Abstract
Tumor necrosis factor-α (TNF), which is an immuno-modulatory cytokine, has been suggested to cause inflammatory responses as well as protection against tissue dysfunction by binding two types of TNF receptor (TNFR1/TNFR2). However, the physiological effects of TNFR2-specific activation remain unclear. We therefore aimed to generate a TNF mutant with full TNFR2-selective agonist activity as a functional analytical tool. In this study, we utilized a phage display technique to create mouse TNFR2 (mTNFR2)-selective TNF mutants that bind specifically to mTNFR2 and show full bioactivity compared with wild-type TNF. A new phage library displaying TNF mutants was created, in which nine amino acid residues at the predicted receptor-binding site were randomized. From this library, an agonistic TNF mutant exhibiting high binding selectivity and bioactivity to mTNFR2 was isolated. We propose that this TNF mutant would be a powerful tool with which to elucidate the functional roles of mTNFR2.
Keywords: Tumor necrosis factor-α, TNF, TNFR2, Phage display technique, Cytokine, Mutant
Highlights
-
•
We generated a TNF mutant with full TNFR2-selective agonist activity.
-
•
This mutant was identified using a phage display technique.
-
•
This agonist exhibited high binding selectivity and bioactivity to mouse TNFR2.
-
•
This would be a powerful tool to elucidate the functional roles of mouse TNFR2.
1. Introduction
TNF, a major inflammatory cytokine, interacts with two receptors: TNF receptor type I (TNFR1, also known as TNFRSF1A or P55) and TNF receptor type II (TNFR2, also known as TNFRSF1B or P75), resulting in stimulation of host immune defense and inflammation [1], [2]. These receptors are expressed on different cell types and transmit cellular signals by their respective distinct pathways. Unlike TNFR1, which is pan-expressed in most tissues, TNFR2 is expressed in a more limited manner, restricted primarily to cells of the immune system such as CD4+ or CD8+ T cells and several other cell types such as oligodendrocytes and endothelial cells [3], [4], [5]. TNFR1 appears to be the mediator of well-known TNF signaling effects such as cytotoxicity [6], [7] and cytokine secretion [8], whereas in the lymphoid system TNFR2 seems to play a major role in the activation of T cells [7], [9]. Recent reports suggest that TNFR2 signaling also mediates myocardial protection [10], [11], neuroprotection [12], [13] and the activity of regulatory T cells (Tregs) [14], [15]. Although the two TNFRs have been shown to have distinct functions in some cells, some researchers have suggested that TNFR2 induces intensive crosstalk with TNFR1 by sharing some adaptor molecules such as TRAF2 [16]. TNFR2 has another shared characteristic with TNFR1, as they both utilize analogous “ligand passing” mechanisms [17]. Analyses of single receptor knockout (KO) mice and KO cells necessarily failed to reveal molecular crosstalk between TNFR1 and TNFR2. Interpretations of the physiological significance of TNFR2 signaling in the presence of both receptors, therefore, require further investigation.
In order to understand TNFR2 signaling, several attempts were made in the past to create TNFR2-selective TNF mutants. Some researchers created TNF mutants by conventional site-directed mutagenesis methods (such as Kunkel's method). These attempts were not very successful in yielding the desired TNF mutant(s) having high receptor specificity and full bioactivity. For example, the human TNFR2 (hTNFR2) binding affinity of the double mutant D143N-A145R was ~5–10 fold less than that of wild-type (wt) TNF [18], [19], [20]. To overcome these problems, we previously applied a phage display technique and optimized a competitive panning method, producing a hTNFR2-selective TNF mutant (R2-7) having high receptor specificity and full bioactivity [21]. However, there are species differences between hTNFR2 and mouse TNFR2 (mTNFR2), and R2-7 did not bind to mTNFR2. Thus, mTNFR2-selective TNF mutants are needed to study its efficacy in an in vivo mouse model. Regarding the mouse TNF mutant (D142N-A144R), analogous to the human TNF mutant (D143N-A145R), only cell culture supernatants were evaluated from these gene-encoded adenovirus vector-transduced cells [22]. Neither receptor specificity nor bioactivity have been evaluated using the relevant recombinant protein. In this study, we created potent mTNFR2-selective agonistic TNF mutants using a phage display technique.
2. Materials and methods
2.1. Cell culture
L-M cells (a mouse fibroblast cell line) were provided by Mochida Pharmaceutical Co. Ltd. (Tokyo, Japan) and were cultured in minimum Eagle's medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 1% (v/v) fetal bovine serum (FBS) and a 1% (v/v) antibiotic mixture (penicillin 10,000 units/ml, streptomycin 10 mg/ml, and amphotericin B 25 μg/ml) (Nacalai Tesque, Kyoto, Japan). According to a previous report, mTNFR2/mFas-preadipocytes (PA) derived from TNFR1−/−R2−/− mice expressing a chimeric receptor consisting of the extracellular and transmembrane domains of mTNFR2, and the intracellular domain of mouse Fas, were produced [23], [24]. These cells were cultured in RPMI 1640 (Sigma-Aldrich Japan, Tokyo, Japan) supplemented with 10% (v/v) FBS, 5 µg/ml Blasticidin S HCl (Invitrogen, Carlsbad, CA), and a 1% (v/v) antibiotics mixture.
2.2. Construction of a novel phage library displaying TNF mutants
The pCANTAB 5E phagemid vector (GE Healthcare Ltd., UK) encoding mutTNF-Lys (-) was used as a template for PCR [25]. This TNF mutant was previously reported to be a fully active, lysine-deficient human TNF mutant. PCR amplification was performed using primers containing the sequence NNS (which encodes all 20 standard amino acids) at the predicted receptor-binding site (9 amino acid residues; 29, 31–33, 143, 145–147 and 149). The PCR products were digested with NcoI and NotI and then ligated into pCANTAB 5E. The resultant phagemid was electroporated into Escherichia coli (E. coli) strain TG1 (Lucigen, Middleton, WI), which allows read-through of the amber stop codon. The phage library displaying TNF mutants on the phage surface, as g3p-fusion proteins, was prepared as described previously [25].
2.3. Panning of the phage display library
The mTNFR2 Fc chimera (R&D Systems, Minneapolis, MN) was immobilized onto a maxisorp immunotube (Thermo Fisher Scientific Inc., Waltham, MA) at 2 µg/ml diluted in a 50 mM carbonate bicarbonate buffer. The phage display library (>1×1012 colony forming units) was reacted with immobilized antigen for 1 h at RT in the presence of the free form of the mTNFR1 Fc chimera at 2 µg/ml (R&D Systems). After binding, the unbound phage was washed away using phosphate buffered saline (PBS) and PBS containing 0.1% (v/v) Tween 20. Bound phage was eluted with 100 mM triethylamine for 5 min at RT. Eluted phage was neutralized with 1 M Tris-HCl (pH 8.0) and then used to infect E. coli TG1 in order to amplify the phage. Infected E. coli were incubated on LB agar/ampicillin plates overnight at 30 °C. Bacterial colonies were resuspended and cultured in 2YT containing 100 µg/ml ampicillin and 2% (w/v) glucose until the OD600 of the culture medium reached 0.4. Then, helper phage (1.8×1011 colony forming units) were added to the culture media for 30 min at 37 °C. The cells were collected by centrifugation and resuspended in fresh medium containing 100 µg/ml ampicillin and 50 µg/ml kanamycin. After overnight growth at 30 °C, cells were removed by centrifugation and 4% (w/v) PEG8000 and 0.5 M NaCl were added to the supernatant. Phage particles were collected by centrifugation (11,600g, 2 min) and resuspended in PBS containing 2% (v/v) glycerol. These panning steps were repeated three times.
2.4. Screening of TNFR2-selective agonist candidates from the phage display library
After the final round of panning, the phage mixture was used to infect E. coli TG1 and plated on LB agar/ampicillin plates. Single clones were picked randomly from the plate and each colony was grown in 2YT containing 100 µg/ml ampicillin and 2% (w/v) glucose for 3 h at 37 °C. After incubation, for induction, 1 mM isopropyl-beta-d-thiogalactopyranoside (IPTG) was added to each medium and incubated for 24 h at 30 °C. Supernatants containing each TNF mutant from each clone were collected and used to determine their affinities for mTNFR2 and mTNFR1 by capture ELISA as described previously [21], and their cytotoxicity mediated via mTNFR2 and mTNFR1 by mTNFR2/mFas-PA and L-M cells cytotoxic assay, respectively. We screened clones having significant affinity and cytotoxicity for mTNFR2 but not for mTNFR1. After this procedure, the phagemid vectors were sequenced using a Big Dye Terminator v3.1 kit and ABI PRISM 3100 (Applied Biosystems Ltd., Pleasanton, CA).
2.5. Expression and purification of recombinant TNF mutants
The protocol for expression and purification of the recombinant proteins was the same as that described previously [25], [26], [27]. Briefly, pCANTAB 5E vectors encoding each TNF mutant were sub-cloned into the expression vector pYas. TNF mutants were produced in E. coli (BL21λDE3). The inclusion body of each TNF mutant was washed in 2.5% (v/v) triton X-100 and solubilized in 6 M guanidine-HCl, 0.1 M Tris-HCl (pH 8.0), and 2 mM ethylenediaminetetraacetic acid (EDTA). Solubilized protein at 10 mg/ml was reduced with 10 mg/ml dithiothreitol for 4 h at RT and refolded by 100-fold dilution in a refolding buffer (100 mM Tris–HCl, 2 mM EDTA, 0.5 M arginine, and 551 mg/L oxidized glutathione, pH 9.5). After dialysis against 20 mM Tris–HCl (pH 7.4) containing 100 mM urea, active trimeric proteins were purified by ion-exchange chromatography and gel-filtration chromatography.
2.6. Cytotoxicity assays
The mTNFR2/mFas-PA were seeded in 96-well flat bottom plates (Thermo Fisher Scientific Inc.) at a density of 1.5×104 cells/well. Serial dilutions of mouse wtTNF and TNF mutants were prepared with 1 µg/ml cycloheximide and added to each well. After 48 h incubation, the cell viabilities were analyzed using a WST-8 assay kit (Nacalai Tesque). L-M cells were cultured in 96-well flat bottom plates in the presence of serially diluted mouse wtTNF and TNF mutants, and for 24 h at 3.0×104 cells/well. Cytotoxicity was then assessed using a methylene blue assay as described previously [26]. Experimental data were analyzed using a logistic regression model to calculate the mean effective concentrations (EC50) with GraphPad Prism version 5 (GraphPad Software, San Diego, CA).
2.7. Surface plasmon resonance (SPR) assay (BiacoreⓇ assay)
The binding kinetics of the mouse wtTNF and TNF mutants were analyzed by SPR (BIAcore T200; GE Healthcare, UK). mTNFR1-Fc or mTNFR2-Fc were immobilized separately on a CM5 sensor chip, resulting in an increase of 3000–3500 RU. During the association phase, wtTNF or TNF mutants diluted in running buffer (HBS-EP) at 100, 50, 25, 12.5 or 6.25 nM were passed over the immobilized ligands for 2 min at a flow rate of 20 µl/min. During the dissociation phase, HBS-EP was run over the sensor chip for 1 min at a flow rate of 20 µl/min. These measurements were performed by single-cycle kinetics. The data were analyzed by global-fitting using the BIAcore T200 Evaluation Software (GE Healthcare) applying a 1:1 Langmuir binding model. The obtained sensorgrams were fitted globally over the range of injected concentrations and simultaneously over the association and dissociation phases.
3. Results and discussion
3.1. Construction of a novel phage library displaying TNF mutants
We designed a phage display library that expressed TNF mutants having 9 amino acid residue replacements (aa 29, 31–33, 143, 145–147, 149) in the predicted receptor-binding site of TNF, as described previously [26], [28]. Although the structures of human TNF and mouse TNF are very similar [29], we selected a human TNF mutant named mutTNF-Lys (-) as a template, which dramatically improved the in vivo stability due to effects of a novel PEGylation technique [25]. To construct the phage library, PCR was performed to replace these amino acids randomly with primers containing NNS sequences. As a result, we confirmed that the phage display library consisted of 5.0×108 independent recombinant clones. Amino acid analysis of eight clones picked randomly from this library revealed that each one was a mutant containing amino acid substitutions (Table 1).
Table 1.
Amino acid sequences of eight clones picked randomly from a phage library displaying TNF mutants.
| Receptor binding domain |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 29 | 31 | 32 | 33 | 143 | 145 | 146 | 147 | 149 | |
| mutTNF-Lys (-) | L (CTG) | R (CGC) | R (CGG) | A (GCC) | D (GAC) | A (GCC) | E (GAG) | S (TCT) | Q (CAG) |
| # 1 | A (GCC) | F (TTC) | G (GGG) | W (TGG) | T (ACC) | V (GTC) | N (AAC) | S (AGC) | N (CTC) |
| # 2 | Y (TAC) | G (GGG) | G (GGG) | R (AGG) | T (ACG) | P (CCC) | N (AAC) | D (GAC) | L (CTC) |
| # 3 | E (GAG) | P (CCC) | S (TCG) | C (TGC) | S (CAC) | S (AGC) | T (ACC) | T (ACC) | P (CCC) |
| # 4 | R (AGG) | P (CCG) | G (GGG) | W (TGG) | H (CAC) | Y (TAC) | P (CCG) | P (CCG) | A (GCG) |
| # 5 | P (CCC) | M (ATG) | V (GTG) | R (AGG) | N (AAC) | T (ACC) | Q(CAG) | N (AAC) | P (CCC) |
| # 6 | R (AGG) | E (GAG) | W (TGG) | C (TGC) | F (TTC) | K (AAG) | Y (TAC) | G (GGG) | R (AGG) |
| # 7 | W (TGG) | C (TGC) | R (AGG) | A (GCG) | H (CAC) | T (ACC) | A (GCC) | H (CAC) | D (GAC) |
| # 8 | S (TCG) | Q (CAG) | R (CGG) | G (GGG) | P (CCG) | N (AAC) | T (ACG) | P (CCC) | G (GGG) |
The corresponding codons are shown in parentheses.
3.2. Selection of mTNFR2-selective agonist candidates from the phage display library
To enrich for the mTNFR2-binding TNF mutants, three rounds of panning were performed against mTNFR2 in competition with mTNFR1-Fc as described in Section 2. The ratios of selected TNF mutants to applied TNF mutants increased at each round of panning (data not shown), suggesting that potent binders to mTNFR2 were concentrated from the library. We then screened for clones that bound specifically to mTNFR2 but not to mTNFR1. Supernatants of 26 single clones randomly selected from E. coli were collected and subjected to screening by capture ELISA. As shown in Fig. 1, most of the selected clones bound to mTNFR2 (Fig. 1(A)). On the other hand, almost none of the clones bound to mTNFR1 (Fig. 1(B)), suggesting a remarkable enrichment of TNFR2-selective clones through each round of panning. We eventually identified 13 clones that strongly and selectively bound to TNFR2, compared to binding of mouse wtTNF (Fig. 1(A) and (B) black bar). Thereafter, to evaluate the agonistic potency against mTNFR2 and mTNFR1, two bioassays were performed using mTNFR2/mFas-PA and L-M cells, which can evaluate mTNFR2 and mTNFR1-mediated cytotoxic effects, respectively. Six clones (clones 3, 7, 8, 9, 10 and 16) showed mTNFR2 cytotoxic effects compared with mouse wtTNF (Fig. 1(C)). However, only clone 3 showed minimal mTNFR1 cytotoxic effects (Fig. 1(D)). Consequently, we identified five candidates (clones 7, 8, 9, 10 and 16) as TNFR2-selective TNF mutants having promising agonistic activity. Amino acid analysis of these five clones revealed that each one had a different protein sequence, and no tendency of amino acids was observed in the mutated region (Table 2).
Fig. 1.
Determination of relative affinities and bioactivities of TNF mutants of mouse TNFRs by ELISA and cytotoxic assays. ELISA assays with an E. coli supernatant containing a TNF mutant detected against (A) mouse TNFR2 and (B) mouse TNFR1. In (A) and (B): gray bar, mutTNF-Lys (-) (shown as Lys (-)); hatched bar, mouse wtTNF (mTNF) as a positive control; white bar, TNF mutants; black bar, TNFR2-selective binding clones. Affinities of TNFR2-selective clones for TNFR2 were more than 100% of the mouse wtTNF value, and the affinity for TNFR1 was less than 100% that of wtTNF value. In the cytotoxic assay, selected TNFR2-selective agonistic clones from TNFR2-selective binding clones were assessed to determine their bioactivities. (C) TNFR2-mediated bioactivity was measured using mTNFR2/mFas-PA. (D) TNFR1-mediated bioactivity was measured using L-M cells. In (C) and (D): gray bar, mutTNF-Lys (-) (shown as Lys (-)); hatched bar, mouse wtTNF; white bar, TNF mutants; black bar, TNFR2-selective agonist candidates. Each column represents the mean±SEM (n=3).
Table 2.
Amino acid sequences of potential TNFR2-selective TNF mutants.
| Receptor binding domain |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 29 | 31 | 32 | 33 | 142 (143) | 144 (145) | 145 (146) | 146 (147) | 148 (149) | |
| mouse TNF | L | Q | R | A | D | A | E | S | Q |
| mutTNF-Lys (-) | L | R | R | A | D | A | E | S | Q |
| Clone 7 | T | H | G | G | N | P | D | S | E |
| Clone 8 | T | I | E | T | T | D | D | R | E |
| Clone 9 | S | R | G | G | N | N | H | P | Q |
| Clone 10 | A | L | G | P | S | T | E | T | N |
| Clone 16 | G | I | G | A | T | S | N | A | E |
Amino acid positions of mutTNF-Lys (-) and each clone in the homologous region are shown in parentheses, because human TNF has one more amino acid than mouse TNF.
3.3. Bioactivity of TNFR2-selective recombinant TNF mutants
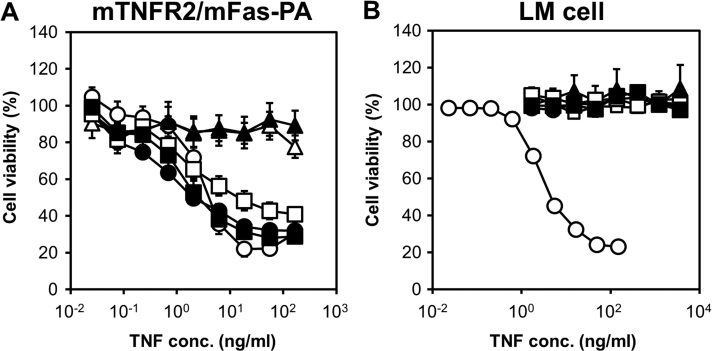
To examine the bioactivities of these five candidates in detail, recombinant proteins were prepared using a general protein expression system as described previously [25], [26], [27]. TNF mutants expressed as an inclusion body in E. coli (BL21λDE3) were denatured and refolded. Next, active TNF mutants were purified by ion-exchange and gel-filtration chromatography. TNF mutant purity was greater than 90%, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and all mutants were confirmed to form homotrimers in the same manner as wtTNF by gel-filtration analysis (data not shown). Previously, we created a TNFR2-selective TNF mutant (R2-7) that bound to only human TNFR2 [21]. However, the in vivo function of TNFR2 must be examined using animals, e.g. in mouse model systems. Therefore, mTNFR2-selective TNF mutants were generated. To discover these mutant TNFs, TNFR2-mediated cytotoxic assays were carried out using the mTNFR2/mFas-PA. Among the candidates, clones 7, 10 and 16 showed dose-dependent cytotoxicity, with EC50 values of 0.48, 1.54 and 1.14 ng/ml, respectively, while the other clones showed almost no cytotoxicity (Fig. 2(A)). These results demonstrated that clones 7, 10 and 16 had similar or higher bioactivities compared to that of mouse wtTNF, because the EC50 value of wtTNF against mTNFR2/mFas-PA cytotoxicity is 3.01 ng/ml. Moreover, the TNFR1-mediated cytotoxic assay was performed using L-M cells. None of the TNF mutants showed cytotoxicity, and their bioactivities were significantly lower than that of mouse wtTNF (Fig. 2(B)). These observations suggested that clones 7, 10 and 16 had mTNFR2-selective agonistic activity.
Fig. 2.
In vitro bioactivity assay of TNF mutants via mTNFR2 or TNFR1. The receptor-specific bioactivity (% viability) was measured following treatment of mTNFR2/mFas-PA or L-M cells with mouse wtTNF or TNF mutants followed by a cytotoxicity assay, as described in Section 2. (A) TNFR2-mediated bioactivity was measured using mTNFR2/mFas-PA. (B) TNFR1-mediated bioactivity was measured using L-M cells. In (A) and (B): open circles, mouse wtTNF; closed circles, clone 7; open triangles, clone 8; closed triangles, clone 9; open squares, clone 10; and closed squares, clone 16. Each point represents the mean±SEM (n=5).
3.4. Binding kinetics of TNFR2-selective TNF mutants
To investigate the binding properties of these TNF mutants in detail, their binding dissociation constants (kinetic on- and off-rates) for mTNFR2 and mTNFR1, respectively, were determined using SPR (Fig. 3(A) and (B)). As shown in Table 3, three clones (7, 10 and 16) bound strongly to mTNFR2 with equilibrium dissociation constants (KD) of 7.61, 20.5 and 18.1 nM, respectively; in contrast, they bound to mTNFR1 with greatly reduced affinity. Thus, clone 7 was identified as the most highly mTNFR2-selective mutant having full bioactivity. The EC50 values of TNF mutants against mTNFR2/mFas-PA cytotoxicity (0.48, 1.14 and 1.54 ng/ml) were correlated with the KD rates against mTNFR2 (7.61×10−9, 18.1×10−9 and 20.5×10−9 M), respectively (Table 3), but this correlation was not observed for mouse wtTNF (EC50:3.01 ng/ml, KD:10.4×10−9 M). Interestingly, the SPR sensorgram indicated that the association and dissociation of wtTNF to mTNFR2 was slow, whereas that of each mutant was quick (Fig. 3(A)). Furthermore, the maximum binding response (Rmax) of each mutant was lower than that of wtTNF. Therefore, specifically focusing on wtTNF and clone 7, we considered that both proteins show almost the same affinity and bioactivity towards mTNFR2, but their modes of binding to mTNFR2 are different.
Fig. 3.
SPR assay of TNF mutants for mTNFR1 and mTNFR2. The receptor-specific affinity of mouse TNF, human TNF, mutTNF-Lys (-) and each TNF mutant was measured using BIAcore T200 for mTNFR1 (A) and mTNFR2 (B), respectively. Each protein (100, 50, 25, 12.5 or 6.25 nM) was sequentially injected by the single-cycle kinetics method.
Table 3.
Binding kinetics of TNF mutants to mTNFR1 and mTNFR2.
|
mTNFR1 |
mTNFR2 |
||||||
|---|---|---|---|---|---|---|---|
| kona (×105 M−1 s−1) | koffb (×10−4 s−1) | Kdc (×10−9 M) | kona (×105 M−1 s−1) | koffb (×10−4 s−1) | Kdc (×10−9 M) | ||
| mouse TNF | 1.66 | 9.69 | 5.84 | 5.37 | 56.0 | 10.4 | |
| mutTNF-Lys (-) | 4.25 | 19.9 | 4.68 | N.D. | |||
| Clone 7 | N.D. | 33.8 | 257 | 7.61 | |||
| Clone 8 | N.D. | 538 | 37,400 | 69.4 | |||
| Clone 9 | N.D. | N.D. | |||||
| Clone 10 | N.D. | 8.43 | 173 | 20.5 | |||
| Clone 16 | N.D. | 9.71 | 176 | 18.1 |
Kinetic parameters for TNFs and TNF mutants were calculated from the respective sensorgrams using the BIAcore T200 Evaluation Software, and taking into consideration that TNF binds as a trimer.
kon is the association kinetic constant.
koff is the dissociation kinetic constant.
KD is the equilibrium dissociation constant (KD= koff/kon).
Our previous report showed that hTNFR2-selective TNF mutants with amino acid substitutions at positions 29, 31 and 32 indeed had identical properties to those of human wtTNF, in which these three amino acids play critical roles in maintaining the binding of wtTNF to hTNFR2 [21]. On the other hand, mTNFR2-selective TNF mutants with amino acid substitutions at the same positions were randomized, and position 32 was consistently a Glycine residue. Following this line of reasoning, a species difference has apparently existed between hTNFR2 and mTNFR2, in which hTNFR2 selective TNF mutants could not bind to mTNFR2. However, the reason why this replacement would increase the selectivity for mTNFR2 is unclear based on results of the present study. We are currently attempting to determine the three-dimensional structure of the mouse TNF-TNFR2 complex by X-ray crystallography, in order to carry out structure-activity relationship studies in the near future.
We have analyzed human TNFR2 and it signaling. By constructing a TNF mutant with human TNFR2-selective agonistic ability, we previously elucidated the three-dimensional structure of the TNF–TNFR2 complex, and identified a new member of the TNFR2 signaling family [30], [31]. In terms of mouse TNFR2, it is difficult to confirm its function using mouse or a mouse-derived cell line, because human TNF cannot selectively stimulate mouse TNFR2. To overcome this species specificity, we generated a TNF mutant that has mTNFR2-selective agonist ability. The mutant can fully activate TNFR2 signaling independently in vitro, and also can be expected to show a synergistic effect in vivo by TNFR2 clustering with the antibody [32]. Furthermore, researchers have developed designer TNF mutants to enhance TNF signaling in a previous study. Such mutants enhance TNF signaling by increasing ligand stability and clustering among ligand–receptor complexes, for example, by increasing internal or external crosslinking of monomeric TNF and oligomerization of trimeric TNF [33], [34], [35]. This approach will be a very effective method of modifying our TNFR2-selective mutant to enhance TNFR2 signaling. This mutant has the potential to be a great analytical tool and a promising drug seed.
Acknowledgements
This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 16K18918. This study also supported in part by the Japan Agency for Medical Research and Development (AMED) and Hoansya Foundation.
Footnotes
Transparency Document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.06.008.
Appendix A. Transparency Document
Supplementary material
.
References
- 1.Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 2.Szlosarek P.W., Balkwill F.R. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4:565–573. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 3.Chan F.K., Lenardo M.J. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur. J. Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Maier O., Fischer R., Agresti C., Pfizenmaier K. TNF receptor 2 protects oligodendrocyte progenitor cells against oxidative stress. Biochem. Biophys. Res. Commun. 2013;440:336–341. doi: 10.1016/j.bbrc.2013.09.083. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesh D., Ernandez T., Rosetti F., Batal I., Cullere X., Luscinskas F.W., Zhang Y., Stavrakis G., Garcia-Cardena G., Horwitz B.H., Mayadas T.N. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-beta autocrine signaling to promote monocyte recruitment. Immunity. 2013;38:1025–1037. doi: 10.1016/j.immuni.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiers W. Tumor necrosis factor-α. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- 7.Sarraf C. Tumor-necrosis-factor and cell-death in tumors (review) Int. J. Oncol. 1994;5:1333–1339. doi: 10.3892/ijo.5.6.1333. [DOI] [PubMed] [Google Scholar]
- 8.Benigni F., Faggioni R., Sironi M., Fantuzzi G., Vandenabeele P., Takahashi N., Sacco S., Fiers W., Buurman W.A., Ghezzi P. TNF receptor p55 plays a major role in centrally mediated increases of serum IL-6 and corticosterone after intracerebroventricular injection of TNF. J. Immunol. 1996;157:5563–5568. [PubMed] [Google Scholar]
- 9.Vandenabeele P., Declercq W., Vercammen D., Van de Craen M., Grooten J., Loetscher H., Brockhaus M., Lesslauer W., Fiers W. Functional characterization of the human tumor necrosis factor receptor p75 in a transfected rat/mouse T cell hybridoma. J. Exp. Med. 1992;176:1015–1024. doi: 10.1084/jem.176.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monden Y., Kubota T., Inoue T., Tsutsumi T., Kawano S., Ide T., Tsutsui H., Sunagawa K. Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H743–H753. doi: 10.1152/ajpheart.00166.2007. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Crisostomo P.R., Markel T.A., Wang Y., Meldrum D.R. Mechanisms of sex differences in TNFR2-mediated cardioprotection. Circulation. 2008;118:S38–S45. doi: 10.1161/CIRCULATIONAHA.107.756890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett H.A., Mason J., Marino M., Suzuki K., Matsushima G.K., Ting J.P. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine V., Mohand-Said S., Hanoteau N., Fuchs C., Pfizenmaier K., Eisel U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J. Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-07-j0001.2002. RC216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X., Baumel M., Mannel D.N., Howard O.M., Oppenheim J.J. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J. Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Subleski J.J., Hamano R., Howard O.M., Wiltrout R.H., Oppenheim J.J. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur. J. Immunol. 2010;40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naude P.J., den Boer J.A., Luiten P.G., Eisel U.L. Tumor necrosis factor-α receptor cross-talk. FEBS J. 2011;278:888–898. doi: 10.1111/j.1742-4658.2011.08017.x. [DOI] [PubMed] [Google Scholar]
- 17.Tartaglia L.A., Pennica D., Goeddel D.V. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J. Biol. Chem. 1993;268:18542–18548. [PubMed] [Google Scholar]
- 18.Yamagishi J., Kawashima H., Matsuo N., Ohue M., Yamayoshi M., Fukui T., Kotani H., Furuta R., Nakano K., Yamada M. Mutational analysis of structure–activity relationships in human tumor necrosis factor-alpha. Protein Eng. 1990;3:713–719. doi: 10.1093/protein/3.8.713. [DOI] [PubMed] [Google Scholar]
- 19.Barbara J.A., Smith W.B., Gamble J.R., Van Ostade X., Vandenabeele P., Tavernier J., Fiers W., Vadas M.A., Lopez A.F. Dissociation of TNF-alpha cytotoxic and proinflammatory activities by p55 receptor- and p75 receptor-selective TNF-alpha mutants. EMBO J. 1994;13:843–850. doi: 10.1002/j.1460-2075.1994.tb06327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Ostade X., Vandenabeele P., Everaerdt B., Loetscher H., Gentz R., Brockhaus M., Lesslauer W., Tavernier J., Brouckaert P., Fiers W. Human TNF mutants with selective activity on the p55 receptor. Nature. 1993;361:266–269. doi: 10.1038/361266a0. [DOI] [PubMed] [Google Scholar]
- 21.Abe Y., Yoshikawa T., Inoue M., Nomura T., Furuya T., Yamashita T., Nagano K., Nabeshi H., Yoshioka Y., Mukai Y., Nakagawa S., Kamada H., Tsutsumi Y., Tsunoda S. Fine tuning of receptor-selectivity for tumor necrosis factor-alpha using a phage display system with one-step competitive panning. Biomaterials. 2011;32:5498–5504. doi: 10.1016/j.biomaterials.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Marr R.A., Hitt M., Gauldie J., Muller W.J., Graham F.L. A p75 tumor necrosis factor receptor-specific mutant of murine tumor necrosis factor alpha expressed from an adenovirus vector induces an antitumor response with reduced toxicity. Cancer Gene Ther. 1999;6:465–474. doi: 10.1038/sj.cgt.7700068. [DOI] [PubMed] [Google Scholar]
- 23.Abe Y., Yoshikawa T., Kamada H., Shibata H., Nomura T., Minowa K., Kayamuro H., Katayama K., Miyoshi H., Mukai Y., Yoshioka Y., Nakagawa S., Tsunoda S., Tsutsumi Y. Simple and highly sensitive assay system for TNFR2-mediated soluble- and transmembrane-TNF activity. J. Immunol. Methods. 2008;335:71–78. doi: 10.1016/j.jim.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Ando D., Kamada H., Inoue M., Taki S., Furuya T., Abe Y., Nagano K., Tsutsumi Y., Tsunoda S. Generation of a sensitive TNFR2-specific murine assays system. Pharm. – Int. J. Pharm. Sci. 2016;71:235–237. [PubMed] [Google Scholar]
- 25.Yamamoto Y., Tsutsumi Y., Yoshioka Y., Nishibata T., Kobayashi K., Okamoto T., Mukai Y., Shimizu T., Nakagawa S., Nagata S., Mayumi T. Site-specific PEGylation of a lysine-deficient TNF-alpha with full bioactivity. Nat. Biotechnol. 2003;21:546–552. doi: 10.1038/nbt812. [DOI] [PubMed] [Google Scholar]
- 26.Shibata H., Yoshioka Y., Ohkawa A., Minowa K., Mukai Y., Abe Y., Taniai M., Nomura T., Kayamuro H., Nabeshi H., Sugita T., Imai S., Nagano K., Yoshikawa T., Fujita T., Nakagawa S., Yamamoto A., Ohta T., Hayakawa T., Mayumi T., Vandenabeele P., Aggarwal B.B., Nakamura T., Yamagata Y., Tsunoda S., Kamada H., Tsutsumi Y. Creation and X-ray structure analysis of the tumor necrosis factor receptor-1-selective mutant of a tumor necrosis factor-alpha antagonist. J. Biol. Chem. 2008;283:998–1007. doi: 10.1074/jbc.M707933200. [DOI] [PubMed] [Google Scholar]
- 27.Nomura T., Abe Y., Kamada H., Inoue M., Kawara T., Arita S., Furuya T., Yoshioka Y., Shibata H., Kayamuro H., Yamashita T., Nagano K., Yoshikawa T., Mukai Y., Nakagawa S., Taniai M., Ohta T., Tsunoda S., Tsutsumi Y. Novel protein engineering strategy for creating highly receptor-selective mutant TNFs. Biochem. Biophys. Res. Commun. 2009;388:667–671. doi: 10.1016/j.bbrc.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 28.Mukai Y., Shibata H., Nakamura T., Yoshioka Y., Abe Y., Nomura T., Taniai M., Ohta T., Ikemizu S., Nakagawa S., Tsunoda S., Kamada H., Yamagata Y., Tsutsumi Y. Structure-function relationship of tumor necrosis factor (TNF) and its receptor interaction based on 3D structural analysis of a fully active TNFR1-selective TNF mutant. J. Mol. Biol. 2009;385:1221–1229. doi: 10.1016/j.jmb.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 29.Baeyens K.J., De Bondt H.L., Raeymaekers A., Fiers W., De Ranter C.J. The structure of mouse tumour-necrosis factor at 1.4 a resolution: towards modulation of its selectivity and trimerization. Acta Cryst. D. Biol. Cryst. 1999;55:772–778. doi: 10.1107/s0907444998018435. [DOI] [PubMed] [Google Scholar]
- 30.Mukai Y., Nakamura T., Yoshikawa M., Yoshioka Y., Tsunoda S., Nakagawa S., Yamagata Y., Tsutsumi Y. Solution of the structure of the TNF-TNFR2 complex. Sci. Signal. 2010;(3) doi: 10.1126/scisignal.2000954. ra83. [DOI] [PubMed] [Google Scholar]
- 31.Inoue M., Kamada H., Abe Y., Higashisaka K., Nagano K., Mukai Y., Yoshioka Y., Tsutsumi Y., Tsunoda S. Aminopeptidase P3, a new member of the TNF-TNFR2 signaling complex, induces phosphorylation of JNK1 and JNK2. J. Cell Sci. 2015;128:656–669. doi: 10.1242/jcs.149385. [DOI] [PubMed] [Google Scholar]
- 32.Bremer E. Targeting of the tumor necrosis factor receptor superfamily for cancer immunotherapy. ISRN Oncol. 2013;2013:371854. doi: 10.1155/2013/371854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ban L., Kuhtreiber W., Butterworth J., Okubo Y., Vanamee E.S., Faustman D.L. Strategic internal covalent cross-linking of TNF produces a stable TNF trimer with improved TNFR2 signaling. Mol. Cell. Ther. 2015;3:7. doi: 10.1186/s40591-015-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krippner-Heidenreich A., Grunwald I., Zimmermann G., Kuhnle M., Gerspach J., Sterns T., Shnyder S.D., Gill J.H., Mannel D.N., Pfizenmaier K., Scheurich P., Single-chain T.N.F. A TNF derivative with enhanced stability and antitumoral activity. J. Immunol. 2008;180:8176–8183. doi: 10.4049/jimmunol.180.12.8176. [DOI] [PubMed] [Google Scholar]
- 35.Fischer R., Maier O., Siegemund M., Wajant H., Scheurich P., Pfizenmaier K. A TNF receptor 2 selective agonist rescues human neurons from oxidative stress-induced cell death. PLOS One. 2011;6:e27621. doi: 10.1371/journal.pone.0027621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material