Abstract
GM2 gangliosidoses are autosomal recessive lysosomal storage diseases (LSDs) caused by mutations in the HEXA, HEXB and GM2A genes, which encode the human lysosomal β-hexosaminidase (Hex) α- and β-subunits, and GM2 activator protein (GM2A), respectively. These diseases are associated with excessive accumulation of GM2 ganglioside (GM2) in the brains of patients with neurological symptoms. Here we established a CHO cell line overexpressing human GM2A, and purified GM2A from the conditioned medium, which was taken up by fibroblasts derived from a patient with GM2A deficiency, and had the therapeutic effects of reducing the GM2 accumulated in fibroblasts when added to the culture medium. We also demonstrated for the first time that recombinant GM2A could enhance the replacement effect of human modified HexB (modB) with GM2-degrading activity, which is composed of homodimeric altered β-subunits containing a partial amino acid sequence of the α-subunit, including the GSEP loop necessary for binding to GM2A, on reduction of the GM2 accumulated in fibroblasts derived from a patient with Tay-Sachs disease, a HexA (αβ heterodimer) deficiency, caused by HEXA mutations. We predicted the same manner of binding of GM2A to the GSEP loop located in the modified HexB β-subunit to that in the native HexA α-subunit on the basis of the x-ray crystal structures. These findings suggest the effectiveness of combinational replacement therapy involving the human modified HexB and GM2A for GM2 gangliosidoses.
Abbreviations: CI-M6PR, cation-independent M6P receptor; CM, conditioned medium; ERT, enzyme replacement therapy; GM2, GM2 ganglioside; GM2A, GM2 activator protein; Hex, β-hexosaminidase; LAMP-1, lysosomal associated membrane protein 1; LSD, lysosomal storage disease; M6P, mannose-6-phosphate; modB, modified HexB; SD, Sandhoff disease; TSD, Tay-Sachs disease
Keywords: Lysosomal storage disease, Gm2 gangliosidosis, Gm2 activator protein, β-hexosaminidase, Enzyme replacement therapy
Highlights
-
•
Purification of recombinant human GM2A proteins by CHO cell line overexpressing GM2A.
-
•
Reduction of GM2 accumulated in GM2A deficiency fibroblasts by GM2A replacement.
-
•
Combined effects of modified HexB and GM2A for HexA deficiency fibroblasts.
-
•
In silico prediction of molecular interaction between modified HexB and GM2A.
1. Introduction
GM2 gangliosidoses, including Tay-Sachs disease (TSD, variant B), Sandhoff disease (SD, variant O), and GM2 ganglioside (GM2) activator protein (GM2A) deficiency (variant AB), are neurodegenerative lysosomal storage diseases (LSDs) associated with excessive accumulation of GM2 [1]. GM2A is known as a co-factor of β-hexosaminidase A (HexA, αβ heterodimer) involved in the degradation of GM2 [2], [3], [4]. Recently, intravenous enzyme replacement therapy involving recombinant human lysosomal enzymes produced by CHO and human HT1080 cell lines was clinically applied for several LSDs [5], [6], so GM2A-based protein replacement therapy should be applicable to patients with variant AB. Previously, we developed a modified human HexB (modB) [7] composed of homodimeric altered β-subunits containing amino acid substitutions, the DL (β452–453) to NR (α423–424) sequences, which are necessary for anionic substrate recognition by the native α-subunit, and the RQNK (β312–315) to GSEP (α280–283) loop sequence, which is required for interaction with GM2A. We also revealed that the modB has GM2-degrading activity in fibroblasts derived from TSD and SD patients. Here, we first demonstrated that co-administration of recombinant GM2A could significantly enhance the reduction of intracellular GM2 accumulated in TSD fibroblasts mediated by modB in the culture system.
2. Materials and methods
2.1. Antibodies
Anti-GM2 mouse monoclonal antibodies (GMB28: IgM) were established previously [8].
2.2. Cells
The CHO-K1 cell line was provided by the RIKEN BioResource Center (Ibaraki, Japan). The use of cultured skin fibroblasts from patients with TSD (F218) [7] and variant AB (F582) [9], and normal ones (F258) were approved by the ethics committee of our institution. The fibroblasts were cultured in nutrient mixture Ham's F-10 (Sigma-Aldrich, St. Louis, MO, USA) medium containing 10% (v/v) fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), 100 μg/mL streptomycin (Sigma-Aldrich), and 70 μg/mL penicillin G (Sigma-Aldrich) at 37 °C in a humidified incubator continuously flushed with a mixture of 5% CO2−95% air.
2.3. Establishment of CHO cell lines expressing GM2A
A DNA fragment encoding GM2A with a histidine-tag (10xHis) and the signal sequence of human lysosomal α-galactosidase A (GLA) was ligated into the pCXN2-Hygro and pCXN2-Neo vectors [10]. Then each vector was used to transform MAX Efficiency DH5α Competent Cells (Life Technologies, Carlsbad, CA, USA). Plasmid DNA-Lipofectamine 2000 (Life technologies) complexes were transfected into CHO cells according to the manufacturer's instructions. Drug-resistant cell lines were established by double selection with hygromycin (Wako, Osaka, Japan) and G418 (Sigma-Aldrich). The CHO cell line stably expressing GM2A was cultured in EX-CELL ACF CHO medium (Sigma-Aldrich). The conditioned medium (CM) derived from each cell line was collected.
Immunoblotting for the expressed GM2A was performed with anti-hGM2A polyclonal antibodies (HPA008063, Sigma-Aldrich). Briefly, aliquots of GM2A fractions were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5% (w/v) acrylamide gels. The proteins were transferred to Immobilon®-P PVDF membranes (Merck, Darmstadt, HE, Germany). After blocking with 50% (v/v) Blocking One (Nacalai Tesque, Kyoto, Japan) in TBS [25 mM Tris (Sigma-Aldrich), 137 mM NaCl, 2.7 mM KCl, pH 7.4] at room temperature (rt) for 1 h, each membrane was treated with anti-hGM2A antibodies diluted with Blocking One/TBS (1:1,000 dilution) overnight at 4 °C. After washing with TBS containing 0.1% (v/v) Tween 20 (Sigma-Aldrich), the membrane was treated with horseradish peroxidase (HRP)-linked anti-rabbit IgG antibodies (#7074, Cell Signaling Technology, Danvers, MA, USA, 1:1000 dilution) at rt for 1 h. After washing, detection of antibody binding was carried out with ECL (PerkinElmer, Waltham, MA, USA) according to the manufacturer's instructions. The protein levels were determined by the DC™ Protein Assay or Bio-Rad Protein Assay (Bio-Rad, Hercules, CA, USA) with BSA (Sigma-Aldrich) as a standard.
For assaying of the presence of N-glycans attached to recombinant GM2A, CM containing GM2A was treated overnight with or without PNGase F (New England BioLabs, Hitchin, Herts, UK) according to the manufacturer's protocol, and then immunoblotting with anti-hGM2A antibodies was performed.
2.4. Purification of recombinant GM2A
For Ni-column chromatography, 5N NaOH (Sigma-Aldrich) was added to the CM to adjust the pH to 8.0–8.3, and then the CM was filtered with a Minisart® Syringe Filter 0.45 µm (Sartorius, Göttingen, NI, Germany). Then samples were applied to Ni Sepharose 6 Fast Flow (GE Healthcare, Little Chalfont, BKM, UK) equilibrated with 25 mM sodium phosphate buffer (NaPB), 0.3 M NaCl (pH 8.0). After washing with 25 mM NaPB, 0.3 M NaCl (pH 7.0 and 6.0), the bound proteins were eluted with 50 mM sodium acetate buffer, 0.3 M NaCl (pH 4.0). Each fraction was subjected to SDS-PAGE on a 12.5% (w/v) acrylamide gel and silver staining with a Dodeca Silver Stain Kit (Bio-Rad). The molecular weight of GM2A was calculated based on those of the APRO markers (APRO Science, Tokushima, Japan).
2.5. Preparation of modified HexB
CHO cell line stably expressing modified HexB [7] was cultured in EX-CELL ACF CHO medium and the CM was collected. Then samples were applied to a TOYOPEARL AF-Blue HC-650 column (Tosoh, Tokyo, Japan), and the bound proteins were eluted with 0.6 M NaCl (Nacalai tesque)/0.1 M Tris-acetate buffer (pH 7.5). The eluate was applied to Phos-tag agarose (Wako) and the bound proteins were eluted with 0.2 M NaPB (pH 6.0). The eluate was applied to a HiTrap SP HP column (GE Healthcare). The eluted fractions containing β-Hex activity were collected.
2.6. Replacement assay for GM2 gangliosidosis fibroblasts
Each type of fibroblast was seeded onto a collagen type I-coated 35 mm dish (AGC Techno Glass, Shizuoka, Japan). GM2A (5 μM) was added, followed by incubation for 4 d. The cells were dissociated and sonicated with phosphate-buffered saline [PBS; 8.1 mM Na2HPO4 (Sigma-Aldrich), 1.5 mM KH2PO4 (Wako), 137 mM NaCl, 2.7 mM KCl, pH 7.4] containing 1% (v/v) Nonidet P-40 and protease inhibitors [1 µM pepstatin A (Peptide Institute, Osaka, Japan), 20 µM leupeptin (Peptide Institute), 2 mM EDTA (Sigma-Aldrich) and 200 µM PMSF (Wako)]. Then each sample (9 µg protein/ lane) was subjected to SDS-PAGE, and immunoblotting with anti-hGM2A (1:1000 dilution) and anti-β-actin antibodies (A5316, Sigma-Aldrich, 1:2000 dilution). The Image J software program (Ver.1.46) [11] was used to quantify the signal intensity.
For immunostaining, variant AB fibroblasts (F582) were seeded onto 8-well Lab-Tek chamber slides (Thermo Fisher Scientific) coated with 0.05% (w/v) atelocollagen (Koken, Tokyo, Japan). Then, GM2A (5 µM) was added, followed by incubation for 7 d. The fibroblasts were fixed with 4% (w/v) paraformaldehyde (PFA, Wako). After blocking with 5% (v/v) goat serum (Cedarlane Labs, Burlington, Ontario, Canada), 1% (w/v) BSA/PBS at rt for 2 h, the intracellular GM2 and LAMP-1 were detected with anti-GM2 (1:20 dilution) and anti-LAMP-1 antibodies (ab24170, Abcam, Cambridge, MA, USA., 1:200 dilution) overnight at 4 °C. After washing, FITC-mouse IgG+M (ab47830, Abcam) and Cy3-rabbit IgG (111-165-006, Jackson ImmunoResearch, West Grove, PA, USA) were treated at rt for 1 h. Nuclei were stained with Hoechst33258 (Sigma-Aldrich). Specimens were viewed under the LSM700 (Carl Zeiss, Oberkochen, BW, Germany).
For GM2-ELISA [12], TSD fibroblasts were seeded onto a collagen type I-coated 96-well plate (AGC Techno Glass, 5×103 cells/well), followed by incubation for 3 d. Then GM2A (4 μg) was added to the culture medium, followed by incubation for 2 d. After a medium change, modified HexB [7] (4-methylumbelliferyl-6-sulfo-N-acetyl-β-D-glucosaminide-degrading activity, 3 μmol h–1) was added, followed by incubation for 2 d. The cells were fixed with 4% (w/v) PFA/PBS overnight at 4 °C. After washing with PBS, the cells were treated with 0.6% (v/v) H2O2 (Sigma-Aldrich)/PBS for 30 min, and 5% (v/v) goat serum/1% (w/v) BSA/PBS at 37 °C for 2 h. Then the cells were immunostained with anti-GM2 antibodies (1:100 dilution) at rt for 1 h. After washing, the cells were treated with biotin-conjugated anti-mouse IgG, IgM antibodies (31,807, Thermo Fisher Scientific, 1:1000 dilution) at rt for 1 h. After washing, the cells were treated with HRP-conjugated streptavidin (P0397, Dako, Glostrup, Hovedstaden, Denmark, 1:2000 dilution) at rt for 1 h. After washing, a peroxidase assay kit for ELISA (Sumitomo Bakelite, Tokyo, Japan) was used with a microtiter plate reader at 450 nm.
2.7. Computation modeling of modified HexB/GM2A complex
The proposed model of the modB/GM2A complex was constructed by means of the protein-protein docking and homology modeling. We first simulated protein-protein docking of the x-ray structure of GM2A (Protein Data Bank ID code: 1pub) with that of the HexA αβ-heterodimer structure (PDB ID code: 2gjx, A & B-chains). ZDOCK version 3.0.2 [13] was used to generate 2000 candidate conformations with the lowest docking energies. The best model was manually selected based on both the ZDOCK score within the top 1%, and the molecular connectivity of GM2 between GM2A and the active site of the HexA α-subunit. The GM2 binding position was refined into the interface between GM2A and the HexA α-subunit with restricted docking to the reference position of the lipid molecule bound in GM2A using MOE (Chemical Computing Group, Montreal, Quebec, Canada). Finally, the HexA αβ-heterodimer structure was replaced with modB including GM2A and GM2 molecules using the homolog modeling in MOE.
2.8. Statistics
Statistical analyses were performed using the SigmaPlot11 software program (Systat Software, San Jose, CA, USA), P<0.05 being considered to be significant. For comparisons of three or more groups, we used one-way ANOVA with a Tukey post-hoc test. We used the 2-tailed unpaired t-test to compare two groups.
3. Results
3.1. Production of GM2A by CHO cell lines and its purification
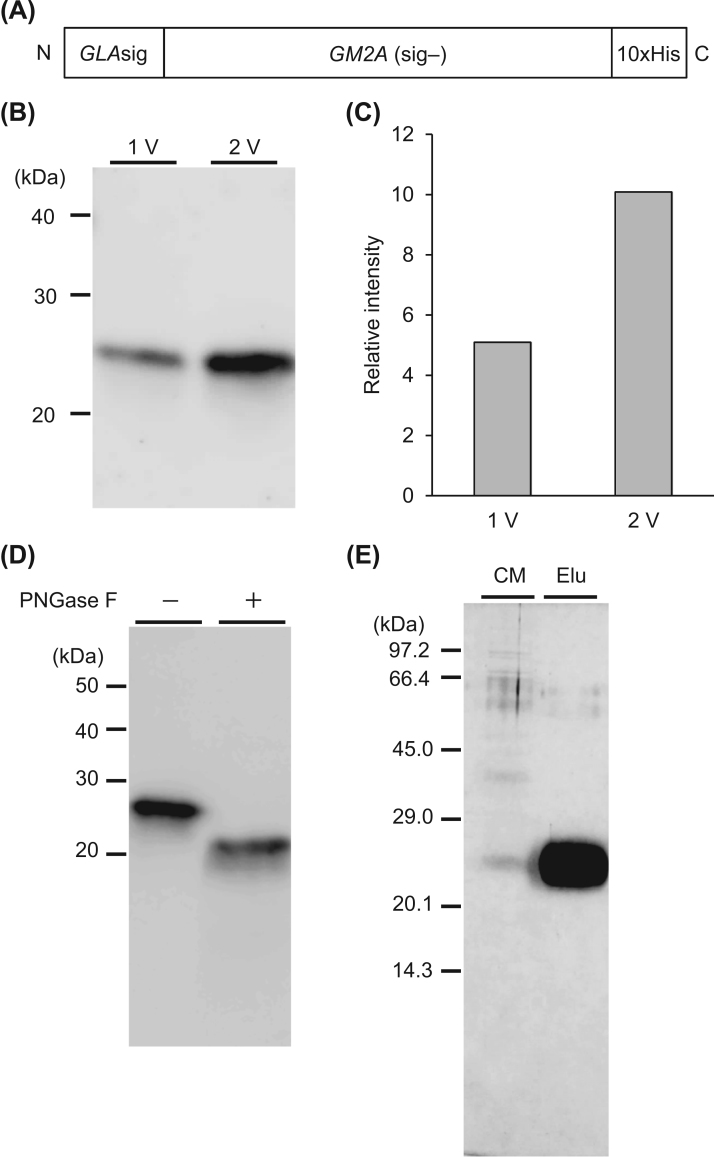
We twice introduced the GM2A gene containing a 10xHis-tag and the GLA signal sequence by utilizing plasmid vectors containing different drug-resistance genes (Fig. 1(A)), and established CHO cell lines stably expressing GM2A by sequential selection with hygromycin and G418. Although the recombinant GM2A-His proteins were secreted from the CHO cells, marked increases in the GM2A level was observed on immunoblotting with anti-GM2A antibodies in serum-free CM derived from the CHO cell line repeatedly transfected with the GM2A expression vectors. The secreted GM2A-immunoreactivity in serum-free CM of the CHO cell line transfected with two different vectors (Fig. 1B, C, lane 2V) was about two times higher than that for the cell line transfected once (Fig. 1B, C, lane 1V). The expressed GM2A migrated to the 23 kDa position. After digestion with PNGase F, non-glycosylated GM2A gave a 20 kDa band, indicated the GM2A contains N-glycans (Fig. 1D). We purified the recombinant GM2A-His by Ni chromatography, and then subjected it to SDS-PAGE. We obtained 6.1 mg of purified GM2A-His per 1 L of CM (Fig. 1E).
Fig. 1.
Expression and purification of GM2A-His with CHO cell lines. (A) Schematic drawing of the GM2A construct. GLAsig: α-galactosidase A signal sequence, GM2A (sig–): GM2A without a signal sequence. (B) Immunoblotting of CM with anti-hGM2A antibodies. Each lane contained 20 μL of CM. (C) GM2A-immunoreactivity indicated as relative signal intensity of GM2A. 1 V: one vector, 2 V: two vectors transfected. (D) Immunoblotting of N-glycosylated GM2A and the digested product with PNGase F. Each lane contained 50 μL of CM. (E) Purification of GM2A by Ni-column chromatography. Each fraction was separated by SDS-PAGE, and then silver staining was performed. Each lane contained 5 μg of protein. CM: conditioned medium. Elu: eluted fraction.
3.2. GM2A replacement and GM2 reduction in patient fibroblasts
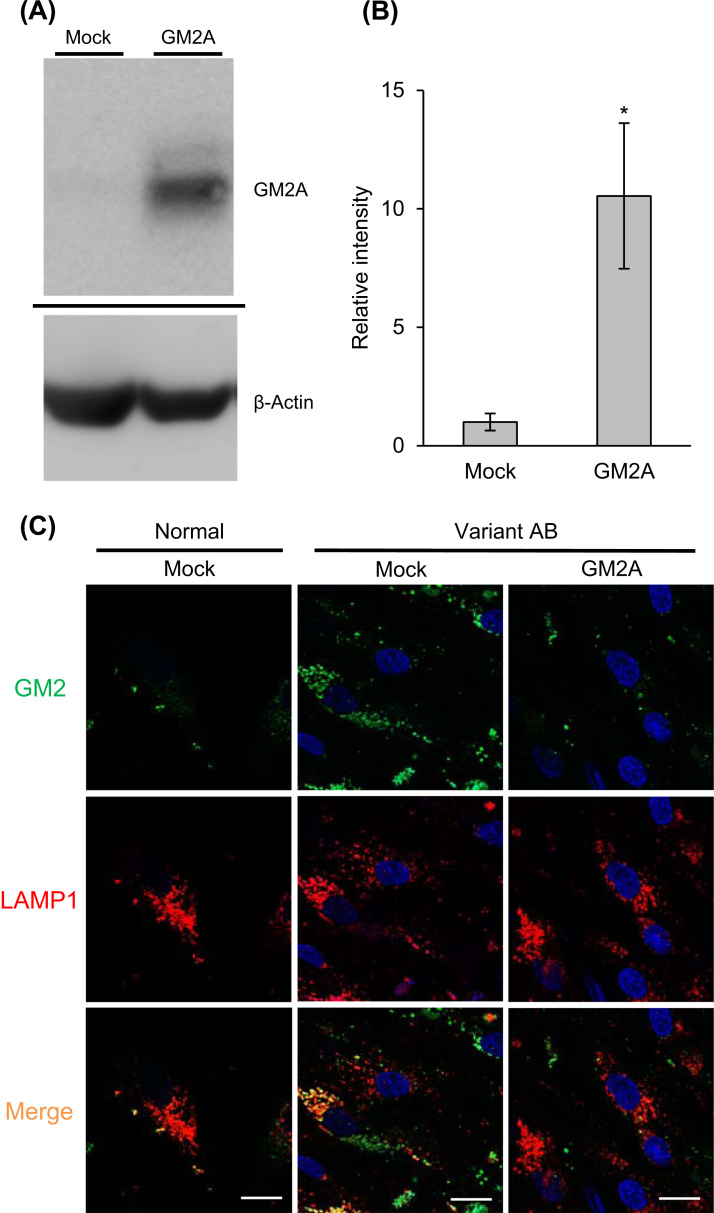
We evaluated the GM2A function in the culture system. Cultured fibroblasts derived from a variant AB patient were treated with recombinant GM2A, and then examined by immunoblotting with anti-GM2A antibodies and immunostaining with anti-GM2 antibodies. Significant restoration of the GM2A-immunoreactivity in a cell extract was observed after treatment with recombinant GM2A (Fig. 2A and B). We detected excessive accumulation of GM2 in lysosomes as punctate fluorescence, co-localized with LAMP-1, in untreated variant AB fibroblasts. After treatment with GM2A, the punctate fluorescence due to GM2 was markedly reduced (Fig. 2C).
Fig. 2.
GM2A replacement effect on variant AB fibroblasts. (A) Immunoblotting of cell extracts with anti-hGM2A antibodies after GM2A replacement. Each lane contained 9 μg protein. (B) Relative signal intensities of GM2A. Error bars show means ±SEM (n=3). Unpaired t-test,*P<0.05. (C) Variant AB fibroblasts treated with GM2A were immunostained for GM2 (green) and LAMP-1 (red), and then examined by confocal laser scanning microscopy. Scale bars indicate 20 µm.
3.3. Combined replacement effects of modified HexB and GM2A on Tay-Sachs disease fibroblasts
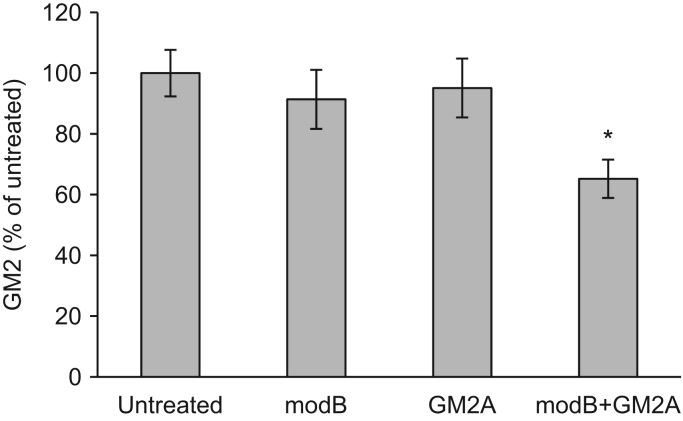
Next, we evaluated the combinational replacement effect of modB and GM2A. TSD fibroblasts were treated with modB and GM2A, and then reduction of the GM2 accumulated in the cells after administration of the recombinant proteins was examined by GM2-ELISA. Accumulated GM2 was not significantly decreased by single replacement with modB or GM2A. In contrast, GM2 was significantly reduced by combinational replacement with modB and GM2A (Fig. 3).
Fig. 3.
Combined replacement effect of modified HexB and GM2A on TSD fibroblasts. Reduction of the GM2 accumulated in TSD fibroblasts was evaluated by GM2-ELISA after treatment with modB or GM2A. Error bars show means ±SEM (n=6–7)., ANOVA with a Tukey post-hoc test,*P<0.05.
3.4. In silico models of the modified HexB and GM2A complex
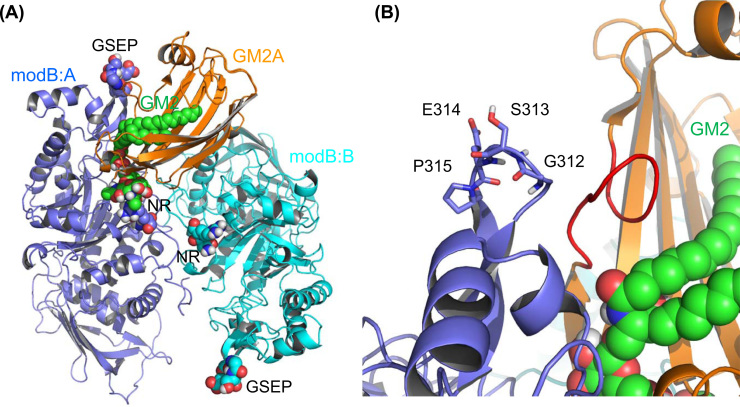
We constructed a complex model composed of modB and GM2A based on the crystal structures of human HexA (PDB ID: 2GJX) [14] and GM2A (PDB ID: 1PUB) [15] in silico (Fig. 4A and B), and predicted the quite similar manner of binding of the GSEP loop sequences located in the altered β-subunit of modB with the loop region (I66–C75) of the mature GM2A compared to that in the Hex α-subunit [16].
Fig. 4.
Homology model of the modified HexB and GM2A complex. Proposed model for the modB/GM2A complex. (A) Overview of the model. Amino acid substitution regions (RQNK 312–315 GSEP and DL 452–453 NR) in modB are shown as sphere models with labels (GSEP and NR). (B) Close-up view of the substitution region of GSEP in the A chain of modB. The GSEP-interacting loop region (I66–C75) of GM2A is shown in red.
4. Discussion
Recently, several therapeutic approaches have been developed for clinical application and trials for LSDs on the molecular bases of the enzymology and structural biology of lysosomal enzymes, including enzyme replacement therapy (ERT) [7], [17], gene therapy [18], [19], [20], pharmacological chaperone therapy [21], and substrate reduction therapy [22], [23]. However, these therapies are not clinically available for GM2 gangliosidoses (TSD, SD and variant AB) at present. In recent years, clinical trials of intrathecal and intracerebroventricular ERT as well as intracranial gene therapy for neurodegenerative LSDs, including mucopolysaccharidosis types I, II and IIIA, Batten disease and metachromatic leukodystrophy, have been performed in the U.S.A. and E.U. according to ClinicalTrials.gov (https://clinicaltrials.gov/, accessed March 3, 2016). These strategies are expected to be applicable to the GM2 gangliosidoses therapy.
GM2 is known to be physiologically degraded in lysosomes by HexA (αβ heterodimer) in co-operation with GM2A [4]. Previously, we developed a genetically engineered human HexA carrying N-glycans with high contents of terminal mannose 6-phosphate (M6P) (Om4HexA) produced by an MNN4-introduced methylotrophic yeast Ogataea minuta strain [17]. We also designed a human modified HexB (modB), which contains substitutions of six amino acids in the α-subunit, based on homology modeling of human HexA (αβ heterodimer, PDB ID: 2gjx) and HexB (ββ homodimer, PDB ID: 1nou), and produced a recombinant modB with a CHO cell line, which can degrade GM2 through interaction with GM2A [7], for brain-directed ERT for TSD and SD. We also demonstrated that both Om4HexA and modB could reduce the GM2 accumulated in the brains of SD (Hexb–/–) mice after intracerebroventricular administration.
In this study, we established a CHO cell line that stably expresses and secretes human GM2A (>10 mg/L CM). The purified GM2A with terminal M6P residues was taken up mainly via CI-M6PR [24] by the fibroblasts derived from a variant AB patient (GM2A deficiency), and reduced the GM2 accumulated in lysosomes of the patient cells. However, in the presence of 5 mM M6P in the CM, not only was the intracellular GM2 reduction partly inhibited but also the GM2A protein level became lower (data not shown). Since there are two mechanisms involved in recapture of extracellular GM2A via CI-M6PR and sortilin receptor [25], the purified recombinant GM2A was considered to be incorporated by the variant AB fibroblasts via both receptors.
Although human GM2A is produced by Escherichia coli [26], methylotrophic yeast Pichia pastoris [27], insect cells [28], and the CHO cell line [9], [25] and through chemical synthesis [29], as previously reported, the only form derived from the CHO cell line contains an N-glycan with M6P residues at the non-reducing termini [25]. So, we examined the combined replacement effect of recombinant modB and GM2A on TSD fibroblasts. It took about 7 days for the modB treatment to reduce the accumulated GM2 (data not shown). In contrast, combined replacement with modB and GM2A had a significant synergistic effect on the reduction of the GM2, it taking only 4 days.
We also predicted through in silico analysis the molecular interaction between the altered Hex β-subunit containing the substituted GSEP loop sequence and GM2A based on the structural information of the human HexA and GM2A complex model. Both of the GSEP loop structures in modB and HexA were suggested to be commonly recognized by the loop domain (Ile66–Cys75) of GM2A, and are responsible for the synergistic effect of co-administration of modB and GM2A on GM2 reduction in TSD fibroblasts. From these findings, we propose a novel combinational ERT involving co-administration of the modified HexB and GM2A for not only TSD and SD but also variant AB patients, although the latter GM2 gangliosidosis is known to be an exceptionally ultra-rare disease. Additional modifications of the Hex protein or GM2A to enhance the protein-protein interaction predicted on in silico analysis may be more effective for this combinational replacement therapy. Furthermore, the combination of GM2A protein replacement and modified HEXB single gene therapy may become an alternative therapy as a more effective therapy for GM2 gangliosidoses in the future.
Acknowledgements
We thank Dr. T. Tai, (The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) for the GM2-specific antibodies. This work was supported by the Program for Promotion of Fundamental Studied Health Sciences of the National Institute of Biomedical Innovation (NIBIO, Osaka, Japan., Grant number 09-15), Japan Science and Technology Agency Exploratory Research for Advanced Technology, the Platform Project for Supporting Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science), the Project for Creation of Research Platforms and Sharing of Advanced Research Infrastructure (Grant no. 25801) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and MEXT/JSPS KAKENHI (Grant nos. 26293120, 25460389, 23390140, and 23659527).
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.04.012.
Appendix A. Transparency document
Transparency document
.
References
- 1.Gravel R.A., Kaback M.M., Proia R.L. The GM2 gangliosidoses. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 3827–3876. [Google Scholar]
- 2.Wu Y.Y., Lockyer J.M., Sugiyama E. Expression and specificity of human GM2 activator protein. J. Biol. Chem. 1994;269:16276–16283. [PubMed] [Google Scholar]
- 3.Smiljanic-Georgijev N., Rigat B., Xie B. Characterization of the affinity of the GM2 activator protein for glycolipids by a fluorescence dequenching assay. Biochim. Biophys. Acta. 1997;1339:192–202. doi: 10.1016/s0167-4838(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 4.Zarghooni M., Bukovac S., Tropak M. An α-subunit loop structure is required for GM2 activator protein binding by β-hexosaminidase A. Biochem. Biophys. Res. Commun. 2004;324:1048–1052. doi: 10.1016/j.bbrc.2004.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton N.W., Brady R.O., Dambrosia J.M. Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 6.Eng C.M., Guffon N., Wilcox W.R. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N. Engl. J. Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka K., Tamura T., Tsuji D. Therapeutic potential of intracerebroventricular replacement of modified human β-hexosaminidase B for GM2 gangliosidosis. Mol. Ther. 2011;19:1017–1024. doi: 10.1038/mt.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotani M., Ozawa H., Kawashima I. Generation of one set of monoclonal antibodies specific for a-pathway ganglio-series gangliosides. Biochim. Biophys. Acta. 1992;1117:97–103. doi: 10.1016/0304-4165(92)90168-t. [DOI] [PubMed] [Google Scholar]
- 9.Sakuraba H., Itoh K., Shimmoto M. GM2 gangliosidosis AB variant: clinical and biochemical studies of a Japanese patient. Neurology. 1999;52:372–377. doi: 10.1212/wnl.52.2.372. [DOI] [PubMed] [Google Scholar]
- 10.Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 11.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji D., Higashine Y., Matsuoka K. Therapeutic evaluation of GM2 gangliosidoses by ELISA using anti-GM2 ganglioside antibodies. Clin. Chim. Acta. 2007;378:38–41. doi: 10.1016/j.cca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Pierce B.G., Hourai Y., Weng Z. Accelerating protein docking in ZDOCK using an advanced 3D convolution library. PLoS One. 2011;6:e24657. doi: 10.1371/journal.pone.0024657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemieux M.J. Crystallographic structure of human β-hexosaminidase A: interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis. J. Mol. Biol. 2006;359:913–929. doi: 10.1016/j.jmb.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright C.S., Zhao Q., Rastinejad F.J. Structural analysis of lipid complexes of GM2-activator protein. Mol. Biol. 2003;331:951–964. doi: 10.1016/s0022-2836(03)00794-0. [DOI] [PubMed] [Google Scholar]
- 16.Mark B.L. Crystal structure of human β-hexosaminidase B: understanding the molecular basis of Sandhoff and Tay-Sachs disease. J. Mol. Biol. 2003;327:1093–1109. doi: 10.1016/s0022-2836(03)00216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji D., Akeboshi H., Matsuoka K. Highly phosphomannosylated enzyme replacement therapy for GM2 gangliosidosis. Ann. Neurol. 2011;69:691–701. doi: 10.1002/ana.22262. [DOI] [PubMed] [Google Scholar]
- 18.Cachón-González M.B., Wang S.Z., Ziegler R. Reversibility of neuropathology in Tay-Sachs-related diseases. Hum. Mol. Genet. 2014;23:730–748. doi: 10.1093/hmg/ddt459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray-Edwards H.L., Brunson B.L., Holland M. Mucopolysaccharidosis-like phenotype in feline Sandhoff disease and partial correction after AAV gene therapy. Mol. Genet. Metab. 2015;116:80–87. doi: 10.1016/j.ymgme.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 20.McCurdy V.J., Rockwell H.E., Arthur J.R. Widespread correction of central nervous system disease after intracranial gene therapy in a feline model of Sandhoff disease. Gene Ther. 2015;22(1):81–89. doi: 10.1038/gt.2014.108. [DOI] [PubMed] [Google Scholar]
- 21.Platt F.M., Neises G.R., Reinkensmeier G. Prevention of lysosomal storage in Tay-Sachs mice treated with N-butyldeoxynojirimycin. Science. 1997;276:428–431. doi: 10.1126/science.276.5311.428. [DOI] [PubMed] [Google Scholar]
- 22.Maegawa G.H., Banwell B.L., Blaser S. Substrate reduction therapy in juvenile GM2 gangliosidosis. Mol. Genet. Metab. 2009;98:215–224. doi: 10.1016/j.ymgme.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Clarke J.T., Mahuran D.J., Sathe S. An open-label Phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants) Mol. Genet. Metab. 2011;102:6–12. doi: 10.1016/j.ymgme.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkes C., Kar S. Insulin-like growth factor-II/mannose-6-phosphate receptor: widespread distribution in neurons of the central nervous system including those expressing cholinergic phenotype. J. Comp. Neurol. 2003;458:113–127. doi: 10.1002/cne.10578. [DOI] [PubMed] [Google Scholar]
- 25.Rigat B., Wang W., Leung A., Mahuran D.J. Two mechanisms for the recapture of extracellular GM2 activator protein: evidence for a major secretory form of the protein. Biochemistry. 1997;36:8325–8331. doi: 10.1021/bi970571c. [DOI] [PubMed] [Google Scholar]
- 26.Klima H., Klein A., van Echten G. Over-expression of a functionally active human GM2-activator protein in Escherichia coli. Biochem. J. 1993;292:571–576. doi: 10.1042/bj2920571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendeler M., Hoernschemeyer J., John M. Expression of the GM2-activator protein in the methylotrophic yeast Pichia pastoris, purification, isotopic labeling, and biophysical characterization. Protein Expr. Purif. 2004;34:147–157. doi: 10.1016/j.pep.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Wendeler M., Lemm T., Weisgerber J. Expression of recombinant human GM2-activator protein in insect cells: purification and characterization by mass spectrometry. Protein Expr. Purif. 2003;27:259–266. doi: 10.1016/s1046-5928(02)00599-5. [DOI] [PubMed] [Google Scholar]
- 29.Sato K., Kitakaze K., Nakamura T. The total chemical synthesis of the monoglycosylated GM2 ganglioside activator using a novel cysteine surrogate. Chem. Commun. 2015;51:9946–9948. doi: 10.1039/c5cc02967h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document