Abstract
TSLP induces Th2 cytokine production by Th2 cells and various other types of cells, thereby contributing to Th2-type immune responses and development of allergic disorders. We found that house dust mite (HDM) extract induced TSLP production by nasal epithelial cells, suggesting that TSLP may be involved in development of HDM-induced allergic rhinitis (AR). To investigate that possibility in greater detail, wild-type and TSLP receptor-deficient (TSLPR−/−) mice on the C57BL/6J background were repeatedly treated intranasally with HDM extract. The frequency of sneezing, numbers of eosinophils and goblet cells, thickness of submucosal layers, serum levels of total IgE and HDM-specific IgG1, and levels of IL-4, IL-5 and IL-13 in the culture supernatants of HDM-stimulated LN cells were comparable in the two mouse strains. Those findings indicate that, in mice, TSLPR is not crucial for development of HDM-induced AR.
Abbreviations: AR, allergic rhinitis; HDM, house dust mites; TSLP, thymic stromal lymphopoietin; TSLPR, thymic stromal lymphopoietin receptor
Keywords: TSLP receptor, Allergy, Mouse, Rhinitis, House dust mite
Highlights
-
•
TSLP is involved in Th2-type immune responses.
-
•
HDM can induce TSLP production by nasal epithelial cells.
-
•
TSLP receptor is not crucial for development of HDM-induced AR.
1. Introduction
The number of patients with allergic rhinitis (AR) has been increasing since around 1965. Its incidence is estimated at 10–20% in advanced countries, and it has become a health problem worldwide. In particular, patients with allergic reactions to house dust mites (HDM) and pollens are increasing. AR is accompanied by three major symptoms, i.e., sneezing, rhinorrhea and nasal congestion, and it is roughly divided into intermittent/seasonal AR (pollinosis) induced by pollens, and persistent/perennial rhinitis induced by HDM-derived antigens [1]. In general, patients with AR show an increased serum concentration of antigen-specific IgE and increased numbers of eosinophils in rhinorrhea. Crosslinking of high-affinity IgE receptors (FcεRI) by antigens and antigen-specific IgE complexes induces degranulation and activation of mast cells and basophils. Those cells then release a variety of proinflammatory mediators, such as histamine, leukotrienes, prostaglandins, cytokines and chemokines, which induce immediate-phase and/or delayed-phase reactions. In immediate-phase reactions, mast cell- and/or basophil-derived chemical mediators cause sneezing by stimulating the sensory nerves, secretion of serous nasal discharge from serous gland cells and nasal congestion associated with interstitial edema and increased vascular volume due to smooth muscle relaxation.
Accumulation of such leukocytes as eosinophils, neutrophils, macrophages and Th2 cells can be observed in the nasal mucosa as a delayed reaction [2]. Th2-cytokines such as IL-4, IL-5 and IL-13 are crucial for development of AR; IL-4, IL-5 and IL-13 are involved in induction of IgE, eosinophilia and mucus secretion, respectively. In support of this, Th2 cells are increased in the blood of patients with AR [3].
Thymic stromal lymphopoietin (TSLP) was identified as an IL-7 family cytokine expressed in thymic stromal cells [4], [5], [6]. Airway and intestinal epithelial cells, mast cells and basophils also express TSLP [4], [5], [6]. TSLP receptors (TSLPR), which consist of the IL-7Rα chain and the TSLPR chain, are expressed on dendritic cells (DCs) and CD4+ T cells [4], [5], [6]. TSLP is considered to be involved in Th2 cell differentiation by acting directly on naïve CD4+ T cells [7] and by promoting DC functions such as increased expression of OX40 ligands on their cell surface [5]. TSLP contributes to host defenses against parasites by augmenting Th2-type immune responses [8].
On the other hand, inappropriate/excessive expression of TSLP is thought to be involved in development of Th2-type allergic disorders [9]. In support of that, TSLP levels were increased in the nasal secretion and nasal epithelial cells of patients with AR induced by HDM [10], [11], [12]. However, the precise roles of TSLP in the development of HDM-induced AR remain poorly understood. Therefore, in the present study, we investigated TSLP using TSLP receptor-deficient (TSLPR−/−) mice as a model of AR induced by HDM.
2. Materials and methods
2.1. Animals
C57BL/6J wild-type mice were purchased from Japan SLC Inc. (Shizuoka, Japan). TSLPR-/- mice on the C57BL/6J background were generated as described previously [13]. Eight- to 10-week-old female mice were used in the experiments. All animals were housed under specific-pathogen-free conditions in an environmentally controlled clean room at The Institute of Medical Science, The University of Tokyo. All animal experiments were approved by the institution's Ethics Committee and conducted in accordance with its ethical safety guidelines (A11-28).
2.2. HDM-induced allergic rhinitis
AR was induced in mice by treatment with HDM extract from Dermatophagoides farinae (Greer Laboratories, Lenoir, NC, USA) as described previously [14].
2.3. Epithelial cell culture
Nasal epithelial cells were harvested from wild-type mice. Red blood cells were removed using a red blood cell removal solution (Sigma-Aldrich, St. Louis, MO, USA). The cells were then suspended in RPMI1640 medium (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Invitrogen, Grand Island, NY, USA), 50 μg/ml streptomycin (Invitrogen), 50 U/ml penicillin (Invitrogen), 5 mg/ml Transferrin (Sigma-Aldrich), 50 pM hydrocortisone (Sigma-Aldrich), 50 pM β-estradiol (Sigma-Aldrich), 10 mM HEPES (Invitrogen), and Insulin Transferrin Selenium (Invitrogen). The cells were cultured in a ϕ10-cm dish at 37 °C for 4 days in a 5% CO2 incubator. Cells were passaged two to four times, and the culture medium was changed every four days. Epithelial cells (2×105 cells/well in a 96-well flat-bottom plate) were cultured in the presence and absence of 50 μg/ml HDM extracts at 37 °C for 24 h, 48 h and 7 days in a 5% CO2 incubator.
2.4. Lymph node cell culture
At 48 h after the last inhalation of HDM or PBS, cervical lymph nodes (LNs) were collected, and LN cells were suspended in RPMI1640 (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Invitrogen), 50 μM 2-mercaptoethanol (Invitrogen), 50 μg/ml streptomycin and 50 U/ml penicillin (Invitrogen). LN cells (5×105 cells/well in 0.2 ml in a 96-well flat-bottom plate) were cultured in the presence and absence of 50 μg/ml HDM extract at 37 °C for 5 days in a 5% CO2 incubator.
2.5. Measurement of cytokines
The levels of TSLP, IL-4, IL-5 and IL-13 in the culture supernatants of nasal epithelial cells and LN cells were evaluated using ELISA kits obtained from BioLegend (San Jose, CA, USA) or Peprotech Inc. (Rocky Hill, NJ, USA).
2.6. Measurement of serum immunoglobulins
Sera were collected from mice 48 h after the last inhalation of HDM, or PBS as a control. The serum levels of total IgE were determined using an ELISA kit (Bethyl Laboratories, Montgomery, TX, USA) in accordance with the manufacturer's instructions. The serum levels of HDM-specific IgG1 were determined using an ELISA kit (Bethyl Laboratories) with 0.1-mg/ml HDM extract as a coating antigen, as described elsewhere [15].
2.7. Histological analysis
At 48 h after the last inhalation of HDM or PBS, mouse heads were severed, fixed and decalcified as described previously [14]. Four-μm coronal paraffin sections were stained with hematoxylin and eosin, and with periodic acid-Schiff (PAS). The numbers of eosinophils and PAS-positive cells and the submucosal thickness were determined as described previously [14].
2.8. Statistical analysis
Data show the mean±SEM. Unless otherwise specified, ANOVA was used for statistical evaluation of results. P values of less than.05 using Graph Pad Prism software (San Diego, CA, USA) were considered statistically significant.
3. Results
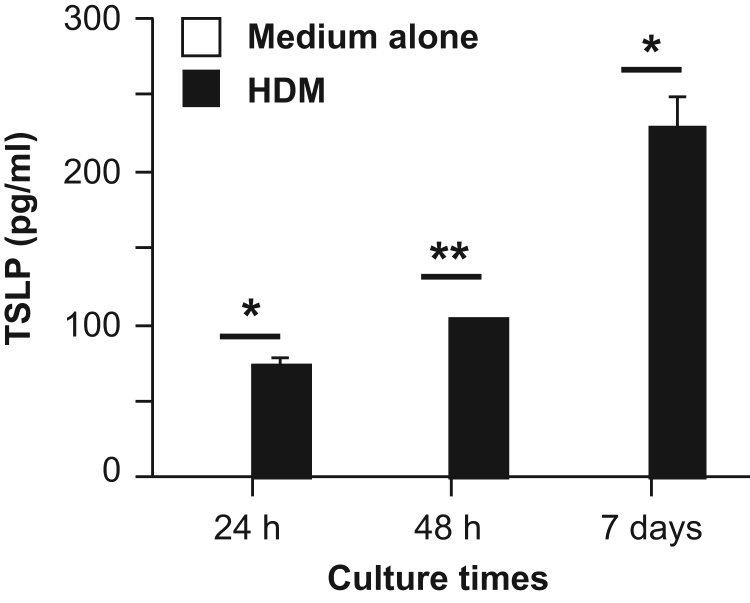
TSLP was reported to be produced by epithelial cells from the skin and lungs [16], [17]. In order to evaluate whether nasal epithelial cells also can produce TSLP, we cultured nasal epithelial cells from wild-type mice in the presence and absence of HDM extract. As shown in Fig. 1, TSLP was increased in the supernatants of nasal epithelial cells cultured in the presence, but not absence, of HDM extract for 24 h, 48 h and 7 days. These observations indicate that HDM extract can directly induce TSLP production by nasal epithelial cells.
Fig. 1.
TSLP induction by nasal epithelial cells in response to HDM extract. Nasal epithelial cells from wild-type mice were cultured in the presence and absence of HDM extract for 24 h, 48 h and 7 days. The levels of TSLP in the culture supernatants were determined by ELISA. Data show the mean±SEM (n=3). *p<0.05 and **p<0.01. The data show representative results from 3 independent experiments.
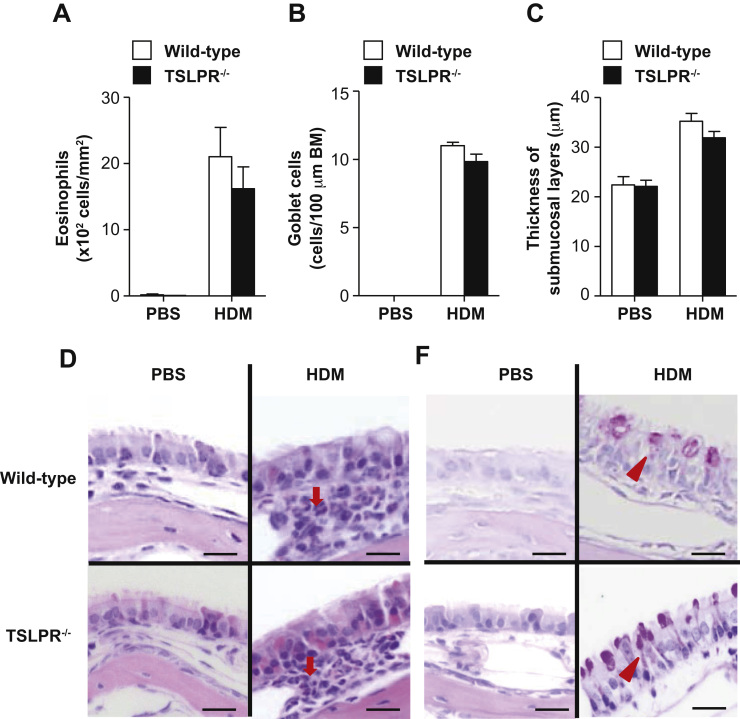
To elucidate the role of TSLP in development of HDM-induced AR, we treated wild-type and TSLPR-/- mice with HDM intranasally. The frequency of sneezing was comparable between the two mouse groups during the 5-minute period after the last HDM treatment (Fig. 2A). Moreover, the serum levels of total IgE and HDM-specific IgG1 were equivalent between the two mouse groups at 48 h after the last HDM inhalation (Fig. 2B and C). The numbers of eosinophils (Fig. 3A, D) and goblet cells (Fig. 3B, D) in the nasal mucosa and the thickness of the submucosal layers (Fig. 3C) were also comparable between the two mouse groups at 48 h after the last HDM inhalation.
Fig. 2.
TSLPR is not essential for immediate reaction during HDM-induced AR. Wild-type and TSLPR-/- mice were treated intranasally with HDM extract or PBS. Sera were collected 48 h after the last inhalation. (A) The frequency of sneezing was counted for 5 min after the last HDM or PBS treatment. (B)The serum levels of total IgE and (C) HDM-specific IgG1 were determined by ELISA. Data show the mean±SEM (wild-type mice, n=4 [PBS] and n=6–8 [HDM]; and TSLPR−/− mice, n=4 [PBS] and n=6-8 [HDM]).
Fig. 3.
TSLPR is not essential for development of HDM-induced AR. Wild-type and TSLPR−/− mice were treated intranasally with HDM extract or PBS. Tissues were harvested 48 h after the last inhalation. (A) The number of eosinophils in the nasal mucosa. (B) The number of goblet cells in the nasal mucosa. (C) The thickness of submucosal layers. (D) H&E and PAS staining of nasal mucosa sections. Arrow = eosinophil; arrowhead=goblet cell. Scale bar=20 µm. Data show the mean±SEM (wild-type mice, n=4 [PBS] and n=6-8 [HDM]; and TSLPR−/− mice, n=4 [PBS] and n=6–8 [HDM]) (A, B and C). Data show representative results from 4 to 6 mice in each group (D).
TSLP is involved in the differentiation and activation of Th2 cells [7]. At 48 h after the last HDM inhalation, we harvested LNs from wild-type and TSLPR−/− mice and then cultured the LN cells with HDM extract in vitro. After the cultivation, the levels of IL-4, IL-5 and IL-13 in the LN cell culture supernatants were also identical between the two mouse groups (Fig. 4), suggesting that TSLPR is not essential for the differentiation and activation of Th2 cells during HDM-induced AR. Taken together, our findings suggest that epithelial cell-derived TSLP is not essential for development of HDM-induced AR.
Fig. 4.
TSLPR is not required for induction and activation of HDM-specific LN cells. Wild-type and TSLPR−/− mice were treated intranasally with HDM extract or PBS. Cervical LNs were harvested 48 h after the last inhalation. LN cells were cultured in the presence of HDM extract for 72 h. The levels of IL-4, IL-5 and IL-13 in the culture supernatants were determined by ELISA. Data show the mean±SEM (wild-type mice, n=4 [PBS] and n=6-8 [HDM]; and TSLPR−/− mice, n=4 [PBS] and n=6-8 [HDM]) (A, B and C).
4. Discussion
Epithelial cell-derived TSLP is a potent cytokine that directly induces Th2 cytokine production by various types of cells such as Th2 cells, mast cells, basophils and/or group 2 innate lymphoid cells [4], [5], [6]. It also indirectly induces Th2 cytokine production via Th2 cell activation through DCs functions such as expression of OX40 ligands on their cell surface [4], [5], [6]. On the other hand, when LN cells from wild-type and TSLPR−/− mice after treatment with HDM antigens were re-stimulated with HDM antigens in vitro, the levels of IL-4, IL-5 and IL-13 in the culture supernatants of LNs were comparable between these groups, as shown in Fig. 4. These observations suggest that Th2 cell activation through DC functions—including antigen presentation and OX 40L expression—was normal even in the absence of TSLPR in our model. It is known that TSLP contributes to development of certain allergic diseases such as asthma [17], [18], [19] and atopic dermatitis [16]. Also, the levels of TSLP were increased in specimens from patients with AR [10], [11], [12], suggesting that TSLP may be involved in development of AR. In support of that notion, nasal goblet cell hyperplasia during OVA-induced AR was suppressed in mice treated with anti-TSLP neutralizing Ab [20]. Moreover, it was recently reported that induction of AR by ragweed pollen (a model of intermittent/seasonal AR) was reduced in TSLPR−/− mice [21]. In the present study, we demonstrated that induction by HDM (a model of persistent/perennial rhinitis) was normal in TSLPR−/− mice. Likewise, allergic airway inflammation induced by OVA was reduced [22], but it developed normally in response to HDM [23], in TSLPR−/− mice. Therefore, TSLP is critical for development of OVA- and ragweed pollen-induced AR and airway inflammation, whereas it is not responsible for HDM-induced AR and airway inflammation in mice. In addition to TSLP, IL-33, which is a member of the IL-1 cytokine family, is known to be produced by epithelial cells and can induce production of type 2 cytokines such as IL-4, IL-5 and/or IL-13 by various types of cells such as Th2 cells, basophils and/or group 2 innate lymphoid cells [24]. It has been reported that IL-33 is crucial for development of HDM-induced allergic airway inflammation [23] and HDM-induced AR [14]. These observations suggest that epithelial cell-derived IL-33, but not TSLP, is crucial for development of HDM-induced allergic diseases such as airway inflammation and rhinitis.
5. Conclusion
TSLP is not crucial for development of HDM-induced AR in mice.
Acknowledgments
We thank Lawrence W. Stiver (Tokyo, Japan) for his critical reading of the manuscript. This work was supported by PRESTO, JST (S. N.).
Footnotes
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.06.003.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.van Cauwenberge P., Bachert C., Passalacqua G., Bousquet J., Canonica G.W., Durham S.R., Fokkens W.J., Howarth P.H., Lund V., Malling H.J., Mygind N., Passali D., Scadding G.K., Wang D.Y. Consensus statement on the treatment of allergic rhinitis. Eur. Acad. Allergol. Clin. Immunol. Allergy. 2000;55:116–134. doi: 10.1034/j.1398-9995.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Skoner D.P. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J. Allergy Clin. Immunol. 2001;108:S2–S8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C.Q., Li T.L., He S.H., Chen X., An Y.F., Wu W.K., Zhou X.H., Li P., Yang P.C. Specific immunotherapy suppresses Th2 responses via modulating TIM1/TIM4 interaction on dendritic cells. Allergy. 2010;65:986–995. doi: 10.1111/j.1398-9995.2009.02295.x. [DOI] [PubMed] [Google Scholar]
- 4.Saenz S.A., Taylor B.C., Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., Malefyt Rde W., Omori M., Zhou B., Ziegler S.F. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 6.Omori-Miyake M., Ziegler S.F. Mouse models of allergic diseases: TSLP and its functional roles, Allergology international: official journal of the Japanese. Soc. Allergol. 2012;61:27–34. doi: 10.2332/allergolint.11-RAI-0374. [DOI] [PubMed] [Google Scholar]
- 7.Omori M., Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J. Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 8.Giacomin P.R., Siracusa M.C., Walsh K.P., Grencis R.K., Kubo M., Comeau M.R., Artis D. Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection. J. Immunol. 2012;189:4371–4378. doi: 10.4049/jimmunol.1200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler S.F. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr. Opin. Immunol. 2010;22:795–799. doi: 10.1016/j.coi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu G., Zhang L., Wang D.Y., Xu R., Liu Z., Han D.M., Wang X.D., Zuo K.J., Li H.B. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010;65:581–589. doi: 10.1111/j.1398-9995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 11.Mou Z., Xia J., Tan Y., Wang X., Zhang Y., Zhou B., Li H., Han D. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009;129:297–301. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 12.Kamekura R., Kojima T., Koizumi J., Ogasawara N., Kurose M., Go M., Harimaya A., Murata M., Tanaka S., Chiba H., Himi T., Sawada N. Thymic stromal lymphopoietin enhances tight-junction barrier function of human nasal epithelial cells. Cell Tissue Res. 2009;338:283–293. doi: 10.1007/s00441-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 13.Carpino N., Thierfelder W.E., Chang M.S., Saris C., Turner S.J., Ziegler S.F., Ihle J.N. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol. Cell. Biol. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi W., Yamaguchi S., Matsuda A., Suzukawa M., Shibui A., Nambu A., Kondo K., Suto H., Saito H., Matsumoto K., Yamasoba T., Nakae S. IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS One. 2013;8:e78099. doi: 10.1371/journal.pone.0078099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps S., Lam C.E., Kaiko G.E., Foo S.Y., Collison A., Mattes J., Barry J., Davidson S., Oreo K., Smith L., Mansell A., Matthaei K.I., Foster P.S. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am. J. Respir. Crit. Care Med. 2009;179:883–893. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 16.Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., Smith K., Gorman D., Zurawski S., Abrams J., Menon S., McClanahan T., de Waal-Malefyt Rd R., Bazan F., Kastelein R.A., Liu Y.J. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 17.Semlali A., Jacques E., Koussih L., Gounni A.S., Chakir J. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J. Allergy Clin. Immunol. 2010;125:844–850. doi: 10.1016/j.jaci.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Al-Shami A., Spolski R., Kelly J., Keane-Myers A., Leonard W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada M., Hirota T., Jodo A.I., Hitomi Y., Sakashita M., Tsunoda T., Miyagawa T., Doi S., Kameda M., Fujita K., Miyatake A., Enomoto T., Noguchi E., Masuko H., Sakamoto T., Hizawa N., Suzuki Y., Yoshihara S., Adachi M., Ebisawa M., Saito H., Matsumoto K., Nakajima T., Mathias R.A., Rafaels N., Barnes K.C., Himes B.E., Duan Q.L., Tantisira K.G., Weiss S.T., Nakamura Y., Ziegler S.F., Tamari M. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:787–793. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyata M., Hatsushika K., Ando T., Shimokawa N., Ohnuma Y., Katoh R., Suto H., Ogawa H., Masuyama K., Nakao A. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur. J. Immunol. 2008;38:1487–1492. doi: 10.1002/eji.200737809. [DOI] [PubMed] [Google Scholar]
- 21.Akasaki S., Matsushita K., Kato Y., Fukuoka A., Iwasaki N., Nakahira M., Fujieda S., Yasuda K., Yoshimoto T. Murine allergic rhinitis and nasal Th2 activation are mediated via TSLP- and IL-33-signaling pathways. Int. Immunol. 2016;28:65–76. doi: 10.1093/intimm/dxv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou B., Comeau M.R., De Smedt T., Liggitt H.D., Dahl M.E., Lewis D.B., Gyarmati D., Aye T., Campbell D.J., Ziegler S.F. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 23.Chu D.K., Llop-Guevara A., Walker T.D., Flader K., Goncharova S., Boudreau J.E., Moore C.L., Seunghyun T., Waserman In, S., Coyle A.J., Kolbeck R., Humbles A.A., Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 2013;131:187–200. doi: 10.1016/j.jaci.2012.08.002. e181–e188. [DOI] [PubMed] [Google Scholar]
- 24.Barlow J.L., McKenzie A.N. Type-2 innate lymphoid cells in human allergic disease. Curr. Opin. Allergy Clin. Immunol. 2014;14:397–403. doi: 10.1097/ACI.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material