense a broad spectrum of invading pathogens by recognize pathogen-associated molecular patterns (PAMPs), unique molecules on these pathogens [7]. Up to date, 13 TLRs have been discovered, from which TLR1-9 are conserved in both human and mouse. Based on the location, these TLRs can be divided into two groups; receptors expressed on the cell surface (TLR1, TLR2, TLR6, TLR4 and TLR5) and intracellular receptors (TLR3, TLR7, TLR8 and TLR9) [7]. To recognize the specific ligand, the TLRs form functional mono-dimers or a functional complex with other component such as MD-2 (for TLR4), except TLR1, TLR2 and TLR6 [7]. Both TLR1 and TLR6 form functional hetero-dimer complex with TLR2 to recognize triacyl- or diacyl-lipopeptides from bacteria, respectively [8]. Upon activation by specific ligand derived from bacteria, fungi, parasites or virus, they will induce innate immune response and strength adaptive immune response to provide protection against pathogens [9]. However, unregulated activation of TLRs could lead to extensive and chronic inflammation, which results in inflammatory disease such as IBD [4], [10]. In agreement with this hypothesis, increased expression of TLRs such as TLR2, TLR3 and TLR4, are found in the colon of IBD patient [11], [12]. In addition, an association was found between the polymorphism of TLRs and the susceptibility of IBD development [13], [14], [15].
Nucleotide-binding oligomerization domain-containing protein (human: NOD; mice Nod) is another PRR family. Two member of this PRR group, NOD1 and NOD2 are located exclusively intracellular and able to detect peptidoglycan, a cell wall component on bacteria [16]. There has been great interest in studying the role of NOD2 and its related receptor NOD1 in IBD development, because NOD2 encoding gene NOD2 is the first gene that has been directly associated with CD and confer great risk for the development of IBD [17], [18]. The CD risk variant NOD2 gene causes ‘loss of function’ resulting in reduced autophagy induction which result in reduced bactria killing and impaired antigen presenting, which could be the trigger to the development of IBD [19].
Experimental animal models have been used to study the pathology of the IBD and clarify the underlying mechanisms [20]. Among these animal models, DSS-induced colitis in mice is most commonly used model to investigate IBD-like colitis due to its simplicity and the ability to induce predictable intestinal inflammation [21], [22]. While single DSS exposure induces colitis modeling acute injury and repair mechanism, repeated DSS exposure cycles interspersed with recovery period mimic the chronic nature of IBD [23], [24].
In our previous study, we have illustrated the colonic expression pattern of PRRs and the T-helper (Th) cell response in mice using a DSS-induced acute colitis model. To extent our knowledge of the expression of these PRRs and activated immune response during chronic colitis, the mRNA expression of these PRRs in the colon during chronic colitis was determined after repeated DSS exposure. In addition, the T cell development during the chronic colitis was monitored by measuring the mRNA expression of T cell-associated master transcription factors and cytokines.
2. Materials and methods
2.1. Animals
Female C57BL/6 mice were purchased from Charles River Laboratories (Maastricht, the Netherlands). All mice were used at 8–12 weeks of age and were housed under standard conditions in the animal facilities at Utrecht University. All animal experiments were approved by and were in accordance with the guidelines of the Dutch Experimental Animal Commission. The approval document is encoded with 2008.II.03.030.
2.2. Experimental colitis
Chronic colitis was induced in groups of 6 mice by administration of 3 cycles 1.5% DSS to the drinking water of the mice for 6 days with a rest period of 10 days. Colitis development was monitored by measuring the bodyweight and scoring the feces condition during the experiment and measuring the colon length/weight ration after sacrificing the mice on the end of each DSS treatment cycle (day 7, day 23 and day 39). The feces condition assessment was started from experimental day 5 until the end of the experiment (day 39). The feces condition score was determined from two parameters: stool consistency (0 = normal, 1 = soft with normal form, 2 = loss of form/diarrhea) and fecal bleeding (0 = no blood, 1 = blood observation using Colo-rectal Test kit (Axon Lab AG, Germany), 2 = blood observation without test).
2.3. Histological evaluation of colon damage and immunohistochemical staining
After sacrificing the mice on the end of each DSS treatment cycle (day 7, day 23 and day 39), colons (n = 3) were taken out for histological evaluation and immunohistochemical staining. The colon was opened longitudinally; half of each colon was washed in the phosphate buffered saline (PBS) and placed on a piece of blotting paper. After fixing in 10% formalin for 24 h, colons were paraffin-embedded as swiss-roles and sectioned (5 µm). Two researchers assessed general inflammatory features blindly after staining sections with hematoxylin and eosin according the assessment system described before [25]. Briefly, the histological assessments included four pathological criteria: the extent of cellular infiltration (0: no infiltration, 1: infiltration between the crypts, 2: infiltration in the submucosa, 3: infiltration in the muscularis externa, 4: infiltration in entire tissue); cover area of cellular infiltration in the region (0: no infiltration, 1: < 25%, 2: 25–50%, 3: 50–75%, 4: >75%); loss of crypts (0: no damage, 1: 30% shortening of crypts, 2: 65% shorting of crypts, 3: total loss of crypts, 4: loss of entire epithelial layer); extent of crypts loss in the region (0: no crypt loss, 1: < 25%, 2: 25–50%, 3: 50–75%, 4: > 75%). Individual scores were obtained for the whole colon from caecum to anus by two researchers who did not know the origin of the sample.
Immunohistochemistry was employed to determine the Ly-6B.2+ cells (neutrophils & some activated macrophages) and Foxp3+ cells. The sections were subjected to a heat-induced epitope retrieval step. Slides were washed with PBS and blocked with rabbit or goat serum before an overnight incubation (4 °C) with primary antibodies against Ly-6B.2 (AbD Serotec, Dusseldorf, Germany) or Foxp3 (eBioscience San Diego, CA USA). For detection, biotinylated goat anti-rat (Dako, Glostrup, DK) secondary antibodies were administered followed by incubation with peroxidase-labeled streptavidin (Vectastain EliteABC kit, Vector, Burlingame, CA USA). The peroxidase activity was visualized using the substrate, DAB (Sigma, Gillingham, UK). The cell nuclei were visualized by a short incubation with Mayer's hematoxylin (Klinipath, Duiven, the Netherlands). Background staining was determined by substituting the primary antibody with a rat IgG isotype control (Abcam, Cambrige, UK, Fig. S6). The neutrophil infiltration score and Foxp3+ cell score were determined using the following criteria: the extent of Ly6B.2+ or Foxp3+ cellular infiltration (0: no infiltration, 1: infiltration between the crypts, 2: infiltration in the submucosa, 3: infiltration in the muscularis externa, 4: infiltration in entire tissue); cover area of cellular infiltration in the region (0: no infiltration, 1: < 25%, 2: 25–50%, 3: 50–75%, 4: > 75%). Individual scores for Ly6B.2+ cellular infiltration were obtained for the whole colon from caecum to anus. Individual scores for Foxp3+ cellular infiltration were tallied for the proximal colon (characterized by bulges in the colon wall) and the distal colon (the region starting from end of proximal portion stretching to the anus). Scoring was performed by two researchers who did not know the origin of the sample
2.4. Assessment of myeloperoxidase concentration in the colon tissue
After sacrificing the mice, colons (n = 3) were taken out for myeloperoxidase (MPO) concentration assessment, marker for neutrophils. The colon was opened longitudinally; half of each colon was separated into proximal colon (characterized by bulges in the colon wall) and distal colon (the region starting from end of proximal portion stretching to the anus). Subsequently, the colons pieces were transferred into RIPA buffer (Thermo Scientific, Rockford, IL USA) and homogenized using a Precellys®24-Dual homogenizer (Precellys, Villeurbanne, France). The homogenates were centrifuged at 14,000 rpm for 10 min at 4 °C and the MPO concentration in the supernatant was measured using an ELISA kit according to the manufacturer's protocol (Hycult biotech, Uden, the Netherlands).
2.5. mRNA expression analysis
After sacrificing the mice, colons (n = 6) were taken out for gene expression analysis. The colon was opened longitudinally and the proximal colon was distinct from the distal colon by its specific structure (bulges in the colon wall). The total RNA was isolated using the RNAeasy kit (Qiagen, Germantown, MD USA) and, subsequently, reverse transcribed into cDNA using the iScript cDNA sythesis kit (BioRad, Hercules, CA USA). Real-time PCR was performed using iQ SYBR Green super mix kit (BioRad, Hercules, CA USA) with the CFX 96 Real-time system (BioRad, Hercules, CA USA) and the relative mRNA expression values were calculated using Bio-Rad CFX manager V1.6. The sequence of specific primers for Nod1 and Nod2, Tlrs, T cell transcription factor genes, and the gene for the household protein ribosomal protein S13 (Rps13) are listed in Table 1. The primers for the cytokines: tumor necrosis factor-α (Tnf), interleukin-1β (Il-1beta), Il-6, monocyte chemotactic protein-1 (Ccl-2), interferon-γ (Ifn-gamma), Il-12p35, Il-4, Il-5, Il-13, Il-23p19, Il-17, Il-10 and transforming growth factor β (Tgf-beta) were purchased from SABioscience (Frederick, MD USA). The final data for the target samples were normalized against the internal control Rps13.
Table 1.
qPCR primer sequence.
|
Primer Sequence 5′→3′ |
||
|---|---|---|
| Forward primer | Reverse primer | |
| Tlr1 | GGTGTTAGGAGATGCTTATGGGG | GATGTTAGACAGTTCCAAACCGA |
| Tlr2 | CCAGACACTGGGGGTAACATC | CGGATCGACTTTAGACTTTGGG |
| Tlr3 | GGGGTCCAACTGGAGAACCT | CCGGGGAGAACTCTTTAAGTGG |
| Tlr4 | GCCTTTCAGGGAATTAAGCTCC | AGATCAACCGATGGACGTGTAA |
| Tlr5 | TCAGACGGCAGGATAGCCTTT | AATGGTCAAGTTAGCATACTGGG |
| Tlr6 | GACTCTCCCACAACAGGATACG | TCAGGTTGCCAAATTCCTTACAC |
| Tlr7 | TCTTACCCTTACCATCAACCACA | CCCCAGTAGAACAGGTACACA |
| Tlr8 | GGCACAACTCCCTTGTGATT | CATTTGGGTGCTGTTGTTTG |
| Tlr9 | ACTCCGACTTCGTCCACCT | GGCTCAATGGTCATGTGGCA |
| Nod1 | GAAGGCACCCCATTGGGTT | AATCTCTGCATCTTCGGCTGA |
| Nod2 | CCGCTTTCTACTTGGCTGTC | GTGATTTGCAGGTTGTGTGG |
| Tbet | GCCAGCCAAACAGAGAAGAC | AAATGTGCACCCTTCAAACC |
| Gata3 | GCGGTACCTGTCTTTTTCGT | CACACAGGGGCTAACAGTCA |
| Foxp3 | CACTGGGCTTCTGGGTATGT | AGACAGGCCAGGGGATAGTT |
| Rorc | TGCAAGACTCATCGACAAGG | AGGGGATTCAACATCAGTGC |
| Rps13 | GTCCGAAAGCACCTTGAGAG | AGCAGAGGCTGTGGATGACT |
2.6. Statistical analysis
Means with SEM are represented in each graph. Statistical analysis was performed using GraphPad Prism version 6.0 for windows (GraphPad Software, San Diego, CA USA). P-values were calculated using either the two-way ANOVA followed by Bonferroni post-tests or a Mann-Whitney test. P-values considered as significant are indicated as *** < 0.001, ** < 0.01, and * < 0.05. a p < 0.05 cycle 2 and cycle 3 control mice compare to cycle 1 control mice. b p < 0.05 DSS-treated mice compare to control mice after each DSS treatment cycle. c p < 0.05 cycle 2 and cycle 3 DSS-treated mice compare to cycle 1 DSS-treated mice d p < 0.05 cycle 3 DSS-treated mice compare to cycle 2 DSS-treated mice.
3. Results
3.1. Repeated DSS treatment induces features of chronic colitis
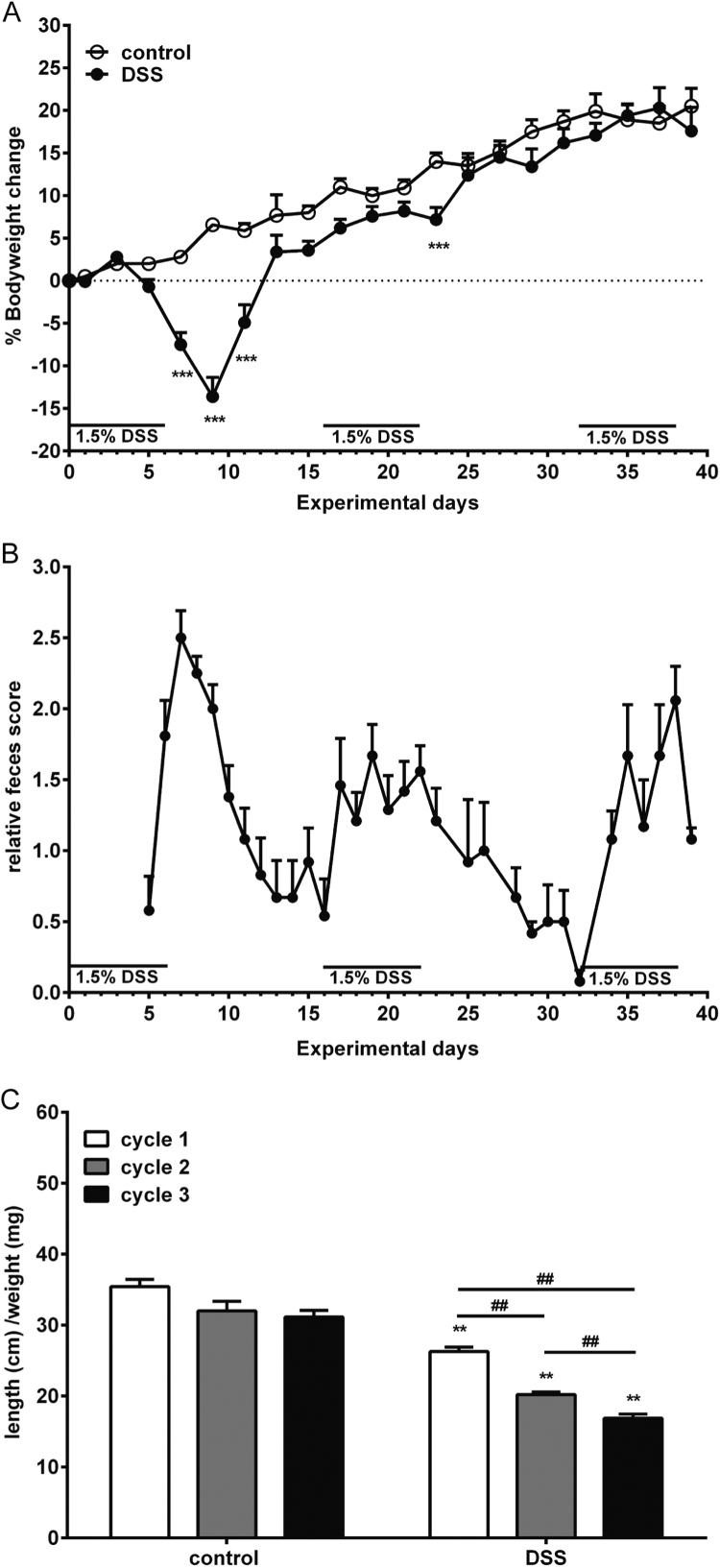
In a previous study, we have induced an acute colitis by adding 1.5% DSS into drinking water of mice for 6 days and found decreased bodyweight and increased feces score [25]. In the current study, we assessed the bodyweight and fecal phenotype changes of mice undergoing repeated DSS treatments. Acute colitis was accompanied with a decreased bodyweight and increased feces score. After repeated DSS treatments, there were no longer inductions of major bodyweight loss as observed after the first DSS treatment cycle (Fig. 1A). During and after all three cycles of DSS treatment increased feces scores were found, although a less extended increase and faster recovery rate were observed during and after second and third DSS treatment (Fig. 1B). After sacrificing the mice, the colon length and weight were measured. There was a decreased colon length/weight ratio after each DSS treatment cycle as compare to healthy mice: this ratio further decreased as the DSS treatment cycle progressed (Fig. 1C).
Fig. 1.
An increased feces score was found after each DSS treatment, while bodyweight loss was only found after acute DSS treatment. A) The bodyweight changes of control and DSS-treated mice during repeated DSS treatment are illustrated in the figure. B) The increased fecal scores of DSS-treated mice as compared to control mice after the first DSS treatment until end of the experiment are shown (n = 18 during cycle 1 DSS treatment, n = 12 during cycle 2 DSS-treatment and n = 6 cycle 3 DSS treatment). C) The colon length (cm) /weight (mg) ratio was determined (n = 6 per group). All results are expressed as + SEM, * indicates significant different between control and DSS-treated group. # Indicates significant different between the DSS-treatment cycles ##, ** P < 0.01, *** P < 0.001.
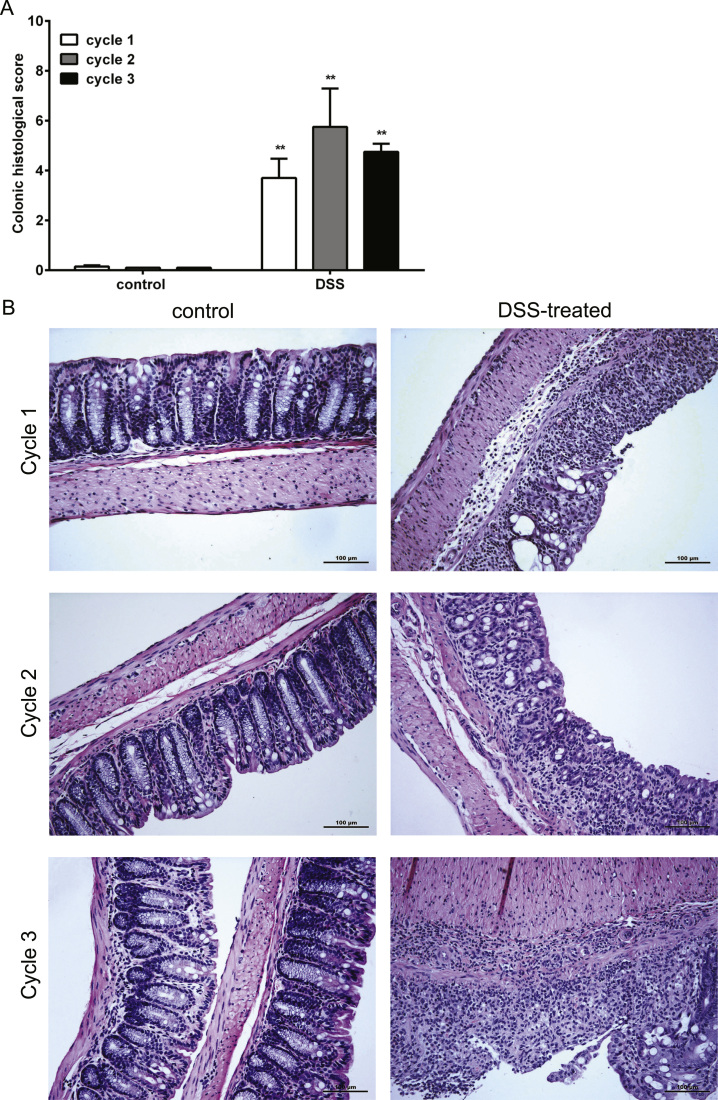
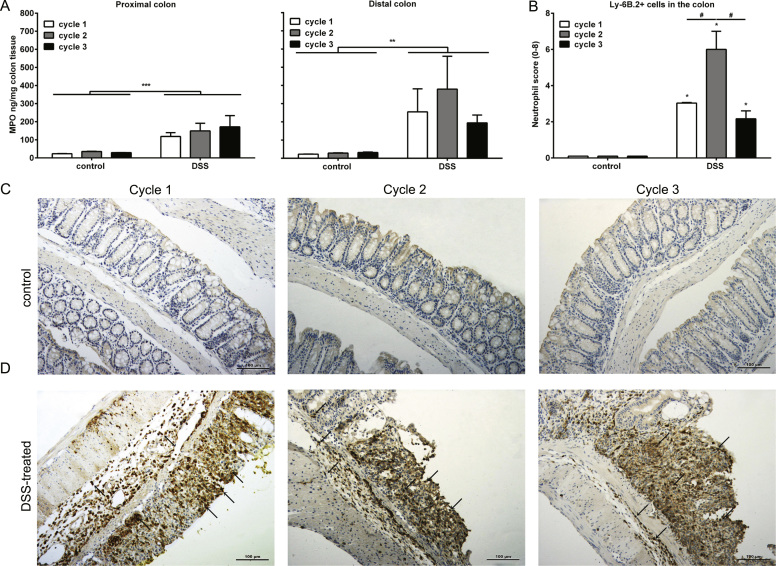
To determine the extent of the inflammation, colonic histological score including tissue damage and cellular infiltration was accessed. The tissue damage and cellular infiltrations were observed in the colon of DSS-treated mice, predominantly in the distal part, after each DSS treatment (Fig. 2). The MPO levels and Ly-6B.2 expressing cells in colon were analyzed to determine the infiltration of neutrophil cells. MPO is abundantly expressed in neutrophil granulocytes [26] and is frequently used as marker of neutrophil infiltration, while Ly-6B.2 expression is particularly high on the surface of neutrophils and some inflammatory macrophages [27]. An increased expression of MPO was found in both proximal and distal part of the colon isolated from DSS-treated mice as compare to healthy control mice after each DSS treatment cycle (Fig. 3A). This result indicates an increased amount of neutrophils in the colon of DSS-treated mice. The increased score of Ly-6B.2 expressing cell in the colon of DSS-treated mice supports this finding (Fig. 3B-D). In addition, a significant increase of neutrophil score was found after second DSS treatment as compared with first and third DSS treatment cycle (Fig. 3B-D).
Fig. 2.
DSS treatment induces damage and cellular infiltration in the colon. Colons were collected for histological assessment as described in material and methods. A) Colonic histological score and B) representative histology staining photos of the colons derived from control or DSS-treated mice after each DSS treatment cycle are shown (n = 3 per group).
Fig. 3.
DSS treatment induces neutrophil influx in the colon. Colons were collected for A) MPO concentration measurement in the proximal and distal colon and B) immunohistochemical assessment of Ly-6b+ cells. Representative histochemical staining picture of Ly-6b+ cells in the colons of C) control and D) DSS-treated mice during each DSS treatment cycle are shown. All results are expressed as + SEM n = 3, * indicates the significant different between control and DSS-treated group. # indicates the significant different between the DSS-treatment cycles #, * P < 0.05, ##, ** P < 0.01, *** P < 0.001.
Taken together, these results indicate that repeated DSS treatments result in chronic colitis accompanied by profound neutrophil infiltration.
3.2. mRNA expression of both Nod 1 and Nod 2 was increased during acute colitis, while different mRNA expression patterns were found during the chronic phase
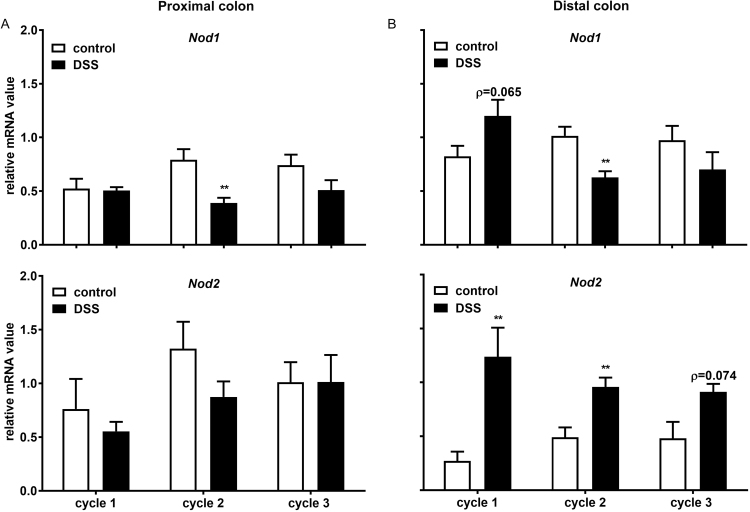
To investigate the mRNA expression of Nod1 and Nod2 during the DSS induced chronic colitis, the specific mRNA expression in both proximal and distal part of the colon isolated from either healthy or DSS-treated mice was determined (Fig. 4). The DSS induced transcriptional modulation was mainly found in the distal part of the colon (Fig. 4B). In the proximal part of the colon, there was no significant change of mRNA expression between healthy and DSS-treated mice, except a decreased Nod1 mRNA expression after second DSS treatment (Fig. 4A). In the distal part of the colon, there were increased mRNA expressions of both Nod1 and Nod2 during acute colitis, although the expression of Nod1 did not reach significance (Fig. 4B). During chronic colitis, the mRNA expression of Nod2 was significantly increased after second DSS treatment and an enhanced trend was observed after third DSS treatment (Fig. 4B). In contrast, there was a significantly reduced mRNA expression of Nod1 during after the second DSS treatment (Fig. 4B)
Fig. 4.
Acute DSS treatment increases both theNod 1andNod 2expression, while repeated DSS treatment induces different effect between Nod1 and Nod2 expression. Colons were collected for the mRNA expression assessment of PRR. The mRNA expressions of Nod 1 and Nod 2 in the A) proximal – and B) distal part of the colon are shown. All results are expressed as + SEM, n = 6. ** P < 0.001.
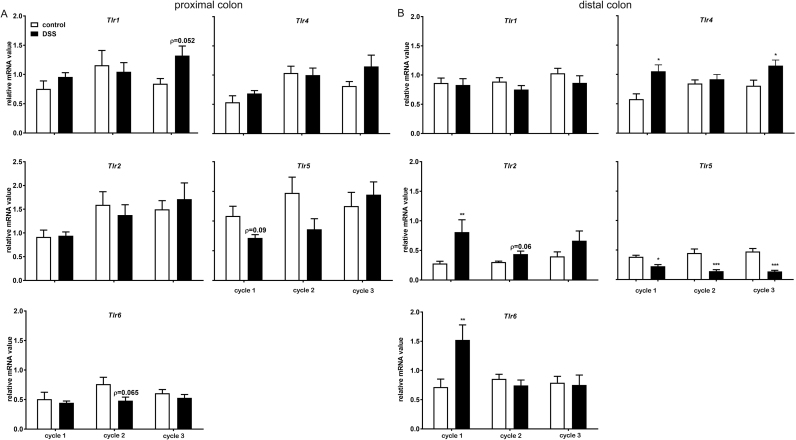
3.3. Tlr5 mRNA expression is the only extracellular Tlr that is structurally modified after every DSS treatments
To further investigate the mRNA expression of PRRs during the DSS induced chronic colitis, we have accessed the mRNA expression of TLRs located on the cell surface (Tlr1, Tlr2, Tlr6, Tlr4 and Tlr5) in both proximal and distal part of the colon isolated from either healthy or DSS-treated mice (Fig. 5). The DSS treatment induced modulatory effects on the transcription of bacterial recognizing TLRs were mainly found in the distal part of colon (Fig. 5B). In the proximal part of colon, there was no significant change of the mRNA expressions, though a tendency of an increased Tlr1 expression after third DSS treatment decreased mRNA expression of Tlr5 after first DSS treatment and Tlr6 after second DSS treatment was observed (Fig. 5A). In the distal part of colon, the acute colitis was accompanied with significant increased mRNA expressions of Tlr2, Tlr6 and Tlr4. The mRNA expression of Tlr1 was unchanged during acute colitis, whereas a significant reduced expression of Tlr5 was observed (Fig. 5B). During chronic colitis, Tlr1 mRNA expression remained unchanged. Interestingly, the significant increased expression of Tlr2 and Tlr6 mRNA during the acute colitis was profoundly reduced after repeated DSS treatment, though Tlr2 tended to be increased after the second DSS treatment. In addition, a significant increased Tlr4 mRNA expression was found after third DSS treatment, but not during second DSS treatment. Tlr5 is the only extracellular TLR that was modulated in a similar way after each DSS treatment: the mRNA expression of Tlr5 was significantly reduced after each DSS treatment (Fig. 5B).
Fig. 5.
The mRNA expression of bacterial PAMP recognizingTlrsis differently modulated by repeated DSS treatment. Colons were collected for mRNA expression assessment of PRR. The mRNA expressions of Tlr 1, Tlr2, Tlr6, Tlr4, Tlr5, and Tlr9 in the A) proximal- and B) distal part of the colon are shown. All results are expressed as + SEM, n = 6. * P < 0.05, ** P < 0.01, *** P < 0.001.
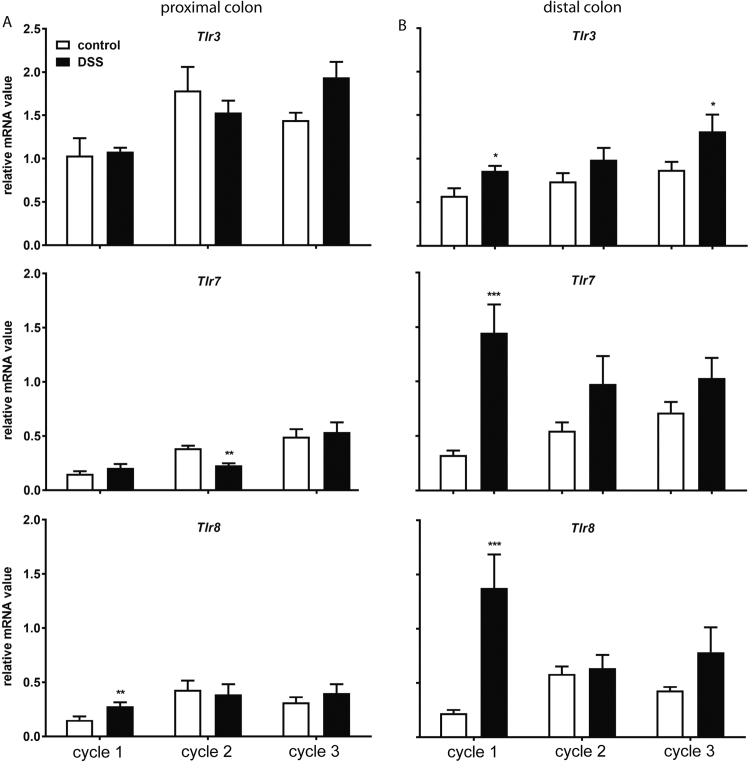
3.4. TLR9 mRNA expression is the only intracellular Tlr that is structurally modified after every DSS treatment
Intracellular TLRs comprise TLR3, TLR7 and TLR8 that recognize single- and double-strand virus RNA (dsRNA) and TLR9 that recognize CpG-rich DNA derived from virus and bacteria [28], [29], [30].
To determine the mRNA expression of this subfamily of TLR during the DSS-induced chronic colitis, the specific mRNA expression was examined in both proximal and distal part of the colon after each DSS treatment cycle (Fig. 6). In the proximal part of the colon, only Tlr8 showed a significantly increased mRNA expression during acute colitis. A significantly decreased mRNA expression of Tlr7 was observed during chronic colitis after the second DSS-treatment (Fig. 6A). In the distal part of the colon, the mRNA expression of all intracellular Tlrs was significantly increased during acute colitis and returned to basal expression levels after chronic DSS treatment, except for Tlr3 and Tlr9 During chronic colitis, Tlr3 mRNA expression was significantly increased after the third DSS, while the mRNA of Tlr9 was elevated after each DSS treatment (Fig. 6B).
Fig. 6.
Repeated DSS treatment increases mRNA expression of viral-associatedTlrsin the distal colon. Colons were collected for mRNA expression assessment of PRR. The mRNA expressions of Tlr3, Tlr7 and Tlr8 in both A) proximal- and B) distal part of the colon are shown. All results are expressed as + SEM, n = 6. * P < 0.05, ** P < 0.01.
3.5. mRNA expression of pro-inflammatory cytokines is significantly increased during acute and chronic colitis
In a previous study, we have demonstrated that acute colitis is accompanied with increased mRNA expression of pro-inflammatory cytokines [25]. To investigate whether the increase also persists during the chronic colitis, mRNA expression of pro-inflammatory cytokines was explored (Table 2). A significantly increased mRNA expression of Tnfα, Ccl2, Il1β and Il6 was observed in both the proximal and distal part of the colon during acute colitis. In addition, a similar increased mRNA expression of these cytokines was observed during chronic colitis, although the expression of Ccl2 in the proximal part of the colon did not reach significance after the second DSS treatment.
Table 2.
DSS treatment increased mRNA expression of pro-inflammatory cytokines.
| Pro-inflammatory cytokines | ||||
|---|---|---|---|---|
| Tnfα | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.06 ± 0.02 | 0.42 ± 0.1** | 0.03 ± 0.01 | 1.01 ± 0.16*** |
| Cycle 2 | 0.16 ± 0.05 | 0.68 ± 0.16* | 0.08 ± 0.03 | 0.83 ± 0.08*** |
| Cycle 3 | 0.16 ± 0.03 | 0.87 ± 0.28** | 0.25 ± 0.14 | 1.15 ± 0.15*** |
| Ccl2 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.01 ± 0 | 0.26 ± 0.08** | 0.02 ± 0 | 1.15 ± 0.25*** |
| Cycle 2 | 0.13 ± 0.03 | 0.25 ± 0.09 | 0.14 ± 0.05 | 0.72 ± 0.18* |
| Cycle 3 | 0.14 ± 0.04 | 0.62 ± 0.33* | 0.17 ± 0.07 | 1.17 ± 0.1*** |
| Il6 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.01 ± 0.01 | 0.45 ± 0.2** | 0.01 ± 0 | 1.09 ± 0.37** |
| Cycle 2 | 0.08 ± 0.01 | 0.28 ± 0.04** | 0.07 ± 0.01 | 0.78 ± 0.23** |
| Cycle 3 | 0.04 ± 0.01 | 0.73 ± 0.36* | 0.03 ± 0 | 1.02 ± 0.22** |
| Il1β | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.01 ± 0 | 0.51 ± 0.18** | 0.01 ± 0 | 1.40 ± 0.43** |
| Cycle 2 | 0.01 ± 0 | 0.15 ± 0.02** | 0.01 ± 0 | 0.68 ± 0.21* |
| Cycle 3 | 0.02 ± 0 | 0.57 ± 0.26* | 0.02 ± 0 | 1.15 ± 0.17** |
Colons were collected for examination of the cytokines mRNA expression level. The mRNA expressions of pro-inflammatory cytokines Tnfα, Ccl2, Il6 and Il1β in the proximal- and distal part of the colon are shown. All results are expressed as ± SEM, n = 6. * indicates the significant different between control and DSS-treated mice.
P < 0.05.
P < 0.01.
P < 0.001.
3.6. Chronic colitis is accompanied with an increased mRNA expression of Th1 cell-associated transcription factors and cytokines
Next, we investigate the mRNA expression of Th cell-associated transcription factors and cytokines during the DSS-induced chronic colitis (Table 2, Table 3, Table 4, Table 5). To accesses the contribution of the Th1 cell response, mRNA expression of Tbet, Ifnγ and Il12 was explored (Table 3). In the proximal part of the colon, there was no significant difference of Tbet mRNA expression between healthy and DSS-treated mice, whereas an increased mRNA expression of Ifnγ was observed in DSS-treated mice during acute colitis as well as chronic colitis, though the expression after third DSS treatment did not reach significance. In contrast to Ifnγ, there was a significant decrease in Il12 mRNA expression after second DSS. In the distal part of the colon, increased mRNA expression of Th1 cell transcription factor Tbet as well as Th1 cell-associated cytokines Ifnγ and Il12 was found during both acute and chronic DSS colitis, though the mRNA expression of Il12 after third DSS treatment did not reach significance.
Table 3.
DSS treatment increased the transcripts associated with Th1 response.
| Th1-associated transcription factor and cytokines | ||||
|---|---|---|---|---|
| Tbet | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.63 ± 0.22 | 0.51 ± 0.12 | 0.38 ± 0.08 | 1.52 ± 0.24** |
| Cycle 2 | 1.45 ± 0.31 | 1.76 ± 0.29 | 0.48 ± 0.09 | 1.65 ± 0.39** |
| Cycle 3 | 1.16 ± 0.22 | 1.20 ± 0.03 | 0.40 ± 0.11 | 1.27 ± 0.11** |
| Ifnγ | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.01 ± 0 | 0.19 ± 0.11* | 0.01 ± 0 | 0.82 ± 0.13* |
| Cycle 2 | 0.05 ± 0.01 | 0.31 ± 0.11* | 0.03 ± 0.01 | 0.67 ± 0.18*** |
| Cycle 3 | 0.04 ± 0.02 | 0.43 ± 0.16ρ = 0.058 | 0.05 ± 0.04 | 0.92 ± 0.12*** |
| Il12 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.20 ± 0.07 | 0.31 ± 0.09 | 0.30 ± 0.09 | 1.23 ± 0.18*** |
| Cycle 2 | 0.52 ± 0.05 | 0.31 ± 0.05* | 0.36 ± 0.03 | 0.89 ± 0.18* |
| Cycle 3 | 0.88 ± 0.18 | 0.64 ± 0.18 | 0.74 ± 0.13 | 1.03 ± 0.11 |
Colons were collected for examination of the mRNA expression level of Th1 transcription factors and associated cytokines. The mRNA expressions of Tbet, Ifnγ and Il12 in the proximal- and distal part of the colon are shown. All results are expressed as + SEM, n = 6. * indicates the significant different between control and DSS-treated mice.
P < 0.05.
P < 0.01.
P < 0.001.
Table 4.
While increased Th2 transcription factor was found after each DSS treatment cycle, only Il4 was increased after acute DSS treatment.
| Th2-associated transcription factor and cytokines | ||||
|---|---|---|---|---|
| Gata3 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.20 ± 0.03 | 0.90 ± 0.28* | 0.24 ± 0.05 | 0.96 ± 0.17** |
| Cycle 2 | 0.35 ± 0.05 | 1.66 ± 0.27*** | 0.24 ± 0.06 | 1.06 ± 0.24* |
| Cycle 3 | 0.21 ± 0.03 | 1.51 ± 0.3*** | 0.12 ± 0.02 | 1.23 ± 0.51** |
| Il4 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.37 ± 0.03 | 0.27 ± 0.06 | 0.47 ± 0.08 | 0.87 ± 0.13* |
| Cycle 2 | 0.90 ± 0.2 | 1.02 ± 0.30 | 0.68 ± 0.1 | 0.49 ± 0.13 |
| Cycle 3 | 1.29 ± 0.22 | 0.63 ± 0.08* | 0.81 ± 0.14 | 0.57 ± 0.04 |
| Il5 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.13 ± 0.06 | 0.04 ± 0.02 | 0.13 ± 0.07 | 0.13 ± 0.08 |
| Cycle 2 | 0.77 ± 0.23 | 0.57 ± 0.23 | 0.59 ± 0.11 | 0.35 ± 0.13 |
| Cycle 3 | 0.93 ± 0.12 | 0.58 ± 0.12ρ = 0.082 | 0.76 ± 0.13 | 0.31 ± 0.05* |
| Il13 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.98 ± 0.29 | 0.51 ± 0.1 | 0.79 ± 0.12 | 1.03 ± 0.13 |
| Cycle 2 | 0.96 ± 0.21 | 1.11 ± 0.46 | 0.66 ± 0.16 | 0.71 ± 0.16 |
| Cycle 3 | 1.21 ± 0.19 | 1.27 ± 0.28 | 0.82 ± 0.2 | 0.87 ± 0.14 |
Colons were collected for examination of the mRNA expression level of Th2 transcription factors and associated cytokines. The mRNA expressions of Gata3, Il4, Il5 and Il13 in the proximal- and distal part of the colon are shown. All results are expressed as + SEM, n = 6. * indicates the significant different between control and DSS-treated mice.
P < 0.05.
P < 0.01.
P < 0.001.
Table 5.
Increased mRNA expression of Il17, but decreased of Th17 associated transcription factors were found after each DSS treatment cycle, Il23 was only increased after acute DSS treatment.
| Th17-associated transcription factor and cytokines | ||||
|---|---|---|---|---|
| Rorc | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.77 ± 0.14 | 0.49 ± 0.02 | 0.95 ± 0.05 | 0.39 ± 0.08*** |
| Cycle 2 | 1.13 ± 0.28 | 1.03 ± 0,23 | 0.94 ± 0.12 | 0.62 ± 0.1ρ = 0.089 |
| Cycle 3 | 0.92 ± 0.11 | 0.98 ± 0.16 | 0.93 ± 0.10 | 0.63 ± 0.08* |
| Il23 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.39 ± 0.17 | 0.54 ± 0.19 | 0.44 ± 0.19 | 1.19 ± 0.23** |
| Cycle 2 | 0.73 ± 0.16 | 0.61 ± 0.10 | 0.84 ± 0.07 | 0.70 ± 0.09 |
| Cycle 3 | 0.64 ± 0.13 | 0.96 ± 0.24 | 0.85 ± 0.14 | 0.85 ± 0.05 |
| Il17 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.02 ± 0 | 0.21 ± 0.08 | 0.02 ± 0 | 1.02 ± 0.19*** |
| Cycle 2 | 0.05 ± 0.02 | 0.28 ± 0.07 | 0.04 ± 0.02 | 0.55 ± 0.2* |
| Cycle 3 | 0.06 ± 0.03 | 0.85 ± 0.48 | 0.05 ± 0.03 | 1.00 ± 0.18*** |
Colons were collected for examination of the mRNA expression level of Th17 transcription factor and associated cytokines. The mRNA expression of Rorc, Il23 and Il17 in the proximal- and distal part of the colon are shown. All results are expressed as + SEM, n = 6. * indicates the significant different between control and DSS-treated mice.
P < 0.05.
P < 0.01.
P < 0.001.
3.7. mRNA encoding Th2 cell-associated cytokines play a less important role during chronic colitis
To examine the involvement of the Th2 cell response, mRNA expression of Gata3, Il4, Il5 and Il13 was determined (Table 4). There was a significant increased mRNA expression of Th2 cell transcription factor Gata3 in both the proximal and the distal part of colon during acute as well as chronic colitis. Interestingly, there was no significant increase in mRNA expression of the Th2 cell-associated cytokines, except Il4 expression in the distal colon during acute colitis. A significant decreased mRNA expression of Il4 in the proximal part of colon and Il5 in the distal part of colon was observed during chronic colitis after the third DSS treatment.
3.8. Chronic colitis was associated with increased transcript of Il17
In contrast to Th1 and Th2 cell transcription factors, the transcription factor of Th17 cells, Rorc was significantly reduced in the distal part of colon during acute colitis. In addition, the expression remained low during the chronic colitis as compared to the expression in the colon of healthy mice, although it did not reach the significance after the second DSS treatment (Table 5). There was significant sustainable increase in Il17 mRNA expressions in both the parts of the colon during chronic colitis. In addition, in the distal part of colon, a significant increased Il23 mRNA expression was observed only during the acute colitis.
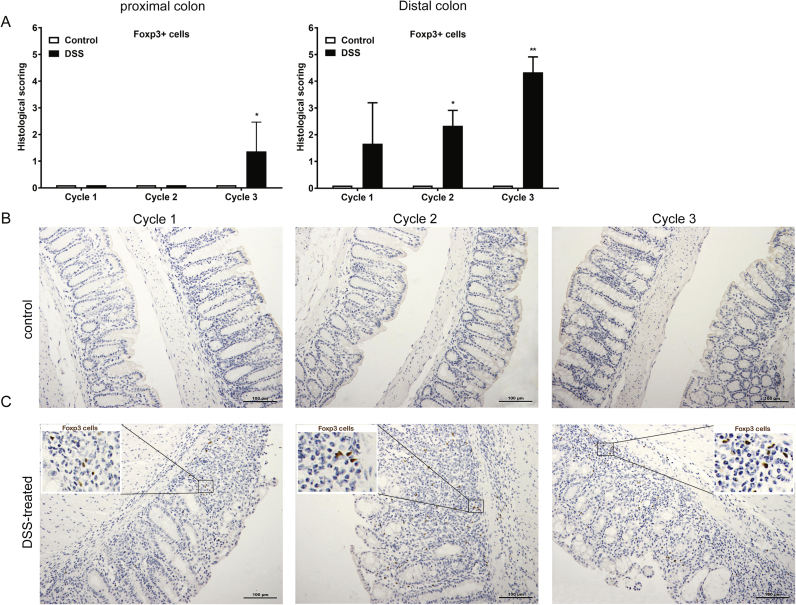
3.9. Chronic colitis increased the mRNA expression of Il10 and enhances the number of Foxp3+ cells in the colon
To determine the contribution of Treg cell-mediated responses, mRNA expression of Foxp3, Tgfβ and Il10 was explored (Table 6). Although there was no change of the Foxp3 mRNA expression between healthy and DSS-treated mice, a significant increased Tgfβ mRNA expression was found in the distal part of colon during acute colitis, which returned to basal levels after three cycles of DSS treatment. Furthermore, there was a significant increased mRNA expression of Il10 in the distal colon during both acute and chronic colitis, though the expression after the third DSS treatment cycle did not reach significance.
Table 6.
Increased Il10 mRNA expression was found after each DSS treatment cycle, while Tgfβ was only increased after acute DSS treatment.
| Treg-associated transcription factor and cytokines | ||||
|---|---|---|---|---|
| Foxp3 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.51 ± 0.15 | 0.38 ± 0.3 | 0.70 ± 0.14 | 0.83 ± 0.07 |
| Cycle 2 | 1.07 ± 0.1 | 0.90 ± 0.14 | 0.80 ± 0.07 | 0.97 ± 0.09 |
| Cycle 3 | 1.24 ± 0.35 | 0.87 ± 0.28 | 0.72 ± 0.1 | 1.03 ± 0.11ρ = 0.082 |
| Tgfβ | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.41 ± 0.05 | 0.41 ± 0.08 | 0.61 ± 0.09 | 1.42 ± 0.19*** |
| Cycle 2 | 0.50 ± 0.05 | 0.34 ± 0.04 | 0.57 ± 0.07 | 0.80 ± 0.13 |
| Cycle 3 | 0.68 ± 0.06 | 0.54 ± 0.09 | 0.75 ± 0.09 | 0.84 ± 0.22 |
| Il10 | Proximal colon | Distal colon | ||
| Control mice | DSS-treated mice | Control mice | DSS-treated mice | |
| Cycle 1 | 0.14 ± 0.04 | 0.18 ± 0.04 | 0.17 ± 0.05 | 0.78 ± 0.10** |
| Cycle 2 | 0.49 ± 0.06 | 0.45 ± 0.07 | 0.53 ± 0.10 | 1.01 ± 0.16* |
| Cycle 3 | 0.58 ± 0.09 | 0.54 ± 0.13 | 0.50 ± 0.13 | 0.90 ± 0.14ρ = 0.076 |
Colons were collected for examination of the mRNA expression level of Treg transcription factor and associated cytokines. The mRNA expression of Foxp3, Tgfβ and Il10 in the proximal- and distal part of the colon are shown. All results are expressed as + SEM, n = 6. * Indicates the significant different between control and DSS-treated mice.
P < 0.05.
P < 0.01.
P < 0.001.
To further analysis the contribution of Treg cells during the chronic colitis, Foxp3+ cells in the colon were examined using histochemistry. There was an increased score of Foxp3+ cell in the colon during chronic colitis, but not in acute colitis (Fig. 7A-C).
Fig. 7.
Increased histological score of Foxp3+ cells after each DSS treatment cycle. Colons were collected for A) immunohistochemical assessment of Foxp3+ cells. Representative histochemical staining pictures of Foxp3+ cells in the colon of B) control and C) DSS-treated mice during each DSS cycle are shown. All results are expressed as + SEM, n = 3.
4. Discussion
In this study, the mRNA expression of colonic PRRs, pro-inflammatory cytokines, T cell-associated transcription factors and cytokines was examined in mice exposed to repeated DSS treatments interspersed with a rest period.
As compared to single DSS exposure-induced colitis study [25], multiple DSS exposure induced chronic colitis in mice shows a higher similarity to IBD that is characterized by remission-relapse course after disease onset [1]. Although we did not found more severe clinical manifestations during the chronic phase of colitis as compared to acute colitis, a gradually decreased colon length and bloody diarrhea were observed during the chronic colitis. The clinical manifestations do not always reflect the severity of intestinal inflammation [22], [26]. Histological analysis shown that chronic inflammation in the colon is characterized by phenotypes such as crypt architecture disarray, pronounced mononuclear leucocytes and neutrophil infiltration, transmural inflammation [26]. All colonic features and the gradually decreased colon length/weight ratio after repeated DSS treatments suggest that our colitis model could be presented as a semi-chronic relapsing colitis model that could provide valuable knowledge to help clarify the development of IBD.
In IBD aberrant and prolonged activation of TLRs can lead to more tissue damage and inflammatory responses [27], [28] resulting in chronic inflammatory disease [11], [12], [29]. Accordingly, the extent and duration of the induction of these PRRs need to be tightly controlled. In this study, the expression of majority of the PRRs, which was enhanced in the inflamed colon region during the acute colitis, including Tlr2, Tlr3, Tlr4, Tlr6, Tlr7 and Tlr8, was not observed after repeated DSS treatments during the chronic colitis, though Nod2 was increased after the second and third cycle DSS, respectively. Although the exact regulation mechanisms still need to be elucidated, the importance of temporary reduced expression or silencing of these receptors was recognized and negative regulation mechanisms at multiple signaling level were reported [30], [31], [32], [33]. For example, activation of NOD2 decreases TLR2-induced IL12 production [34], while NOD2 signaling can be stimulated by TNFα or IFNγ [35]. Since activation of TLRs lead to production of cytokines including TNFα and IFNγ [36], the increased Nod2 may be a part of TLR suppression mechanism during the chronic DSS colitis.
In contrast to other Tlrs, the colonic mRNA expression of Tlr5 and Tlr9 showed a decreased or increased expression trend, respectively, during the chronic phase of colitis. TLR5 recognize bacterial-derived flagellin and is exclusively located on the basolateral [32], [37]. Prolonged exposure of TLR5 to flagellin has been shown to be responsible for flagellin-induced tolerance accompanied by internalization of TLR5 receptors [38]. In addition, IFNγ induces down-regulate Tlr5 expression in mice colon cells in a dose and time dependent manner [33]. Taken together, the reduced Tlr5 expression in this study could be the result of combined effects of the overexposure to bacterial-derived flagellin and increased IFNγ expression. As result, the clearance of infiltrated gut bacteria is reduced leading to continuous expression of pro-inflammatory cytokines and chronic intestinal inflammation. This hypothesis is supported by the observation that TLR5-/- mice develop spontaneous colitis [39] and the finding of a strong anti-flagellin antibody response in IBD patients [40], [41].
TLR9 recognize bacterial DNA and activation of TLR9 will induce the expression of pro-inflammatory cytokines such as TNFα, CCL2, IL1β and IL6 [42], [43]. In the colon of chronically DSS exposed mice, the upregulated Tlr9 expression is associated with enhanced expression of mentioned cytokines mRNA. A recent study demonstrated that Tlr9 signaling is important for the Th1 immune response in mice gut-associated lymphoid tissue (GALT) [44]. In line with these data, enhanced expression of the pro-inflammatory cytokines and Th1 cell-associated cytokine Ifnγ were observed in inflamed colon of mice chronically exposed to DSS.
Besides the increased mRNA expression of pro-inflammatory cytokines and Ifnγ, only the Il17 gene showed a persistent increased trend during the chronic colitis. The increased expression of other T cell-associated cytokine genes was abolished after either acute colitis (Il4, Il23, Tgfβ) or after repeated DSS treatment cycle (Il12, Il10, Il5). These data suggest that the chronic inflammation in this colitis model is mainly directed by the Th1 and Th17 cell immune responses, but not by Th2 cell-mediated immune responses. Furthermore, the decreased IL4 and IL5 mRNA expression could derive from the Th1/Th2 cell paradigm; increased Th1 cell differentiation leads to reduced Th2 cell responses [45], [46]. The increased mRNA expression of Il10 and presence of Foxp3+ cells may indicate to an unsuccessful attempt of Treg cells to control the inflammatory response. Although the mouse Foxp3+ cells are regarded as Treg cells, a subset of Foxp3 expressing CD8+ cytotoxic T cells were found to be involved in early Th17 cell-mediated immune response to enteric pathogen in mice [47]. Furthermore, IL17- and IFNγ secreting Foxp3+ T cells have been demonstrated in peripheral lymph nodes and the spleen [48]. These findings suggest that the Foxp3+ cells observed in this study might not always represent an increased number of Treg cells, but could also be pro-inflammatory cytotoxic T helper cell subpopulations. Further research is needed to confirm this hypothesis.
In this study, we have illustrated the expression profile of colonic PRRs and T cell-associated cytokines during the chronic colitis development using a semi-chronic colitis model that mimics the development of IBD. The current accepted T cell subset associations with the two major forms of IBD are Th1/Th17 cells with CD, while Th2 cell with ulcerative colitis [49], [50]. Human studies have demonstrated the presence of IL-17A in the sera of CD patient and both IFN-γ and IL-17A producing T cells were found [51]. The elevated expression of Il17 and Ifnγ during the chronic DSS colitis suggests this colitis model could provide valuable information for the investigation of the development of CD. In addition, the persistent increased Tlr9 and decreased Tlr5 expression during the chronic colitis and their possible involvement in the aberrant immune response suggest that these TLRs could be potential targets for CD treatment.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.08.009.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.08.009.
Appendix A. Transparency document
Supplementary material
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015 doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 3.Brown S.J., Mayer L. The immune response in inflammatory bowel disease. Am. J. Gastroenterol. 2007;102(9):2058–2069. doi: 10.1111/j.1572-0241.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 4.Walsh D. Pattern recognition receptors-Molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine Growth Factor Rev. 2012 doi: 10.1016/j.cytogfr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 6.Simpson H.L., Campbell B.J., Rhodes J.M. IBD: microbiota manipulation through diet and modified bacteria. Dig. Dis. 2014;32(Suppl 1):S18–S25. doi: 10.1159/000367821. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T., Akira S. TLR signaling. Semin. Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Farhat K. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukoc. Biol. 2008;83(3):692–701. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- 9.Brikos C., O'Neill L.A. Signalling of toll-like receptors. Handb. Exp. Pharmacol. 2008;183:21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- 10.Fukata M., Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6(3):451–463. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown M.R.L., Robins A., Mahida Y.R. PTU-114 expression of toll-like receptor (TLR)-2 and -4 in the intestinal crypt epithelial cells in inflammatory bowel disease (IBD) Gut. 2012;61(Suppl 2):A232. [Google Scholar]
- 12.Ostvik A.E. Expression of Toll-like receptor-3 is enhanced in active inflammatory bowel disease and mediates the excessive release of lipocalin 2. Clin. Exp. Immunol. 2013;173(3):502–511. doi: 10.1111/cei.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valverde-Villegas J.M. G2848A and T-1237C polymorphisms of the TLR9 gene and susceptibility to inflammatory bowel disease in patients from southern Brazil. Tissue Antigens. 2014;83(3):190–192. doi: 10.1111/tan.12302. [DOI] [PubMed] [Google Scholar]
- 14.Kathrani A. Polymorphisms in the TLR4 and TLR5 gene are significantly associated with inflammatory bowel disease in German shepherd dogs. PLoS One. 2010;5(12):e15740. doi: 10.1371/journal.pone.0015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierik M. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm. Bowel Dis. 2006;12(1):1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- 16.Rubino S.J. Nod-like receptors in the control of intestinal inflammation. Curr. Opin. Immunol. 2012;24(4):398–404. doi: 10.1016/j.coi.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Hugot J.P. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 18.Ogura Y. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411(6837):603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 19.Brain O., Allan P., Simmons A. NOD2-mediated autophagy and Crohn disease. Autophagy. 2010;6(3):412–414. doi: 10.4161/auto.6.3.11389. [DOI] [PubMed] [Google Scholar]
- 20.Powrie F., Uhlig H. Animal models of intestinal inflammation: clues to the pathogenesis of inflammatory bowel disease. Novartis Found. Symp. 2004;263:164–174. (discussion 174-8, 211-8) [PubMed] [Google Scholar]
- 21.Gottfries J., Melgar S., Michaelsson E. Modelling of mouse experimental colitis by global property screens: a holistic approach to assess drug effects in inflammatory bowel disease. PLoS One. 2012;7(1):e30005. doi: 10.1371/journal.pone.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melgar S. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int. Immunopharmacol. 2008;8(6):836–844. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Egger B. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62(4):240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 24.Breynaert C. Unique gene expression and MR T2 relaxometry patterns define chronic murine dextran sodium sulphate colitis as a model for connective tissue changes in human Crohn's disease. PLoS One. 2013;8(7):e68876. doi: 10.1371/journal.pone.0068876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng B. Transcriptional modulation of pattern recognition receptors in acute colitis in mice. Biochim. Biophys. Acta. 2013;1832(12):2162–2172. doi: 10.1016/j.bbadis.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Perse M., Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J.Y., Zhao L., Hwang D.H. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr. Rev. 2010;68(1):38–61. doi: 10.1111/j.1753-4887.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Levin A., Shibolet O. Toll-like receptors in inflammatory bowel disease-stepping into uncharted territory. World J. Gastroenterol. 2008;14(33):5149–5153. doi: 10.3748/wjg.14.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo T., Kawai T., Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33(9):449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Shibolet O., Podolsky D.K. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(6):G1469–G1473. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- 32.Crellin N.K. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 2005;175(12):8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 33.Ortega-Cava C.F. Epithelial toll-like receptor 5 is constitutively localized in the mouse cecum and exhibits distinctive down-regulation during experimental colitis. Clin. Vaccine Immunol. 2006;13(1):132–138. doi: 10.1128/CVI.13.1.132-138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe T. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 2004;5(8):800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstiel P. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124(4):1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 36.Tak P.P., Firestein G.S. NF-kappaB: a key role in inflammatory diseases. J. Clin. Investig. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee S.H. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc. Natl. Acad. Sci. USA. 2005;102(38):13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J. Flagellin-induced tolerance of the Toll-like receptor 5 signaling pathway in polarized intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(3):G767–G778. doi: 10.1152/ajpgi.00447.2006. [DOI] [PubMed] [Google Scholar]
- 39.Vijay-Kumar M. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Investig. 2007;117(12):3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodes M.J. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Investig. 2004;113(9):1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Targan S.R. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128(7):2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 42.Anders H.J. Bacterial CpG-DNA aggravates immune complex glomerulonephritis: role of TLR9-mediated expression of chemokines and chemokine receptors. J. Am. Soc. Nephrol. 2003;14(2):317–326. doi: 10.1097/01.asn.0000042169.23931.73. [DOI] [PubMed] [Google Scholar]
- 43.Bhan U. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J. Immunol. 2007;179(6):3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 44.Minns L.A. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J. Immunol. 2006;176(12):7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 45.Fuss I.J. Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm. Bowel Dis. 2008;14(Suppl 2):S110–S112. doi: 10.1002/ibd.20683. [DOI] [PubMed] [Google Scholar]
- 46.Kanhere A. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat. Commun. 2012;3:1268. doi: 10.1038/ncomms2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubino S.J. Constitutive induction of intestinal Tc17 cells in the absence of hematopoietic cell-specific MHC class II expression. Eur. J. Immunol. 2013;43(11):2896–2906. doi: 10.1002/eji.201243028. [DOI] [PubMed] [Google Scholar]
- 48.Esposito M. IL-17- and IFN-gamma-secreting Foxp3+ T cells infiltrate the target tissue in experimental autoimmunity. J. Immunol. 2010;185(12):7467–7473. doi: 10.4049/jimmunol.1001519. [DOI] [PubMed] [Google Scholar]
- 49.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 50.Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58(8):1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 51.Sakuraba A. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137(5):1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material