Abstract
The non-essential amino acid L-glutamine (Gln) displays potent anti-inflammatory activity by deactivating p38 mitogen activating protein kinase and cytosolic phospholipase A2 via induction of MAPK phosphatase-1 (MKP-1) in an extracellular signal-regulated kinase (ERK)-dependent way. In this study, the mechanism of Gln-mediated ERK-dependency in MKP-1 induction was investigated. Gln increased ERK phosphorylation and activity, and phosphorylations of Ras, c-Raf, and MEK, located in the upstream pathway of ERK, in response to lipopolysaccharidein vitro and in vivo. Gln-induced dose-dependent transient increases in intracellular calcium ([Ca2+]i) in MHS macrophage cells. Ionomycin increased [Ca2+]i and activation of Ras → ERK pathway, and MKP-1 induction, in the presence, but not in the absence, of LPS. The Gln-induced pathways involving Ca2+→ MKP-1 induction were abrogated by a calcium blocker. Besides Gln, other amino acids including L-phenylalanine and l-cysteine (Cys) also induced Ca2+ response, activation of Ras → ERK, and MKP-1 induction, albeit to a lesser degree. Gln and Cys were comparable in suppression against 2, 4-dinitrofluorobenzene-induced contact dermatitis. Gln-mediated, but not Cys-mediated, suppression was abolished by MKP-1 small interfering RNA. These data indicate that Gln induces MKP-1 by activating Ca2+→ ERK pathway, which plays a key role in suppression of inflammatory reactions.
Abbreviations: AP-1, activating protein 1; Ala, alanine; Asp, aspartate; BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetraacetoxymethylester; CaM, calmodulin; CaR, Ca2+-sensing receptor; CD, contact dermatitis; cPLA2, cytoplasmic phospholipase A2; DMSO, dimethyl sulfoxide; DNFB, 1-fluoro-2,4-dinitrobenzene; ESR, ear swelling response; ERK, extracellular signal-regulated kinase; Gln, L-glutamine; Glu, glutamate; Gly, glycine; H&E, hematoxylin and eosin; [Ca2+]i, intracellular calcium concentration; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharides; MAPK, mitogen activated protein kinase; MKP-1, MAPK phosphatase-1; PEI, polyethyleneimine; siRNA, small interfering RNA
Keywords: Ras/c-Raf/MEK/ERK, extracellular-signal-regulated kinase; L-Glutamine; MAPK Phosphatase-1; Mitogen-activated protein kinase
1. Introduction
The non-essential amino acid L-Glutamine (Gln) is an energy substrate for most cells [1], [2], and is important in multiple ways in the nitrogen- and carbon-skeleton exchange among different tissues [3]. Several studies have demonstrated that Gln has an anti- inflammatory activity in humans [4] and animals [5], [6].
Regarding the molecular mechanism of anti- inflammatory activity of Gln, we have shown that Gln was beneficial against endotoxin shock as well as bronchial allergic asthma by inhibiting phosphorylation and activity of cytoplasmic phospholipase A2 (cPLA2) [7], [8], which has a high selectivity for liberating arachidonic acid that is subsequently metabolized by a panel of downstream enzymes for eicosanoid production [9], [10]. We have subsequently demonstrated that Gln deactivates p38 and c-Jun N-terminal kinase(JNK) mitogen-activated protein kinases (MAPKs) by a rapid induction of MAPK phosphatase 1 (MKP-1) protein in an extracellular signal-regulated kinase (ERK)-dependent way, which was beneficial against fatal endotoxic shock [11], steroid-resistant airway neutrophilia in allergic asthma [12] and, in particular, dermatitis [13], [14] in mice.
MKP-1, a member of the MKP family, plays a pivotal role in the negative control of p38 and JNK [15], [16]. Given that p38 has a role in the production of inflammatory molecules [17], [18], MKP-1 has been known to functions as a critical negative regulator of inflammation. MKP-1 is a labile protein that is normally degraded via the ubiquitin/proteasome pathway, and its phosphorylation reduces its ubiquitination and degradation [19], [20], [21]. ERK MAPK phosphorylates MKP-1 on two carboxyl-terminal serine residues -serine 359 and serine 364, which stabilizes MKP-1 by preventing the degradation from ubiquitin/proteasome pathway [19]. We have also demonstrated that ERK inhibitors blocked Gln-induced MKP-1 phosphorylation and protein induction [11], [12], [13], further supporting a role for ERK in Gln induction of MKP-1.
In this study, we investigated the precise mechanism of Gln-mediated MKP-1 induction. We found that Gln upregulates MKP-1 expression by activating initial Ca2+ response, followed by Ras/c-Raf/MEK/ERK pathway.
2. Materials and methods
2.1. Animals
Female BALB/c mice (7–8 weeks old, 16–18 g body weight) were purchased from the Samtako Bio Korea, and kept in our animal facility for at least 1 week before use. All animals used in this study were handled using the protocol approved by the Institutional Animal Care and Use Committee of the Chonbuk National University Medical School.
2.2. Chemicals and reagents
DNFB, LPS derived from Escherichia coli O127:B8 (L3024), and all L-amino acids used in this study were obtained from Sigma-Aldrich (St. Louis, MO, USA). U0126, a specific inhibitor of MEK1/2, was obtained from Calbiochem (Madison, WI, USA). U0126 dissolved in DMSO (12.5 mg/kg) [11]was injected i.p. 24 h before LPS treatment. The control group received vehicle. The intracellular calcium chelator, BAPTA-AM was purchased from Calbiochem (Madison, WI, USA). Fluo 4/AM were purchased from Molecular Probes (Eugene, OR, USA). Primary antibodies (rabbit anti-Ras, anti-phospho-c-Raf (Ser338), anti-phospho-MEK1/2 (Ser217/221), anti-phospho-ERK1/2, and anti-phospho-MKP-1) were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-MKP-1 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-phospho-MBP (clone P12) was from Millipore Corporation (Billerica, MA, USA).
2.3. Cell culture
MHS murine alveolar macrophage cells (ATCC CRL-2019), were maintained in RPMI 1640 supplemented with 10% heat inactivated FBS (Invitrogen, Carlsbad, CA, USA) and 1% antibiotics (Invitrogen, Carlsbad, CA, USA) at 37 °C in a 5% CO2 atmosphere.
2.4. Immunoblotting
Mice were sacrificed by cervical dislocation. Ear samples were frozen in liquid nitrogen and were stored in −70 °C until analyzed. Ear samples were homogenized in the Phosphosafe Extraction Reagent (Novagen, Madison, WI). Immunoblotting analysis was performed as described previously[13].
2.5. Immunoprecipitation
Lungs and cells were lysed in non-denaturing lysis buffer containing 20 mM Tris–HCl, pH 8, 137 mM NaCl, 10% glycerol, and 1% Triton X-100, 2 mM EDTA, protease inhibitor cocktail, and phosphatase inhibitor. Equal amounts of cell or tissue extracts were incubated with anti-phospho ERK1/2 at a dilution of 1:50 for 4 h at 4 °C in the same total volume of lysis buffer thereafter, protein A/G conjugated agarose beads (Santa Cruz Biotechnology) was added and incubated overnight. The agarose beads containing the immunoprecipitate was then washed with the lysis buffer five times and finally collected by centrifugation. After keeping a small amount of the beads for the kinase assay, the rest of the beads were suspended in sample buffer and boiled for Western blot analysis.
2.6. Assay of ERK activity
After challenge, lung samples were weighed (100 mg) and homogenized in 1 ml of in non-denaturing lysis buffer containing 20 mM Tris–HCl, pH 8, 137 mM NaCl, 10% glycerol, and 1% Triton X-100, 2 mM EDTA, protease inhibitor cocktail, and phosphatase inhibitor. Homogenates were then centrifuged at 12,000 g for 20 min at 4 °C to obtain the supernatant. Equal amounts of cell or tissue extracts were incubated with anti- ERK1/2 at a dilution of 1:50 for 4 h at 4 °C in the same total volume of lysis buffer thereafter, protein A/G conjugated agarose beads (Santa Cruz Biotechnology, CA, USA) was added and incubated overnight. The agarose beads containing the immunoprecipitate was then washed with the lysis buffer five times and finally collected by centrifugation. The washed precipitate was resuspended in 30 µl kinase buffer (15 mM Tris/HCl, pH 7.2, 15 mM MgCl2, and 1 mM dithiothreitol). ERK activity assay was analyzed using MAP kinase assay kits (Merckmilipore, Darmstadt, Germany) according to the manufacturer's instructions. The assay is based on the ability of ERK to phosphorylate the specific substrate, myelin basic protein, (MBP). The phosphorylated MBP is then analyzed by immunoblot analysis, probing with a monoclonal Phospho-specific MBP antibody.
2.7. Assay of Ras activation
Ras activation was evaluated by measuring an increase in intracellular Ras protein levels as described elsewhere [22], [23].
2.8. Measurement of [Ca2+]I
MHS cells were washed with Hanks’ balanced salt solution (HBSS; 2 mM CaCl2, 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM D-glucose, 20 mM HEPES, pH 7.3) containing 1% bovine serum albumin (BSA). MHS cells were incubated with 5 µM Fluo 4/AM (Molecular Probes) in Hanks’ balanced salt solution for 45 min at 37 °C. The cells were washed three times with HBSS. The MHS cells were placed on the stage of confocal microscope (Nikon, Tokyo, Japan) and Fluo 4/AM loaded cells were excited at excitation wavelength (F488 nM) and an emission fluorescence was measured at 530 nm. For the calculation of [Ca2+]i, the method of by Tsien et al.[24]was used with the following equation: [Ca2+]i=Kd(F-Fmin)/(Fmax-F), where Kdis 345 nM for Fluo-4, respectively, and F is the observed fluorescence levels. Each tracing was calibrated for the maximal intensity (Fmax) by the addition of ionomycin (10 µM) and for the minimal intensity (Fmin) by the addition of EGTA (50 mM) at the end of each measurement. The specific inhibitor for intracellular Ca2+ chelator (BAPTA-AM) was incubated at a suboptimal concentrations of 50 µM. The inhibitor was diluted into DMSO. To study Ca2+ entry in cells, Ca2+ free conditions were used.
2.9. Induction of CD
Induction of CD was performed as described previously [13].
2.10. Measurement of ESR
ESR was measured as described previously [13].
2.11. Histological analysis
Histological analysis was performed as described previously [13].
2.12. Measurement of cytokines and LTB4
Supernatants from ear samples were prepared as described previously [13]. Levels of IFN-γ, IL-1β and TNF-α were analyzed by ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. ELISA kit for LTB4 was from Cayman Chemical Company (Ann Arbor, MI, USA).
2.13. siRNA interference
siRNA strands for mouse MKP-1 and controls were obtained from Santa Cruz Biotechnology. In vivo delivery of siRNA was performed using in vivo-jet polyethyleneimine (PEI, Polyplus-transfection) (BP 90,018, F-67401 ILLKIRCH CEDEX, France), according to the manufacturer's instructions. To confirm that the MKP-1 siRNA used really blocked the synthesis of its target, an immunoblotting analysis was performed.
2.14. Statistical analyses
All data are shown as mean ± error of the mean (±SEM). Statistical comparison was performed using one-way ANOVA followed by the Fisher test. Significant differences between the groups were determined using the unpaired Student's t-test. A P-value <0.05 was considered statistically significant.
3. Results
3.1. Gln increases ERK phosphorylation and activity
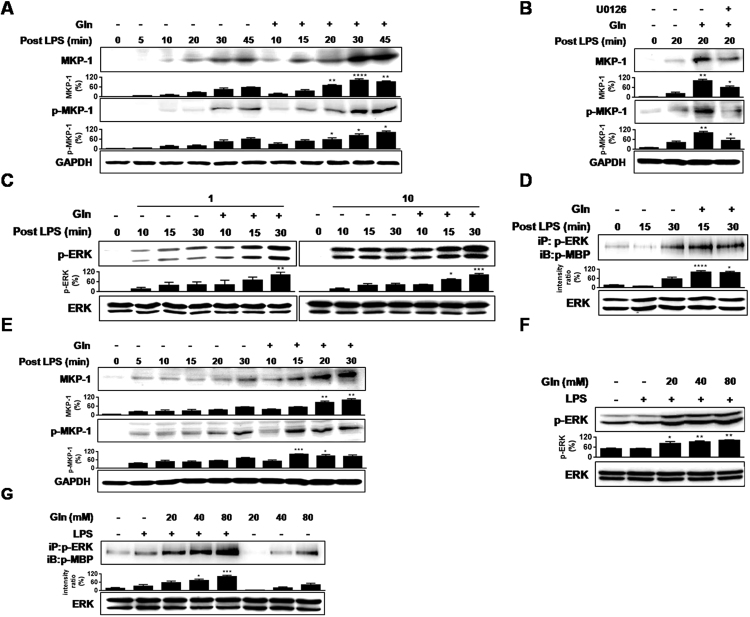
Systemic administration of Gln 5 min after exposure to lipopolysaccharide (LPS) resulted in appearance of MKP-1 phosphorylation and protein as early as 5 min in the lungs (Fig. 1(A)). The effects were abrogated by the MEK inhibitor, U0126 (Fig. 1(B)), confirming our previous findings that Gln induction of MKP-1 is ERK-dependent [11], [12], [13]. To investigate the underlying mechanism of ERK dependency, we assessed the possibility that Gln could affect ERK activity. Gln was administered i.p. at 5 min post-LPS, and ERK phosphorylation and activity were observed at 10, 15, and 30 min post-LPS. Gln increased ERK phosphorylation, which was more prominent at the relatively lower concentrations of LPS (1 µg/mouse) than at a higher concentration (10 µg/mouse) (Fig. 1(C)). Gln also increased ERK activity with a similar LPS concentration kinetics, as was the case for ERK phosphorylation (Fig. 1(D)). We also examined the effect of Gln in the murine alveolar macrophage cell line (MHS). LPS-induced MKP-1 phosphorylation and protein induction at around 30 min, and addition of Gln at 5 min after LPS stimulation resulted in early phosphorylation and protein induction of MKP-1 as early as within 5 min (Fig. 1(E)). Additionally, Gln increased not only ERK phosphorylation (Fig. 1(F)), but also ERK activity (Fig. 1(G)) in dose-dependent manners, as was evident in vivo.
Fig. 1.
Gln induces MKP-1 induction in an ERK-dependent way. (A) Lungs were removed at the indicated times after i.v. injection of LPS (50 µg/kg). Gln (750 mg/kg) was given i.p. 5 min after LPS. (B) U0126 (12.5 mg/kg) was injected i.p. 24 h before LPS injection. Lungs were removed at 20 min (C) Gln (750 mg/kg) was given i.p. 5 min after LPS (1 µg or 10 µg/mouse). (D) ERK activity against MBP was performed as described in Materials and Methods. A representative of three to five independent experiments with 3–5 mice/time point/experiment is shown.(E). Gln (40 mM) was added to MHS cells (2×106) 5 min after LPS (1 ng/ml) stimulation. (F) and (G). Gln (20 mM, 40 mM or 80 mM) was added to MHS cells (2×106) 5 min after LPS (1 ng/ml) stimulation. Cells were stimulated for 15 min A representative of three independent experiments is shown.
3.2. Gln activates Ras/c-Raf/MEK/ERK pathway in response to LPS
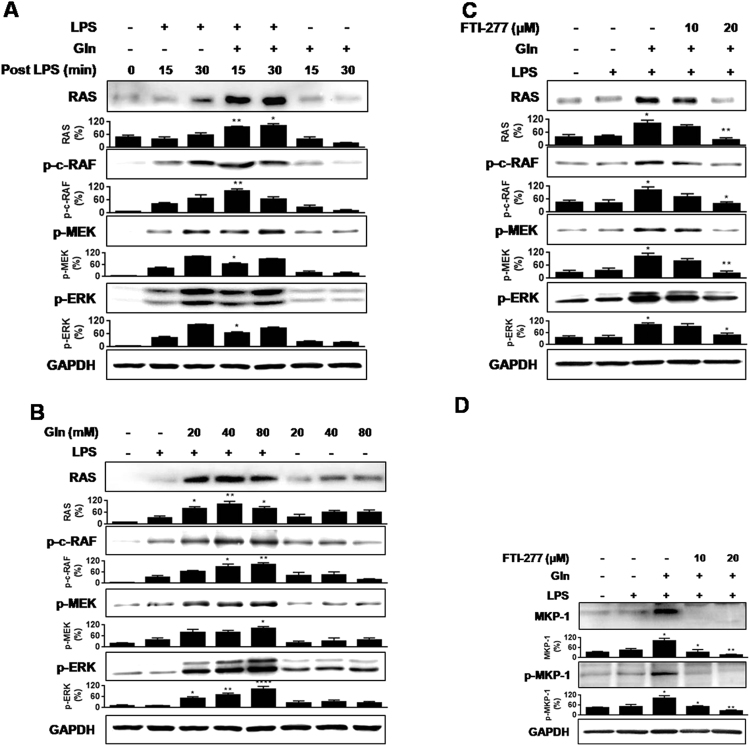
Regarding the upstream pathway of ERK activation, the Ras/Raf/MEK/ERK pathway (ERK cascade) is well established [25], [26], [27], [28]. Therefore, we assessed whether Gln can activate the pathway. Gln increased the levels Ras protein, and phosphorylations of c-Raf (Gln failed to phosphorylate b-Raf, data not shown) and MEK in vivo (Fig. 2(A)) as well as in vitro (Fig. 2(B)). Gln did not exert such effects in the absence of LPS especially in vivo. The Ras inhibitor, farnesyl transferase inhibitor (FTI)-277, inhibited Gln-mediated increases of Ras, phosphorylation of c-Raf, MEK, and ERK (Fig. 2(C)), as well as MKP-1 phosphorylation and protein induction (Fig. 2(D)) in MHS cells. The data indicate that Gln induction of MKP-1 is mediated via activation of ERK cascade.
Fig. 2.
Gln activates Ras/c-Raf (Ser338)/MEK/ERK pathway in response to LPS. (A) Lungs were removed at the indicated times after i.v. injection of LPS (50 µg/kg). Gln (750 mg/kg) was given i.p. 5 min after LPS. A representative of five independent experiments with 3–5 mice/time point/experiment is shown. (B) Gln (40 mM) was added to MHS cells (2×106) 5 min after LPS (1 ng/ml) stimulation (C) and (D) Serum starved MHS cells were pretreated with FTI (10 µM or 20 µM) for 1 h before stimulation with LPS (1 ng/ml). Gln (40 mM) was added to MHS cells 5 min after LPS. Cells were stimulated for 15 min A representative of three to five independent experiments is shown.
3.3. Gln activates ERK cascade by increasing intracellular Ca2+ level
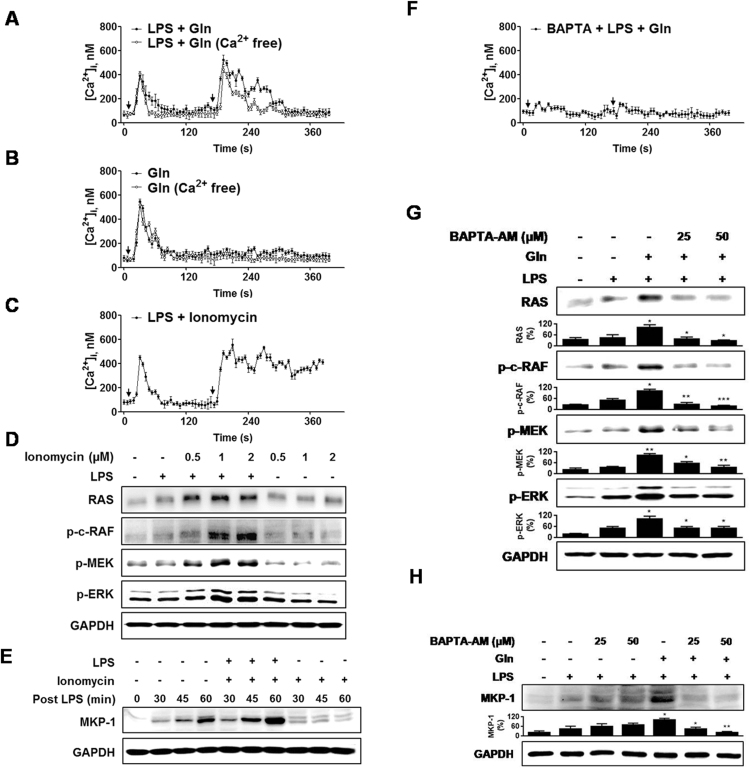
How can Gln modulate the Ras signal? Given that L-amino acids have been reported to increase intracellular calcium ([Ca2+]i) level [29], [30], [31]and the positive modulation of Ras signaling pathways by an increase in [Ca2+]i [32], [33], [34], [35], we investigated the possible involvement of Ca2+ in MHS cells. LPS-induced a transient rise in [Ca2+]i, and addition of Gln 3 min after LPS-induced another strong Ca2+ increase (Fig. 3(A)). In the absence of LPS, Gln also induced a transient rise in [Ca2+]i (Fig. 3(B)). In both cases, Gln-mediated elevation in [Ca2+]I was not significantly affected by removal of extracellular Ca2+.
Fig. 3.
Gln activates ERK cascade via increasing [Ca2+]i. (A) MHS cells (2×106) were loaded with Fluo 4/AM and then stimulated with the LPS (1 ng/ml) and then with Gln (40 mM) 3 min later. (B) [Ca2+]i response in the absence of LPS. (C) Ionomycin (1 µM), 3 min after LPS. (D) Five min after LPS, cells were stimulated with Ionomycin for 15 min (E) Ionomycin, 5 min after LPS. (F) Cells were pretreated with BAPTA-AM (50 µM) for 30 min before LPS. (G) and (H) Serum starved cells were pretreated with BAPTA-AM for 30 min before LPS and then Gln 5 min later. Cells were cultured for 15 min A representative of three to five independent experiments is shown. Each point represents mean ± S. E. of [Ca2+]i(A–C and F).
We next examined whether an increase in [Ca2+]i is responsible for the Gln-induced activation of the pathways involving Ras → ERK → MKP-1 using ionomycin. Ionomycin increased the [Ca2+]ilevel in the presence of LPS (Fig. 3(c)) and increased phosphorylation of Ras and ERK (Fig. 3(D)) and induction of MKP-1 (Fig. 3(E)), in the presence, but not in the absence, of LPS. Furthermore, intracellular calcium chelator, BAPTA-AM, abrogated Gln-induced Ca2+ response (Fig. 3(F)), phosphorylation of Ras, c-Raf, MEK, and ERK (Fig. 3(G)), and MKP-1 induction (Fig. 3(H)). Taken together, the data indicate that Gln induction of MKP-1 is attributed to its ability to induce a rise in [Ca2+]i, which in turn activates ERK cascade.
3.4. Comparison of other amino acids in their abilities to activate Ca2+/Ras/ERK/MKP-1 pathway
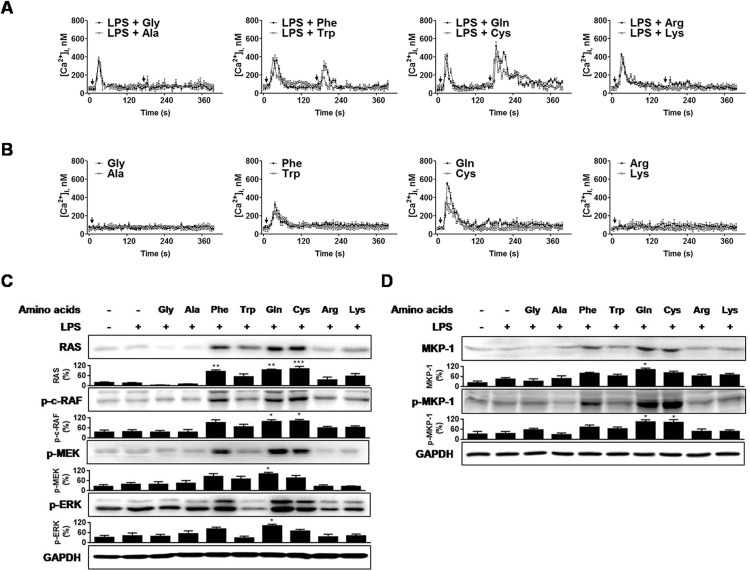
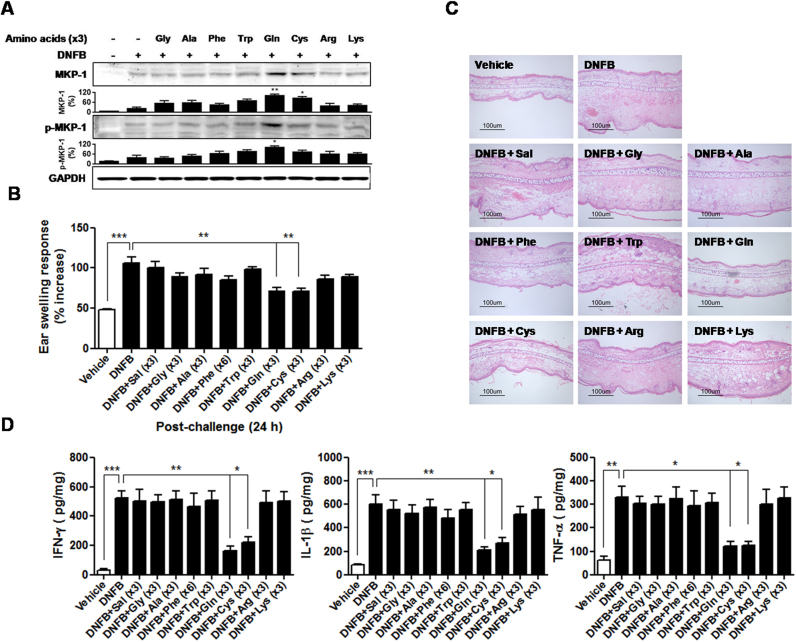
We further investigated the abilities of other amino acids to activate the pathways, Ca2+/Ras/ERK/MKP-1. Besides Gln, we included the following seven l-amino acids according to their differences in R groups: Glycine (Gly) and alanine (Ala) (nonpolar, aliphatic), phenylalanine (Phe) and tryptophan (Trp) (aromatic), lysine (Lys) and arginine (Arg) (positively charged), and cysteine (Cys) (polar, uncharged). Gln has a polar, uncharged R group like Cys. We excluded the negatively charged group, aspartate and glutamate because of their poor solubility. In the presence of LPS, only Gln, Cys, and Phe-induced a transient Ca2+ increase (Fig. 4(A)) in MHS cells, in the order of their effectiveness: Gln > Cys > Phe. In the presence of LPS, Gln, Cys, Phe, and Trp were capable of mobilizing Ca2+, in the order of their effectiveness: Gln > Cys > Phe > Trp (Fig. 4(B)). Other amino acids were inactive. Taken together, these data indicated that the polar, uncharged group (Gln and Cys) was effective in inducing Ca2+ mobilization among the amino acids tested, and aromatic group (Phe and Trp) also induced Ca2+ response, but these responses were significantly smaller than that evoked by Gln.
Fig. 4.
Effects of l-amino acids on [Ca2+]i and the Ras/ERK/MKP-1 pathway. (A) and (B) l-amino acids-induced [Ca2+]i with or without LPS (1 ng/ml). MHS cells (2×106) were loaded with fluorescence probe Fluo 4/AM. (C) and (D) L-amino acids (40 mM) were added MHS cells (2×106) 5 min after LPS (1 ng/ml) stimulation. Cells were cultured for 15 min A representative of three independent experiments is shown. Each point represents mean ± S. E. of [Ca2+]i(A and B).
We next compared the effects of amino acids on activation of ERK cascade and MKP-1 induction. Gln, Cys, and Phe, which showed Ca2+ provoking activities in the presence of LPS, activated the ERK cascade (Fig. 4(C)) and potentiated MKP-1 inductionand MKP-1 phosphorylation(Fig. 4(D)).
3.5. Comparison of other amino acids in their activities to suppress contact dermatitis (CD)
After 2, 4-dinitrofluorobenzene (DNFB) challenge, Gln and seven other amino acids (4% in saline) were topically applied on the ears of mice three times at 5, 10, and 15 min MKP-1 induction in the ears was assessed. Gln and Cys-induced MKP-1 induction, albeit to a lesser degree in the latter case. In contrast to in vitro, Trp failed to trigger MKP-1 induction (Fig. 5(A)). Application of DNFB to the ears increased ear swelling response (ESR) by approximately 120% compared with ears of mice that were treated with vehicle. Among the eight amino acids, only Gln and Cys significantly inhibited not only DNFB-induced ESR measured at 24 h (Fig. 5(B)), and marked spongiosis and extensive leukocyte infiltration in the swollen dermis (Fig. 5(C)), but also protein levels of interferon-gamma (IFN-γ), interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α) in the ears (Fig. 5(D)).
Fig. 5.
Effects of amino acids with enhancing [Ca2+]i on suppression of CD. L-amino acids (4% in saline) were topically applied onto the ears three times at 5, 10, and 15 min (A) Ears were removed at 30 min after DNFB challenge. A representative of five independent experiments with 3–5 mice/time point/experiment is shown. (B) ESRs were measured at 0 and 24 h after challenge. (C) H&E staining of the ear sections (scale bar =100 µm). (D) Cytokine levels in the ear homogenates. Data in (B) and (D) represent the mean ± standard deviation of three independent experiments with five mice per group. *P<0.05;**P<0.01;***P<0.001.
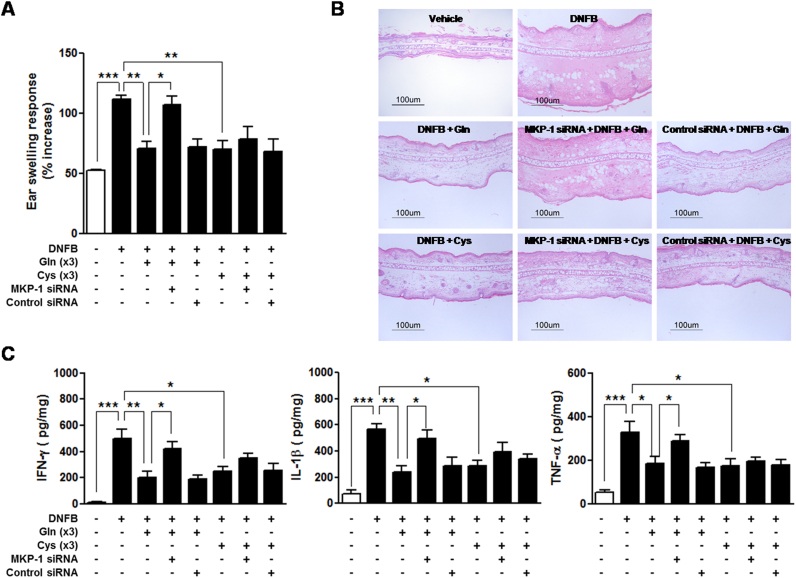
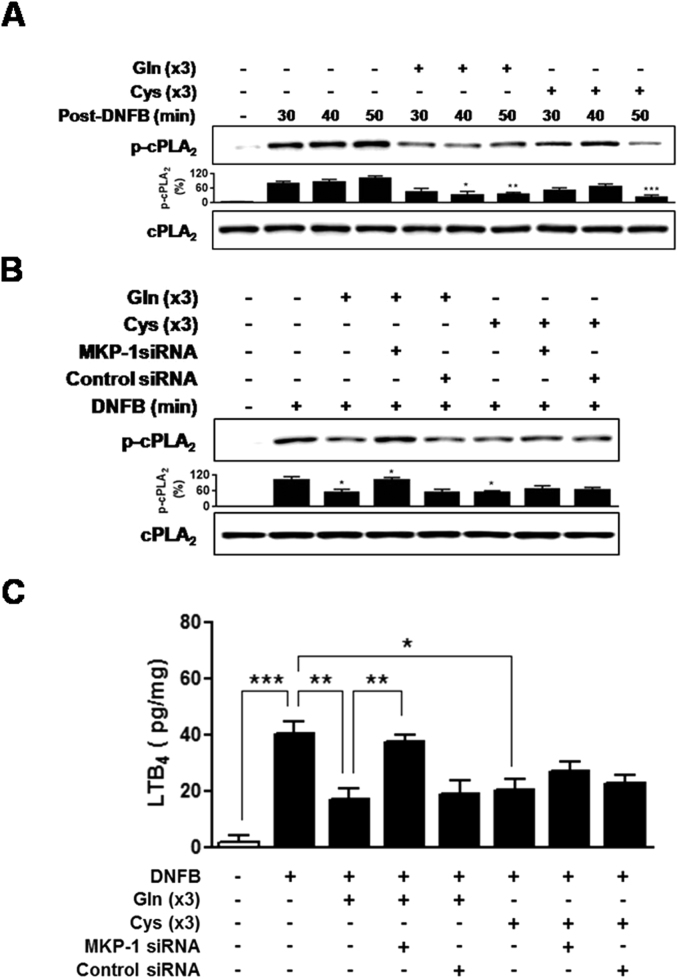
MKP-1 dependency of Gln and Cys suppression of CD was assessed. MKP-1 small interfering RNA (siRNA) significantly abrogated the Gln inhibition of ear inflammation (Fig. 6(A)-(B)) as well as ear levels of cytokines (Fig. 6(C)). In contrast, MKP-1 siRNA did not significantly affect Cys-mediated inhibition of ear inflammation (Fig. 6(A)-(B)) and ear levels of cytokines (Fig. 6(C)). Given that one of mechanisms of Gln inhibition of inflammation is cPLA2 inhibition [7], [8], [14], effects of Gln and Cys on cPLA2 phosphorylation were examined. Gln or Cys application after (DNFB) challenge resulted in inhibition of cPLA2 phosphorylation (Fig. 7(A)). However, whereas Gln-induced inhibition was reversed by MKP-1 siRNA, Cys-induced inhibition was not (Fig. 7(B)). Likewise, although Gln and Cys exerted similar inhibitory activities against the levels of a cPLA2 metabolite, leukotriene B4(LTB4), in the ear tissues, MKP-1 siRNA abrogated the activity of Gln, but not that of Cys (Fig. 7(C)). Taken together, among the amino acids tested, only Gln suppressed CD in a MKP-1-dependent manner.
Fig. 6.
MKP-1 siRNA abrogates suppressive activity of Gln against DNFB-induced CD. Mice were i.v. injected with 200 µl of the PEI mixture containing 0.4 nmol siRNA 24 h before challenge of DNFB. Gln or Cys was applied three times (×3) in the ear skin after DNFB challenge. (A) ESRs were measured at 0 and 24 h after challenge. (B) After measurement of ESR at 24 h, the mice were sacrificed, and the ear sections were stained with H&E (scale bar =100 µm). (C) Measurement of cytokine levels in the ear tissues were performed. Data in (A) and (C) represent the mean ± standard deviation of three independent experiments with five mice per group. *P<0.05;**P<0.01;***P<0.001.
Fig. 7.
Gln and Cys inhibit cPLA2 phosphorylation, but MKP-1 siRNA abrogates only Gln-induced inhibition.(A) The ears were removed at the indicated time points after DNFB challenge. Gln or Cys was applied three times (×3) in the ear skin after DNFB challenge. A representative of three independent experiments with 3–5 mice/time point/experiment is shown. (B) The ears were removed at 30 min post challenge. (C) LTB4 level in the ear samples at 24 h after DNFB challenge were calculated as pg of protein/mg of homogenized tissue solution. Data in (C) represent the mean ± standard deviation of three independent experiments with five mice per group. *P<0.05;**P<0.01;***P<0.001.
4. Discussion
Gln was previously found to show a strong anti-inflammatory activity via ERK-dependent MKP-1 induction. In this study, we found that this appears to be attributable to its ability to trigger sequential events – the steps that increase Ca2+ mobilization, followed by activation of Ras → ERK pathway.
Given that Gln-mediated MKP-1 induction is ERK-dependent [11], [13], we investigated whether Gln can potentiate ERK signaling. Gln increased ERK phosphorylation and activity. MKP-1 phosphorylation and protein induction occurs as early as 5 min after Gln administration. Similarly, increases of ERK phosphorylation and activity by Gln were seen within 5 min, suggesting that increase of ERK activity resulted in MKP-1 induction.
How can Gln exert such effects? Extracellular stimuli such as growth factors, serum, hormones, and cytokines lead to activation of ERK MAPK through a signaling cascade [25], [36], [37], [38], [39], [40], [41]. The signaling via this cascade is usually initiated by the activation of cell surface small G proteins, Ras [42], which is localized to the inner leaflet of the plasma membrane and transmit the signal by recruiting Raf kinases (A-Raf, B-Raf, and C-Raf) to the plasma membrane, where they can be activated. Activated Raf, in particular C-Raf (formally Raf-1) binds to and phosphorylates the down-stream dual specificity kinases MEK1 and MEK2, which, in turn, phosphorylate ERK1/2 within a conserved Thr-Glu-Tyr motif in their activation loop (known as the ERK cascade)[25], [26], [39], [43], [44]. Therefore, we explored whether Gln can activate the upstream kinases of ERK. Gln increased the levels of Ras protein and phosphorylation of, c-Raf, and MEK, indicating that Gln activates Ras-ERK pathway. In this study, we employed anti-phospho-c-Raf (Ser338) to examine Raf activation. However, given the complexity of Raf regulation by homo-and hetero-dimerisation as well as phosphorylation [45], we can not completely rule out the role of other Raf kinase.
What is the possible mechanism by which Gln activates Ras? In addition to a variety of extracellular stimuli leading to activation of the ERK cascade described above, a linkage between Ras activation and Ca2+has been described reported in neurons [32], [33], [34], [35], [46]. One of mechanisms is that these Ca2+ signals are mediated by Ca2+/calmodulin (CaM) protein kinase II (CaMKII), a ubiquitous serine/threonine protein kinase that is activated by Ca2+ and CaM to phosphorylate diverse substrates involved in metabolism, neurotransmitter release and cell cycle control. For example, the activation of the Ras/Raf/MEK/ERK signal pathway by calcium/calmodulin is well established [47]. These observations led us to explore possible cross-talk between these two signal pathways. Consistent with these findings, we demonstrated that Gln evoked Ca2+ mobilization regardless of the presence of LPS in MHS macrophages. Gln also stimulated significant Ca2+ response in the absence of extracellular Ca2+, suggesting that it mainly triggers the release of Ca2+ from an intracellular store. Ionomycin was comparable to Gln in terms of activation of Ras → ERK and MKP-1 induction in vitro. However, both Gln and ionomycin failed to activate the reactions in the absence of LPS, indicating that just Ca2+ increases are not sufficient to trigger such reactions. Ca2+ blocker abrogated not only Gln-mediated Ca2+ mobilization as well as the ERK cascade activation, but also Gln-mediated MKP-1 phosphorylation and protein induction. It has been reported that l-amino acids, particularly aromatic amino acids enhance the stereoselective sensitivity of the CaR to its agonists [30]. However, Gln was the most effective in this study. This may be attributed to the differences in the concentrations of amino acids (2–3 mM vs. 20–40 mM in this study) or cell type (human embryonic kidney cell vs. mouse macrophage cell). Taken together, our data indicate that Gln raises [Ca2+]i concentration and activates Ras → ERK pathway, leading to MKP-1 phosphorylation, which prevents the degradation of MKP-1 protein from ubiquitin/proteasome pathway, resulting in early and/or sustained induction of MKP-1.
Regarding the effects of other amino acids on triggering the Ca2+ → MKP-1 pathway, three amino acids – Gln, Cys (polar, uncharged) and Phe (aromatic) – were capable of mobilizing Ca2+ and activation of ERK cascade and MKP-1 induction in the presence of LPS in MHS cells, indicating that Ca2+ mobilizing activity is closely associated with its activity to trigger Ras-ERK-MKP-1 pathway. Interestingly, only the amino acids with Ca2+ mobilizing activity (Gln, Cys, and Phe) were able to activate Ras-ERK-MKP-1 pathway, indicating that, besides Gln, other amino acids including Cys and Phe were capable of MKP-1 induction via Ca-dependent activation of ERK cascade in vitro.
In contrast to the findings in vitro, Gln was the only amino acid to trigger MKP-1 induction significantly in a murine model of CD. Furthermore, only Gln and Cys significantly suppressed CD to a similar extent in terms of ear inflammation and ear levels of cytokines. Interestingly, Gln and Cys also similarly inhibited DNFB challenge-induced cPLA2 phosphorylation and the levels of a cPLA2 metabolite, LTB4, in the ear. When we assessed MKP-1 dependency of their suppressions using MKP-1 siRNA, MKP-1 siRNA reversed all the Gln's suppressive activities, confirming our previous findings [11], [12], [13], whereas Cys's suppressive activities were not significantly affected by MKP-1 siRNA. Therefore, our data indicate that Cys operates other mechanism rather than a mechanism mediated by MKP-1 in suppression of CD.
Ca2+-Ras pathway is activated by a great variety of extracellular stimuli, including growth factors, hormones, and cell? extracellular matrix contacts [48]. Guanine nucleotide binding protein (G protein)-coupled receptors (GPCRs) form one of the largest protein families found in nature, thus, representing the largest family of cell-surface receptors [49], [50]. Many GPCRs possess distinct allosteric sites that can be targeted by exogenous substances to modulate the receptors’ functions. These allosteric modulators include a variety of amino acids, ions, lipids, peptides, and accessory proteins [51]. Therefore, it is possible that, besides CaR [30], [31], Gln binds to allosteric sites on GPCR, resulting in increasing intracellular Ca2+ via activation of the known phospholipase C/ inositol 1,4,5-trisphosphate pathway. The identification of the Gln-binding GPCR requires further study.
5. Conclusions
We have previously demonstrated that Gln deactivates the two important enzymes involved in inflammatory reactions, p38 and cPLA2, by a rapid induction of MKP-1 protein in an ERK-dependent way. In this study, we demonstrate that 1) Gln induction of MKP-1 is attributed to its ability to enhance Ca2+ and subsequently activate the Ras/c-Raf/MEK/ERK pathway, and 2) Gln is the only amino acid to suppress CD in an exclusively MKP-1-dependent manner.
This study provides important information to improve our understanding of the mechanisms underlying Gln induction of MKP-1 and to establish an easier approach to therapeutic strategies for a variety of human inflammatory diseases as steroid-replacing and/or steroid-sparing agent.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea Government (MSIP) (No. 2008–0062279 and 1401000584).
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.05.011.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Fox R.E., Hopkins I.B., Cabacungan E.T., Tildon J.T. The role of glutamine and other alternate substrates as energy sources in the fetal rat lung type II cell. Pedia. Res. 1996;40:135–141. doi: 10.1203/00006450-199607000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Encarnacion S., Calderon J., Gelbard A.S., Cooper A.J., Mora J. Glutamine biosynthesis and the utilization of succinate and glutamine by Rhizobium etli and Sinorhizobium meliloti. Microbiology. 1998;144(Pt 9):2629–2638. doi: 10.1099/00221287-144-9-2629. [DOI] [PubMed] [Google Scholar]
- 3.Kovacevic Z., McGivan J.D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol. Rev. 1983;63:547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths R.D., Jones C., Palmer T.E. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13:295–302. [PubMed] [Google Scholar]
- 5.Newsholme E.A., Crabtree B., Ardawi M.S. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J. Exp. Physiol. 1985;70:473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- 6.Wischmeyer P.E., Lynch J., Liedel J., Wolfson R., Riehm J., Gottlieb L., Kahana M. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit. Care Med. 2001;29:2075–2080. doi: 10.1097/00003246-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.S., Kim G.Y., Kim J.H., You H.J., Park Y.M., Lee H.K., Yu H.C., Chung S.M., Jin Z.W., Ko H.M., Cho B.H. Glutamine inhibits lipopolysaccharide-induced cytoplasmic phospholipase A2 activation and protects against endotoxin shock in mouse. Shock. 2006;25:290–294. doi: 10.1097/01.shk.0000194041.18699.6f. [DOI] [PubMed] [Google Scholar]
- 8.Ko H.M., Kang N.I., Kim Y.S., Lee Y.M., Jin Z.W., Jung Y.J., Im S.Y., Kim J.H., Shin Y.H., Cho B.H., Lee H.K. Glutamine preferentially inhibits T-helper type 2 cell-mediated airway inflammation and late airway hyperresponsiveness through the inhibition of cytosolic phospholipase A(2) activity in a murine asthma model. Clin. Exp. Allergy. 2008;38:357–364. doi: 10.1111/j.1365-2222.2007.02900.x. [DOI] [PubMed] [Google Scholar]
- 9.Niknami M., Patel M., Witting P.K., Dong Q. Molecules in focus: cytosolic phospholipase A2-alpha. Int. J. Biochem Cell Biol. 2009;41:994–997. doi: 10.1016/j.biocel.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson B., Dahlen S.E., Lindgren J.A., Rouzer C.A., Serhan C.N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 11.Ko H.M., Oh S.H., Bang H.S., Kang N.I., Cho B.H., Im S.Y., Lee H.K. Glutamine protects mice from lethal endotoxic shock via a rapid induction of MAPK phosphatase-1. J. Immunol. 2009;182:7957–7962. doi: 10.4049/jimmunol.0900043. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.H., Kim H.K., Kim J.M., Ayush O., Im S.Y., Oh D.K., Lee H.K. Glutamine suppresses airway neutrophilia by blocking cytosolic phospholipase A(2) via an induction of MAPK phosphatase-1. J. Immunol. 2012;189:5139–5146. doi: 10.4049/jimmunol.1201599. [DOI] [PubMed] [Google Scholar]
- 13.Ayush O., Lee C.H., Kim H.K., Im S.Y., Cho B.H., Lee H.K. Glutamine suppresses DNFB-induced contact dermatitis by deactivating p38 mitogen-activated protein kinase via induction of MAPK phosphatase-1. J. Invest Dermatol. 2013;133:723–731. doi: 10.1038/jid.2012.373. [DOI] [PubMed] [Google Scholar]
- 14.Jin Z.W., Kim H.K., Lee C.H., Jung S.W., Shin S.J., Im S.Y., Cho B.H., Lee H.K. Glutamine suppresses dinitrophenol fluorobenzene-induced allergic contact dermatitis and itching: inhibition of contact dermatitis by glutamine. J. Dermatol. Sci. 2012;67:88–94. doi: 10.1016/j.jdermsci.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Franklin C.C., Kraft A.S. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J. Biol. Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 16.Hammer M., Mages J., Dietrich H., Servatius A., Howells N., Cato A.C., Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J. Exp. Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kracht M., Saklatvala J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine. 2002;20:91–106. doi: 10.1006/cyto.2002.0895. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S., Boehm J., Lee J.C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug. Disco. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 19.Brondello J.M., Pouyssegur J., McKenzie F.R. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 20.Orlowski R.Z., Small G.W., Shi Y.Y. Evidence that inhibition of p44/42 mitogen-activated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. J. Biol. Chem. 2002;277:27864–27871. doi: 10.1074/jbc.M201519200. [DOI] [PubMed] [Google Scholar]
- 21.Pearson G., Robinson F., Beers Gibson T., Xu B.E., Karandikar M., Berman K., Cobb M.H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 22.Lv Z., Yang L. MiR-124 inhibits the growth of glioblastoma through the downregulation of SOS1. Mol. Med. Rep. 2013;8:345–349. doi: 10.3892/mmr.2013.1561. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.K., Kim I.H., Choi Y.H., Choi J.W., Kim Y.M., Nam T.J. The proliferative effects of Pyropia yezoensis peptide on IEC-6 cells are mediated through the epidermal growth factor receptor signaling pathway. Int. J. Mol. Med. 2015;35:909–914. doi: 10.3892/ijmm.2015.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsien R.Y., Pozzan T., Rink T.J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982;295:68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- 25.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 26.Whelan J.T., Hollis S.E., Cha D.S., Asch A.S., Lee M.H. Post-transcriptional regulation of the Ras-ERK/MAPK signaling pathway. J. Cell Physiol. 2012;227:1235–1241. doi: 10.1002/jcp.22899. [DOI] [PubMed] [Google Scholar]
- 27.Giehl K., Skripczynski B., Mansard A., Menke A., Gierschik P. Growth factor-dependent activation of the Ras-Raf-MEK-MAPK pathway in the human pancreatic carcinoma cell line PANC-1 carrying activated K-ras: implications for cell proliferation and cell migration. Oncogene. 2000;19:2930–2942. doi: 10.1038/sj.onc.1203612. [DOI] [PubMed] [Google Scholar]
- 28.Chang F., Steelman L.S., Lee J.T., Shelton J.G., Navolanic P.M., Blalock W.L., Franklin R.A., McCubrey J.A. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 29.Ho Y.P., Yang M.W., Chen L.T., Yang Y.C. Relative calcium-binding strengths of amino acids determined using the kinetic method. Rapid Commun. Mass. Spectrom. 2007;21:1083–1089. doi: 10.1002/rcm.2927. [DOI] [PubMed] [Google Scholar]
- 30.Conigrave A.D., Franks A.H., Brown E.M., Quinn S.J. l-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur. J. Clin. Nutr. 2002;56:1072–1080. doi: 10.1038/sj.ejcn.1601463. [DOI] [PubMed] [Google Scholar]
- 31.Conigrave A.D., Quinn S.J., Brown E.M. l-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl. Acad. Sci. U S A. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cullen P.J., Lockyer P.J. Integration of calcium and Ras signalling. Nat. Rev. Mol. Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- 33.Marambaud P., Dreses-Werringloer U., Vingtdeux V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009;4:20. doi: 10.1186/1750-1326-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farnsworth C.L., Freshney N.W., Rosen L.B., Ghosh A., Greenberg M.E., Feig L.A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 35.Fan W.T., Koch C.A., de Hoog C.L., Fam N.P., Moran M.F. The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr. Biol. 1998;8:935–938. doi: 10.1016/s0960-9822(07)00376-4. [DOI] [PubMed] [Google Scholar]
- 36.Kyriakis J.M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 37.Dong C., Davis R.J., Flavell R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson R.J., Keyse S.M. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J. Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 39.Marshall C.J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Gibson T.B., Robinson F., Silvestro L., Pearson G., Xu B., Wright A., Vanderbilt C., Cobb M.H. MAP kinases. Chem. Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 41.Krishna M., Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol. Life Sci. 2008;65:3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiariello M., Vaque J.P., Crespo P., Gutkind J.S. Activation of Ras and Rho GTPases and MAP Kinases by G-protein-coupled receptors. Methods Mol. Biol. 2010;661:137–150. doi: 10.1007/978-1-60761-795-2_8. [DOI] [PubMed] [Google Scholar]
- 43.Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Micro. Mol. Biol. Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimoto S., Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–786. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu J., Stites E.C., Yu H., Germino E.A., Meharena H.S., Stork P.J., Kornev A.P., Taylor S.S., Shaw A.S. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell. 2013;154:1036–1046. doi: 10.1016/j.cell.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen L.B., Ginty D.D., Weber M.J., Greenberg M.E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 47.Illario M., Cavallo A.L., Bayer K.U., Di Matola T., Fenzi G., Rossi G., Vitale M. Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. J. Biol. Chem. 2003;278:45101–45108. doi: 10.1074/jbc.M305355200. [DOI] [PubMed] [Google Scholar]
- 48.Agell N., Bachs O., Rocamora N., Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 49.Bockaert J., Pin J.P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda S., Kadowaki S., Haga T., Takaesu H., Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 51.van der Westhuizen E.T., Valant C., Sexton P.M., Christopoulos A. Endogenous allosteric modulators of G protein-coupled receptors. J. Pharm. Exp. Ther. 2015;353:246–260. doi: 10.1124/jpet.114.221606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material