Abstract
Background
Intravascular haemolysis has been associated with acute kidney injury (AKI) in different clinical settings (cardiac surgery, sickle cell disease). Haemolysis occurs frequently in critically ill burn patients. The aim of this study was to assess the predictive value of haptoglobin at admission to predict major adverse kidney events (MAKE) and AKI in critically ill burn patients.
Methods
We conducted a retrospective, single-centre cohort study in a burn critical care unit in a tertiary centre, including all consecutive severely burned patients (total burned body surface > 20% and/or shock and/or mechanical ventilation at admission) from January 2012 to April 2017 with a plasmatic haptoglobin dosage at admission.
Results
A total of 130 patients were included in the analysis. Their mean age was 49 (34–62) years, their median total body surface area burned was 29% (15–51%) and the intensive care unit (ICU) mortality was 25%. Early haemolysis was defined as an undetectable plasmatic haptoglobin at admission. We used logistic regression to identify MAKE and AKI risk factors. In multivariate analysis, undetectable haptoglobin was associated with MAKE and AKI (respectively, OR 6.33, 95% CI 2.34–16.45, p < 0.001; OR 8.32, 95% CI 2.86–26.40, p < 0.001).
Conclusions
Undetectable plasmatic haptoglobin at ICU admission is an independent risk factor for MAKE and AKI in critically ill burn patients. This study provides a rationale for biomarker-guided therapy using haptoglobin in critically ill burn patients.
Keywords: Haptoglobin, Intravascular haemolysis, Acute kidney injury, Burn patients, Major adverse kidney event
Background
Acute kidney injury (AKI) during an intensive care unit (ICU) stay is associated with increased mortality and morbidity [1, 2]. The pathophysiology of AKI in critically ill patients remains poorly understood. Preventive or curative strategies for AKI are lacking and urgently needed. In the specific population of critically ill burn patients, the prevalence of acute AKI has been reported to be as high as 53%, with mortality rates ranging from 35% to 70% [3–5]. Although haemodynamic alterations, including hypovolaemic shock and low cardiac output, may precipitate the development of AKI [6], other factors are likely to participate [7].
Among the factors contributing to AKI in critically ill burn patients, intravascular haemolysis is a potential candidate. Severe burns have been associated with haemolysis at the early stage of injury [8, 9]. Haemolysis has also been described as a strong causal factor for AKI in other situations such as sickle cell disease [10, 11] or after cardiopulmonary bypass for cardiovascular surgery [12, 13]. The pathophysiology of renal toxicity is complex and multifactorial, involving (1) cell-free haemoglobin (fHb), which is a scavenger of nitric oxide (NO), thereby decreasing its bioavailability and inducing systemic vasoconstriction; and (2) a direct toxicity of fHb that aggregates into casts in the tubular lumen [14, 15]. Better and earlier identification of haemolysis-related AKI may allow the selection of patients who could benefit from specific and innovative treatments such as intravenous haptoglobin administration [16]. The objective of this study was to evaluate the association between plasma haptoglobin level—a widely available biomarker of intravascular haemolysis—and occurrence of major adverse kidney events (MAKE) and AKI in critically ill burn patients.
Methods
Study design and population
We conducted a single-centre cohort study in the burn unit of St. Louis Hospital (Assistance Publique - Hôpitaux de Paris), Paris, France. The study was approved by our local ethics committee (PRONOBURN study, comité de protection des personnes IV, St-Louis Hospital; Institutional Review Board 00003835, protocol 2013/17NICB). All medical records of the patients admitted to our intensive care burn unit between January 2012 and April 2017 were screened. All burn patients meeting the following criteria were included in the study: total body surface area (TBSA) burned > 20%, and/or mechanical ventilation at admission, and/or catecholamine infusion at admission, and a measurement of haptoglobin upon admission.
Outcomes
The primary endpoint of the study was MAKE at day 90. The secondary endpoints were AKI and death within 90 days. MAKE was defined as a composite of the following criteria: death within 90 days, new renal replacement therapy (RRT) during ICU stay, and/or no renal recovery (defined as a ratio of serum creatinine at ICU discharge to serum creatinine at admission > 125%) [17]. AKI was defined and staged according to the Kidney Disease: Improving Global Outcomes criteria [17]. Serum creatinine (Screat) at hospital admission was used to define the baseline Screat.
Measurements
We collected the following data: age, sex, body mass index, TBSA, full-thickness body surface area burned, mechanism of injury and patient characteristics, Simplified Acute Physiology Score II (SAPS II), Abbreviated Burn Severity Index (ABSI) [18], Unit Burn Standard [19], treatments administered during the first 7 days after admission (hydroxocobalamin, aminoglycosides, vasopressors), and 28- and 90-day mortality. Haptoglobin measurement was performed using a haptoglobin assay (Hitachi modular P analyser; Roche, Paris, France) that is based on the principle of immunological agglutination. Anti-haptoglobin antibodies react with antigen in the sample to form antigen-antibody complexes, which, after agglutination, can be determined turbidimetrically. The reference values of haptoglobin are from 0.3 g/L to 2 g/L; the detection limit is 0.1 g/L; and the linearity limit is 5.2 g/L.
Patient management
Patients were resuscitated according to the St. Louis Hospital Intensive Care Burn Unit resuscitation protocol with the following haemodynamic targets: mean arterial blood pressure > 65 mmHg, 0.5 ml/kg/h less than urine output < 1 ml/kg/h, 2.5 L/minute/m2 less than cardiac index < 3 L/minute/m2 and central venous oxygen saturation > 70%. Norepinephrine was administered when required (diastolic arterial blood pressure < 50 mmHg and/or systemic vascular resistance index < 1250 dyn/second/cm−5/m2). Patients received initial fluid resuscitation using an intravenous bolus of Ringer’s lactate 0.25 ml/kg/%TBSA/h (which corresponds to the 2 ml/kg/%TBSA in the first 8 h of the Parkland formula) with fluid infusion adjusted to reach pre-defined haemodynamic targets. Cardiac function was systematically assessed on admission by echocardiography. Cardiac index was measured by transpulmonary thermodilution using a PiCCO2 monitor (Pulsion Medical Systems AG, Munich, Germany). The PiCCO monitor was calibrated every 2 h during the first 48 h.
Albumin 20% was administered to patients with TBSA > 30% after the sixth hour after thermal injury to reach a serum albumin concentration of 30–35 g/L. When mechanical ventilation was initiated, tidal volume was limited to 6–7 ml/kg to maintain an inspiratory plateau pressure < 30 cmH2O and a transpulmonary driving pressure < 15 cmH2O. Early enteral nutrition was initiated within 24 h of admission. Glycaemic control was adjusted to maintain glucose levels between 5 and 9 mmol/L. Surgical treatment included escharotomy or fasciotomy as needed and early coverage of excised burn wounds with autografts and/or allografts within the first 7 days after admission.
Statistical analysis
Continuous variables are reported as mean and SD or median (25th–75th percentile range) as appropriate. Categorical variables are expressed as count (percent). Categorical variables were compared using the chi-square test or Fisher’s exact test as appropriate. Continuous variables were compared using Student’s t test or the Mann- Whitney U test as appropriate.
Variables associated with MAKE and AKI in univariate analysis were entered in a multivariable logistic regression model with Lasso penalization [20] to identify the factors independently associated with the outcome. Inference was obtained using the post-selection inference method for L 1-penalized models described by Taylor and Tibshirani [21]. Considering the rule of thumb suggesting at least five to ten events for each predictor variable included in the model [22], only the most clinically relevant were included in the multivariate model: creatinine at admission, SAPS II, ABSI, undetectable haptoglobin, need for catecholamine during the first 7 days, and administration of hydroxocobalamin. Model performance was estimated using the cross-validated (tenfold) AUC. Mortality during the first 90 days was described using the Kaplan-Meier estimate and modelled using a Cox proportional hazards model. Survival curves were compared using the log-rank test. In all comparisons, a p value < 0.05 was considered statistically significant. All analyses were performed using R software version 3.3.3 for Mac (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
Between January 2012 and April 2017, 1029 patients were admitted (Fig. 1). Of these, 899 did not meet the inclusion criteria. The characteristics of the 130 patients included in the study are summarised in Table 1.
Fig. 1.

Flowchart
Table 1.
Patient characteristics
| All patients (n = 130) | MAKE (n = 41) | No MAKE (n = 89) | p Value | |
|---|---|---|---|---|
| Age, years | 48.5 (34–61.8) | 53 (47–67) | 44 (29–58) | 0.02 |
| Sex, n (%) | ||||
| Male | 80 (61.5) | 24 (58.5) | 56 (62.9) | 0.7 |
| Female | 50 (38.5) | 17 (41.5) | 33 (37.1) | |
| BMI, kg/m2 | 25.1 (22.7–28.5) | 26.2 (22.8–30.6) | 24.9 (22.7–27.5) | 0.06 |
| Co-morbidities, n (%) | ||||
| Hypertension | 27 (20.8) | 13 (31.7) | 14 (15.7) | 0.06 |
| Diabetes mellitus | 16 (12.3) | 8 (19.5) | 8 (9) | 0.15 |
| Chronic kidney disease | 2 (1.5) | 1 (2.4) | 1 (1.1) | 0.53 |
| Vascular disease | 8 (6.2) | 3 (7.3) | 5 (5.6) | 0.71 |
| Obesity | 21 (16.1) | 12 (29.3) | 9 (10.1) | 0.01 |
| Smoking | 14 (10.8) | 2 (4.9) | 12 (13.5) | 0.22 |
| Alcohol consumption | 15 (11.5) | 5 (12.2) | 10 (11.2) | 1 |
| Psychiatric | 17 (13.1) | 6 (14.6) | 11 (12.4) | 0.78 |
| Burn type, n (%) | ||||
| Thermal | 123 (94.6) | 41 (100) | 82 (92.1) | 0.1 |
| Electrical | 7 (5.4) | 0 (0) | 7 (7.9) | |
| Body surface area burned (%) | ||||
| Total | 28.5 (15–50.8) | 50 (22.5–70) | 20 (12–40) | <0.0001 |
| Full thickness | 15 (4–32.2) | 25 (15–56) | 8 (1.8–25) | <0.0001 |
| ICU length of stay, days | 27.5 (10–42.5) | 18 (3–38) | 29 (13.2–45) | 0.42 |
| Death in ICU, n (%) | 33 (25.4) | 33 (80.5) | 0 (0) | <0.0001 |
| Day of death | 15 (3–32) | 15 (3–32) | – | |
| SAPS II | 33 (19–49) | 45 (35–72) | 24 (15–39) | <0.0001 |
| UBS | 67 (24–146) | 110 (65–224) | 46 (16–113) | <0.0001 |
| ABSI | 8 (6–10) | 11 (8–12) | 7 (5–9) | <0.0001 |
| Serum creatinine on admission, μmol/L | 67 (65–113) | 88 (67–118) | 64 (54–77) | <0.0001 |
| Maximal blood lactate concentration within 7 days, mmol/L | 4.3 (2.8–7) | 7 (5.2–9.4) | 3.2 (2.2–4.7) | <0.0001 |
| Minimal serum haptoglobin level, g/L | 0.6 (0.1–1.2) | 0.1 (0.1–0.5) | 0.8 (0.5–1.4) | <0.0001 |
| Undetectable haptoglobin level, n (%) | 39 (30) | 28 (68.3) | 11 (12.4) | <0.0001 |
Abbreviations: BMI Body Mass Index, ICU Intensive Care Unit, SAPS II Simplified Acute Physiology Score II, UBS Unit Burn Standard, ABSI Abbreviated Burn Severity Index
Data are expressed as median ± 25th–75th interquartile range for continuous variables and count (percent) for discrete variables
Outcomes
Forty-one patients developed MAKE, including 33 (80.5%) patients who died within 90 days. Twenty-six patients required RRT, and 25 patients had no renal recovery. Seventy-three patients developed AKI during the first 7 days, including 29 (39.7%) patients with stage 1 AKI, 17 (23.3%) with stage 2 and 27 (37%) with stage 3 AKI (Table 2). Only one patient developed AKI after 7 days. Among the 73 patients who developed AKI during the first 7 days, 32 (44%) died during their ICU stay.
Table 2.
Renal outcomes
| All patients (n = 130) | MAKE (n = 41) | No MAKE (n = 89) | p Value | |
|---|---|---|---|---|
| Kidney aggression factors within 7 days, n (%) | ||||
| Aminoglycoside | 5 (3.9) | 5 (12.2) | 0 (0) | 0.0026 |
| Hydroxocobalamin | 24 (18.5) | 14 (34.1) | 10 (11.2) | 0.0031 |
| Shock (use of catecholamine) | 58 (44.6) | 34 (82.9) | 24 (27) | <0.0001 |
| AKI within 7 days, n (%) | 73 (56.1) | 39 (95.1) | 34 (38.2) | <0.0001 |
| KDIGO stage of patients with AKI within 7 days | 2 (1–3) | 3 (2–3) | 1 (1–2) | <0.0001 |
| Serum creatinine, μmol/L | ||||
| Admission | 67 (54–90) | 88 (67–118) | 64 (54–77) | <0.0001 |
| Maximum within 7 days | 80 (65–113) | 135 (85–206) | 74 (64–86) | <0.0001 |
| ICU discharge (alive, without RRT) | 49 (35–63) | 64 (43–85) | 49 (35–61) | 0.07 |
| Oliguria within 7 days, n (%) | 63 (48.5) | 35 (85.4) | 28 (31.5) | <0.0001 |
| RRT n (%) | ||||
| Within 7 days | 18 (13.8) | 18 (44) | 0 (0) | <0.0001 |
| During ICU stay | 26 (20) | 26 (63.4) | 0 (0) | <0.001 |
| Alive at ICU discharge | 1 (0) | 1 (2.4) | – | |
Abbreviations: AKI Acute kidney injury, KDIGO Kidney Disease Improving Global Outcomes, ICU Intensive care unit, RRT Renal replacement therapy
Data are expressed as median ± 25th–75th interquartile range for continuous variables and number (percent) for discrete variables
Haptoglobin and outcomes
Undetectable plasma haptoglobin was observed in 39 patients (30%) at ICU admission. All but one had a total burned surface area > 20%. Of these 39 patients, 34 (87.2%) developed AKI and 29 (74.4%) developed MAKE. The minimal haptoglobin concentration was significantly lower in patients with MAKE than in patients without (p = 0.002) (Fig. 2). The frequency of MAKE by haptoglobin quintile is illustrated in Fig. 3.
Fig. 2.

Box plot displaying minimal haptoglobin level between major adverse kidney events (MAKE) and no MAKE
Fig. 3.
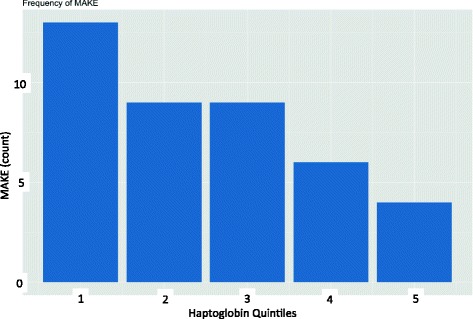
Major adverse kidney event (MAKE) count by haptoglobin quintiles
In univariate analysis, undetectable haptoglobin was strongly associated with MAKE (OR 15.27, 95% CI 6.34–39.66, p < 0.001). In multivariate analysis, undetectable haptoglobin (OR 8.32, 95% CI 2.86–26.40, p < 0.001), admission creatinine (OR 1.02, 95% CI 1.00–1.03, p = 0.05), need for catecholamine during the first 7 days (OR 4.71, 95% CI 1.54–15.49, p = 0.01) and SAPS II score (OR 1.04, 95% CI 1.01–1.07, p = 0.02) were independently associated with MAKE (Table 3) (model discrimination AUC 0.88, 95% CI 0.80–0.96).
Table 3.
Multivariable Lasso penalized logistic model: variables associated with major adverse kidney events
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Admission creatinine | 1.02/μmol/L | 1.00–1.03 | 0.03 |
| SAPS II | 1.03/1 point | 1.00–1.06 | 0.05 |
| ABSI | 1.14/1 point | 0.88–1.35 | 0.19 |
| Shock in the first 7 days | 4.18 | 1.44–10.59 | 0.02 |
| Haptoglobin undetectable | 6.33 | 2.34–16.45 | <0.001 |
SAPS II Simplified Acute Physiology Score II, ABSI Abbreviated Burn Severity Index
After adjustment, admission creatinine (OR 1.02, 95% CI 1.00–1.03, p = 0.05), SAPS II (OR 1.04, 95% CI 1.01–1.07, p = 0.02), shock within 7 days (OR 4.71, 95% CI 1.54–15.49, p = 0.01) and undetectable haptoglobin (OR 8.32, 95% CI 2.86–26.40, p < 0.001) were independently associated with the occurrence of AKI. In patients with undetectable haptoglobin, the risk of death during the first 90 days was higher (23 [59%] of 39 versus 9 [10%] of 91; p < 0.0001) (Fig. 4). This remained true after adjusting on severity (HR 5.11, 95% CI 2.21–11.78, p < 0.001).
Fig. 4.
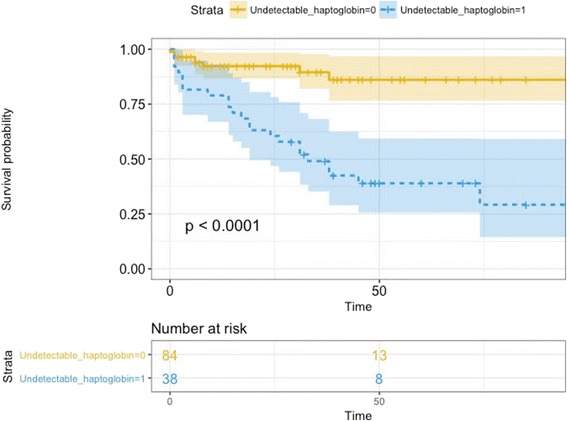
Kaplan-Meier curve between patients with detectable and undetectable haptoglobin in the 72 first h
Factors associated with undetectable haptoglobin
In multivariable analysis, TBSA was independently associated with undetectable haptoglobin (OR 1.04, 95% CI 1.00–1.07, p = 0.03), whereas ABSI, smoke inhalation and SAPS II were not (respectively, OR 1.16, 95% CI 0.89–1.5, p = 0.27; OR 1.88, 95% CI 0.72–4.95, p = 0.20; and OR 1.01, 95% CI 0.98–1.03, p = 0.54).
Discussion
We observed 30% of undetectable plasma haptoglobin in our cohort of critically ill burn patients. The main finding of this study is that undetectable haptoglobin on admission after burn injury is strongly and independently associated with the occurrence of MAKE, AKI and 90-day mortality in critically ill burn patients.
The association between haemolysis and AKI after major aortic surgery [12] and in sickle cell disease [11] has already been described. This is the first study, to our knowledge, describing the association between haptoglobin level and MAKE or AKI in a population of critically ill burn patients. In 1943, Shen et al. [8] described a 25% incidence of haemolysis in 40 patients with combined second-degree and third-degree thermal burns over 15–65% of the body area. Eleven patients developed haemolysis as evidenced by the presence of haemoglobinuria. In the literature on thermal burns, haemolysis is infrequently reported as a complication of severe third-degree burns [8]. In the present study, 38 (>97%) of 39 cases with undetectable haemolysis had > 20% TBSA. TBSA was strongly associated with undetectable haptoglobin, even after adjustment for confounding factors. Physiopathology of renal toxicity of haemolysis is probably multifactorial, including (1) the role of the fHb azote monoxide (NO) scavenger, decreasing its bioavailability and therefore inducing systemic vasoconstriction; and (2) direct toxicity of fHb aggregating into casts in the tubular lumen [14, 15].
We describe a high incidence of AKI in the first week (56%), which is close to the incidence reported by Palmieri et al., who described an incidence of 53% in a retrospective cohort of 60 severely burned patients (>20% TBSA) [3]. In their study, they did not describe the prevalence of MAKE; however, AKI was strongly associated with mortality because 34% of patients with AKI died, whereas no deaths were reported among patients without AKI. In the present study, we chose to use MAKE as the primary endpoint [17]. Of note, MAKE were driven largely by RRT and mortality in our cohort. Moreover, the prognosis of patients needing RRT was very poor, with a 90-day mortality of 78%, which is again in accord with the available literature. Yoon et al. recently reported 84% mortality in burn patients receiving RRT [23].
Recently, in a single-centre, retrospective, observational Japanese study, intra-operative administration of haptoglobin was independently associated with a lower risk of AKI after cardiovascular surgery [16], suggesting a protective role of haptoglobin in binding fHB and therefore preventing its potential toxicity on the kidney. As far as we know, the use of haptoglobin in burn patients has been described only in a case report [24] of a 24-year-old man with 100% TBSA and in a series of five patients [25]. In the case report, haptoglobin was administered three times over the first 24 h after the detection of haemoglobinuria. Despite the severity of the initial burn, the patient did not develop AKI in the five 5 days, suggesting a protective effect of intravenous haptoglobin administration [24].
Our study has some limitations. First, this was an observational study, and thus it describes an association between haemolysis, MAKE and AKI, but not necessarily a causal relationship. Second, it was a single-centre study. This may limit the generalisability of the results. However, our results rely on a strong pathophysiological background and may open a window for establishing methods to prevent MAKE and AKI in this population. Third, low haptoglobin level could arise from conditions other than haemolysis. However, the differential diagnoses appear to be very unlikely in our population. Haptoglobin was measured upon admission, before hepatic dysfunction occurs in critically ill patients. Finally, even though burn patients are frequently transfused after surgery for excision, none were transfused in the early phase (first 96 h), and none had associated trauma or haemorrhage.
Conclusions
This study shows that undetectable haptoglobin was strongly associated with the development of MAKE, AKI and 90-day mortality in a large cohort of patients with severe burns. Further multicentre studies should be performed to confirm these results. Interventional studies using recombinant haptoglobin should be considered in the future to improve outcome in patients with severe burns.
Acknowledgements
None.
Funding
None.
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- ABSI
Abbreviated Burn Severity Index
- AKI
Acute kidney injury
- BMI
Body mass index
- fHb
Cell-free haemoglobin
- ICU
Intensive care unit
- KDIGO
Kidney Disease: Improving Global Outcomes
- MAKE
Major adverse kidney event
- RRT
Renal replacement therapy
- SAPS II
Simplified Acute Physiology Score II
- Screat
Serum creatinine
- TBSA
Total body surface area
- UBS
Unit Burn Standard
Authors’ contributions
FD collected data, performed analysis and interpretation of the data, and drafted the manuscript. CD collected data and contributed to interpretation of the data as well as to drafting the manuscript. CDT collected data and contributed to interpretation of the data as well as to drafting the manuscript. MChau collected data and contributed to interpretation of the data as well as to drafting the manuscript. AB contributed to interpretation of the data and to drafting the manuscript. AF collected data and contributed to interpretation of the data as well as to drafting the manuscript. NM performed haptoglobin dosage and contributed to interpretation of the data as well as to drafting the manuscript. AC collected data and contributed to interpretation of the data as well as to drafting the manuscript. SS collected data and contributed to interpretation of the data as well as to drafting the manuscript. MB collected data and contributed to interpretation of the data as well as to drafting the manuscript. AM conceived of the study and contributed to interpretation of the data as well as to drafting the manuscript. KS collected data and contributed to interpretation of the data as well as to drafting the manuscript. MChao collected data and contributed to interpretation of the data as well as to drafting the manuscript. JPG performed haptoglobin dosage and contributed to interpretation of the data as well as to drafting the manuscript. RP performed the statistical analysis and supervised the interpretation of the data as well as the drafting of the manuscript. ML conceived of the study, performed interpretation of the data and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by our local ethics committee (PRONOBURN study, comité de protection des personnes IV, St-Louis Hospital; Institutional Review Board 00003835, protocol 2013/17NICB). Informed consent was waived by the ethics committee for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
François Dépret, Email: depret.francois@gmail.com.
Chloé Dunyach, Email: chloe.dunyach@gmail.com.
Christian De Tymowski, Email: chrisdetymowski@gmail.com.
Maïté Chaussard, Email: maite.chaussard@gmail.com.
Aurélien Bataille, Email: aurelbataille@gmail.com.
Axelle Ferry, Email: axelleferry@gmail.com.
Nabila Moreno, Email: nabila.moreno@aphp.fr.
Alexandru Cupaciu, Email: alexandru_cupaciu@yahoo.com.
Sabri Soussi, Email: sabri.soussi@gmail.com.
Mourad Benyamina, Email: mourad.benyamina@aphp.fr.
Alexandre Mebazaa, Email: alexandre.mebazaa@aphp.fr.
Kevin Serror, Email: kserror@gmail.com.
Marc Chaouat, Email: marc.chaouat@gmail.com.
Jean-Pierre Garnier, Email: jean-pierre.garnier@aphp.fr.
Romain Pirracchio, Email: romainpirracchio@yahoo.fr.
Matthieu Legrand, Phone: +33 (0)1 42 49 43 48, Email: matthieu.legrand@aphp.fr.
References
- 1.Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: an update and primer for the intensivist. Crit Care Med. 2010;38:261–75. doi: 10.1097/CCM.0b013e3181bfb0b5. [DOI] [PubMed] [Google Scholar]
- 2.Clec’h C, Gonzalez F, Lautrette A, Nguile-Makao M, Garrouste-Orgeas M, Jamali S, et al. Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: a competing risks analysis. Crit Care. 2011;15:R128. doi: 10.1186/cc10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmieri T, Lavrentieva A, Greenhalgh DG. Acute kidney injury in critically ill burn patients: risk factors, progression and impact on mortality. Burns. 2010;36:205–11. doi: 10.1016/j.burns.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Emara SS, Alzaylai AA. Renal failure in burn patients: a review. Ann Burns Fire Disasters. 2013;26:12–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Clark A, Neyra JA, Madni T, Imran J, Phelan H, Arnoldo B, et al. Acute kidney injury after burn. Burns. 2017;43:898–908. doi: 10.1016/j.burns.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Mason SA, Nathens AB, Finnerty CC, Gamelli RL, Gibran NS, Arnoldo BD, et al. Hold the pendulum: rates of acute kidney injury are increased in patients who receive resuscitation volumes less than predicted by the Parkland equation. Ann Surg. 2016;264:1142–7. doi: 10.1097/SLA.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G, Xiao Y, Wang C, Hong X, Sun Y, Ma B, et al. Risk factors for acute kidney injury in patients with burn injury: a meta-analysis and systematic review. J Burn Care Res. 2017;38:271–82. doi: 10.1097/BCR.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 8.Shen SC, Ham TH, Fleming EM. Studies on the destruction of red blood cells: mechanism and complications of hemoglobinuria in patients with thermal burns: spherocytosis and increased osmotic fragility of red blood cells. N Engl J Med. 1943;229:701–13. doi: 10.1056/NEJM194311042291901. [DOI] [Google Scholar]
- 9.Siah S, Elmaataoui A, Messaoudi N, Ihrai I, Kamili ND. The mechanism of non-immune haemolytic anaemia in burns patient [in French] Ann Biol Clin (Paris) 2010;68:603–7. doi: 10.1684/abc.2010.0473. [DOI] [PubMed] [Google Scholar]
- 10.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 11.Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol. 2015;11:161–71. doi: 10.1038/nrneph.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windsant ICV, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913–20. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 13.Mamikonian LS, Mamo LB, Smith PB, Koo J, Lodge AJ, Turi JL. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr Crit Care Med. 2014;15:e111–9. doi: 10.1097/PCC.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg. 2011;142:1–11. doi: 10.1016/j.jtcvs.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Kubota K, Egi M, Mizobuchi S. Haptoglobin administration in cardiovascular surgery patients: its association with the risk of postoperative acute kidney injury. Anesth Analg. 2017;124:1771–6. doi: 10.1213/ANE.0000000000002093. [DOI] [PubMed] [Google Scholar]
- 17.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 18.Tobiasen J, Hiebert JM, Edlich RF. The Abbreviated Burn Severity Index. Ann Emerg Med. 1982;11:260–2. doi: 10.1016/S0196-0644(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 19.Bull JP, Squire JR. A study of mortality in a burns unit: standards for the evaluation of alternative methods of treatment. Ann Surg. 1949;130:160–73. doi: 10.1097/00000658-194908000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective: regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol. 2011;73:273–82. doi: 10.1111/j.1467-9868.2011.00771.x. [DOI] [Google Scholar]
- 21.Taylor J, Tibshirani R. Post-selection inference for ℓ1-penalized likelihood models. Can J Stat. doi:10.1002/cjs.11313. [DOI] [PMC free article] [PubMed]
- 22.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 23.Yoon J, Kim Y, Yim H, Cho YS, Kym D, Hur J, et al. Analysis of prognostic factors for acute kidney injury with continuous renal replacement therapy in severely burned patients. Burns. 2017. doi:10.1016/j.burns.2017.03.015. [DOI] [PubMed]
- 24.Imaizumi H, Tsunoda K, Ichimiya N, Okamoto T, Namiki A. Repeated large-dose haptoglobin therapy in an extensively burned patient: case report. J Emerg Med. 1994;12:33–7. doi: 10.1016/0736-4679(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 25.Yoshioka T, Sugimoto T, Ukai T, Oshiro T. Haptoglobin therapy for possible prevention of renal failure following thermal injury: a clinical study. J Trauma. 1985;25:281–7. doi: 10.1097/00005373-198504000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.


