Abstract
The cytosolic form of phosphoenolpyruvate carboxykinase (PCK1) plays a regulatory role in gluconeogenesis and glyceroneogenesis. The role of the mitochondrial isoform (PCK2) remains unclear. We report the partial purification and kinetic and functional characterization of human PCK2. Kinetic properties of the enzyme are very similar to those of the cytosolic enzyme. PCK2 has an absolute requirement for Mn2+ ions for activity; Mg2+ ions reduce the Km for Mn2+ by about 60 fold. Its specificity constant is 100 fold larger for oxaloacetate than for phosphoenolpyruvate suggesting that oxaloacetate phosphorylation is the favored reaction in vivo. The enzyme possesses weak pyruvate kinase-like activity (kcat=2.7 s−1). When overexpressed in HEK293T cells it enhances strongly glucose and lipid production showing that it can play, as the cytosolic isoenzyme, an active role in glyceroneogenesis and gluconeogenesis.
Keywords: Human mitochondrial phosphoenolpyruvate carboxykinase (PCK), Purification, Kinetics, Gluconeogenesis, Glyceroneogenesis
Highlights
-
•
Purification of recombinant human PCK2 has been performed.
-
•
Its kinetic behavior is very similar to that of human PCK1.
-
•
PCK2 overexpression increases gluconeogenesis and glyceroneogenesis in cell cultures.
1. Introduction
Phosphoenolpyruvate carboxykinase (PCK) catalyzes the decarboxylation and subsequent phosphorylation of oxaloacetate to yield phosphoenolpyruvate. GTP is used as phosphoryl donor in this reaction [1]. The reverse reaction is possible; however, it is difficult to occur in vivo. The concentration ranges of oxaloacetate and GTP in tissues [2], [3] are similar to their Km values in human PCK1 [4]. Nevertheless, the Km for PEP and GDP are higher than their physiological concentrations [5]. Two divalent ions are needed for this reaction; one binds directly to the enzyme active site and the second, to the nucleotide substrate [1], [6]. PCK plays several metabolic roles in mammals, with its most known regulatory role in gluconeogenesis and glyceroneogenesis [7]. There exist two isoforms of the enzyme, a cytosolic and a mitochondrial one [8]; their relative contribution to total PCK activity varies between species. Whereas in mice and rat PCK1, the cytosolic isoenzyme, accounts for over 90% of total PCK activity, in both pigs and humans each isoenzyme is responsible for about 50% of total PCK activity [9]. The cytosolic isoform has been extensively studied, and even serves even as a model enzyme to study transcriptional regulation [10], but much less information is available on the precise role of the mitochondrial isoform. However, in the past two years the involvement of the mitochondrial isoenzyme (PCK2) in pathological processes such as cancer and diabetes has been highlighted by well-grounded works in several laboratories [11], [12], [13], [14]. Despite these studies, there exist no report on the purification and kinetic characterization of the human PCK2. In this work we present a protocol for the partial purification of recombinant human PCK2, and we determine the kinetic constants for its five substrates. In addition, we present evidence of the gluconeogenic and glyceroneogenic capacity of human PCK2 in cell cultures.
2. Materials and methods
2.1. Cloning of PCK2
PCK2 gene (Invitrogen, USA) was amplified by PCR using Pfu Ultra II HS DNA polymerase (Agilent Technologies, USA) according to manufacturer's instructions. The following primers were used:
PCK2-BamHI-F:
5′-CTAGGATCGGATCCATGGCCGCATTGTACCGCCCTG-3′.
PCK2-NotI-R:
5′-CTAGGATCGCGGCCGCTCACATTTTGTGCACACGTCTCTCC-3′.
PCK2-NheI-F:
5′-CTAGGATCGCTAGCATGGCCGCATTGTACCGCCCTG-3′.
PCK2-myc-NotI-R:
5′-CTAGGATCGCGGCCGCTCACAGGTCTTCTTCAGAGATCAGTTT
CTGTTCCATTTTGTGCACACGTCTCTCC-3′
The last primer included a myc tag sequence to detect the protein in cell culture by western blot. PCR products were digested at 37 °C overnight using either BamHI and NotI or NheI and NotI (New England Biolabs, USA) and cloned into either plasmid pET22bSUMO or pCDNA3.1(-) using T4 DNA ligase (Invitrogen, USA) at 16 °C overnight. Constructs were sequenced and transformed into Arctic Express Escherichia coli strain.
2.2. Purification of PCK2
PCK2 had an unstable behavior during purification steps, either rendering insoluble or inactive protein. Several E. coli strains and purification protocols were used to obtain soluble active protein. The most successful protocol that yielded soluble active PCK2 was the following.
Arctic Express E. coli cells were grown in 100 mL of 2xYT medium supplemented with ampicillin (100 µg/mL) at 37 °C overnight and 220 rpm. This bacterial preculture was poured into 2 L of the same medium and incubated at 37 °C and 160 rpm until OD600 reached 0.6. Then, cells were induced using IPTG at a final concentration of 1 mM and incubated at 12.5 °C for 72 h and 160 rpm. Cells were harvested by centrifugation at 4 °C, 8000×g for 15 min and resuspended in buffer A (25 mM HEPES, pH 8, 500 mM NaCl, 10% glycerol, 1 mM TCEP and 10 mM imidazole) with 1 mg lysozyme, 1 µM PMSF, 10 µM benzamidine, 0.5 µM leupeptin and 0.1% benzonase (Sigma-Aldrich, USA). Cells were lysed with a Vibra-Cell sonicator (Sonics & Materials, USA) performing 10 cycles, each for 30 s on-30 s off, on ice. The lysate was clarified by centrifugation at 18,000×g for 15 min and 4 °C. Finally, the pellet was discarded and the supernatant was filtered through a 0.45 µm filter and used in further purification steps.
Protein was loaded into a 5 mL FF crude HisTrap column (GE Healthcare, USA) equilibrated in buffer A. Protein was eluted with buffer B (same as buffer A but with 300 mM imidazole) using a 0–100% gradient. Aliquots were analyzed by SDS-PAGE. Fractions were concentrated using Amicon Ultra Centrifugal Filters 30 kDa (Millipore, Germany). After two washes in buffer C (25 mM HEPES pH 8, 150 mM NaCl, 1 mM TCEP) protein was diluted up to 10 mL in buffer C and quantified using NanoDrop 1000 (Thermo Scientific, USA). PCK2 was incubated with SUMO protease (Thermo Scientific, 1:100 mg protease/mg protein) for 6 h at 4 °C. The protein was then loaded into a HisTrap column equilibrated in buffer C. PCK2 was collected in the flow-through. PCK2 was detected and quantified by SDS-PAGE and using NanoDrop1000. Since PCK2 was partially purified with chaperone 60 (Cpn60), Image J (NIH, USA) was used to quantify the portion of PCK2 in the mixture, comparing the intensity with that of known standards. Total protein (2 mg/mL) aliquots were flash-frozen in liquid nitrogen and stored at −80 °C.
To avoid the potential interference of metals (Mn2+ and Mg2+) derived from purified PCK2 preparations we performed a control purification including a metal chelating step. Purified PCK2 was mixed with 5% Chelex 100 chelating resin (Sigma-Aldrich, USA) and was gently shaken for 1 h at 4 °C. The sample was then decanted to remove the chelating resin. Total manganese and magnesium contents were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) using IRIS Intrepid Radial Thermo-Elemental (Thermo Scientific, USA). The detection limit of the method was 1 µg/L.
2.3. Kinetic properties of PCK2
All kinetic assays were performed at 30 °C using a Unicam UV500 spectrophotometer (Thermo Scientific, USA) in a total volume of 1 mL. Readings were measured at 340 nm. The maximum duration of the assays was 10 min. We report no activity if no change in absorbance above 0.001 units was detected after the maximum assay duration. Three different reactions were performed:
-
a)
Oxaloacetic acid (OAA) decarboxylation. OAA+GTP→PEP+CO2+GDP.
The reaction consisted of 100 mM HEPES pH 7.4, 1 mM ADP (Sigma-Aldrich, USA), 10 mM DTT (Sigma-Aldrich, USA), 0.5 mM GTP (Sigma-Aldrich, USA), 0.2 mM MnCl2 (Panreac, Spain), 2 mM MgCl2 (Panreac, Spain), 0.2 mM NADH (Sigma-Aldrich, USA), 5 units each of pyruvate kinase and lactate dehydrogenase (Sigma-Aldrich, USA), 1 µg PCK2 and 0.4 mM OAA (Sigma-Aldrich, USA). Reaction was started by adding OAA.
-
b)
Phosphoenolpyruvate (PEP) carboxylation. PEP+GDP+ CO2→OAA+GTP.
The reaction consisted of 100 mM HEPES pH 7,4, 2 mM PEP (Sigma-Aldrich, USA), 100 mM KHCO3 (Panreac, Spain), 2 mM GDP (Sigma-Aldrich, USA), 2 mM MgCl2, 2 mM MnCl2, 10 mM DTT, 0,2 mM NADH, 2 units of malic dehydrogenase (Sigma-Aldrich, USA) and 1 μg of PCK2.
-
c)
Pyruvate formation. OAA+(GTP or GDP)→Pyruvate+CO2+GDP.
These reactions were identical to reaction (a) but without addition of ADP and pyruvate kinase to the mixture.
2.4. Gluconeogenic and glyceroneogenic activity of PCK2 in cell cultures
HEK293T cells (2×105 per well) were seeded on 24-well plates in complete medium (DMEM supplemented with 10% FBS, 10 mM l-glutamine, 0.1 mg/mL streptomycin and 100 U/mL penicillin). Poly-l-lysine (Sigma-Aldrich, USA) was used to attach cells to the culture surface in glyceroneogenic assays. Cells were incubated at 37 °C and 5% CO2 for 24 h. Then, cells were transfected using GeneJuice (Novagen, UK) following manufacturer's instructions. The amount of DNA used per well was 1 µg.
For glucose production assays cells were incubated for 48 h after transfection. Medium was replaced with 1 mL of DMEM without glucose and phenol red and supplemented with 2 mM sodium pyruvate (Sigma-Aldrich, USA) and 20 mM sodium lactate (Sigma-Aldrich, USA). After a 6-hour incubation at 37 °C, half of the medium was collected and a colorimetric assay was performed (GAGO20, Sigma-Aldrich, USA).
For glyceroneogenic assays, 24 h post-transfection DMEM medium was replaced with complete DMEM medium, as described above, supplemented with 250 µM palmitic acid. After 24 h, cells were washed with 1xPBS once and fixed in 1xPBS with 3.7% formaldehyde for 1 h at room temperature. Cells were washed twice with distilled water. Water was discarded and 60% isopropanol was added. They were then incubated for 5 min, dried, and treated with Oil Red-O stain (Sigma-Aldrich, 0.2% in 60% isopropanol) for 30 min. Stain was discarded and cells were washed four times with distilled water. Oil Red-O stain was dissolved in 1 mL of isopropanol for 1 h with shaking and readings were performed at 500 nm.
Data was normalized to the total protein content of whole cell lysates. Units are mg glucose/mg protein and absorbance units/mg protein for gluconegenic and glyceroneogenic assays, respectively.
2.5. Data analysis
All kinetic data were analyzed using Origin Pro (OriginLab, USA) adjusting kinetic data to the Michaelis-Menten equation using a molecular weight for PCK2 of 70,700 Da. Data normality was checked by Shapiro-Wilk tests. Two-sample t-tests were performed to analyze glucose production experiments and one-way ANOVA following a Tukey post-hoc test was performed to analyze glyceroneogenic activity data.
3. Results and discussion
3.1. Cloning of PCK2
The full-length cDNA of human PCK2, including the mitochondrial targeting sequence, was cloned in a pET22bSUMO plasmid. The DNA sequence encoding an N-terminal histidine-tagged Saccharomyces cerevisiae SMT3 (SUMO protein) was synthesized and codon-optimized by GenScript to be expressed in E. coli. The DNA contained at the 5′ end a recognition sequence for NdeI, and at the 3′ end a polylinker sequence for BamHI, EcoRI, NcoI, SacI, SalI, HindIII, NotI, and XhoI, and was cloned into the pUC57 vector (GenScript). Following digestion with NdeI and XhoI, the construct was subcloned into the protein expression vector pET22b, resulting in the plasmid pET22bSUMO. The DNA sequence encoding residues 403-621 of ScUlp1 was also synthesized and codon-optimized by GenScript to be expressed in E. coli. The DNA, containing at the 5′ end a recognition sequence for NdeI and a histidine tag, and at the 3′ end a sequence for XhoI and a stop codon, was cloned into the pUC57 vector (GenScript). Following digestion with NdeI and XhoI the construct was subcloned into the protein expression vector pET22b, resulting in the expression plasmid pET22bScUlp1. Sequencing of the cloned cDNA showed no differences with published human PCK2 sequence [15].
3.2. PCK2 purification
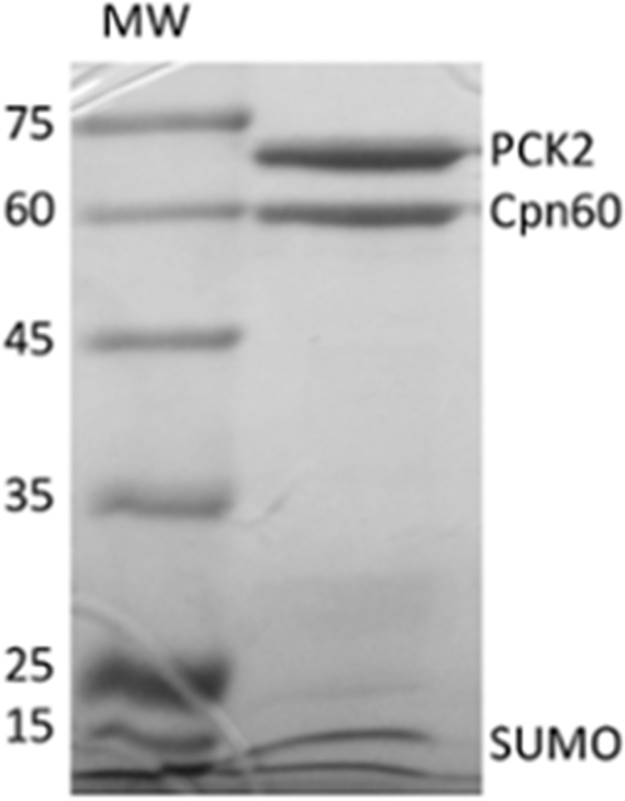
After several unsuccessful attempts to purify PCK2 using E. coli BL21 DE3 Star or Rosetta-gami strains with several plasmid constructs that yielded insoluble or inactive PCK2 (data not shown), we decided to use Artic Express E. coli cells. This strain overexpresses chaperones Cpn10 and Cpn60 from Oleispira antarctica, which form one of the most important intracellular folding machines that facilitate protein folding and stability [16]. Fig. 1 shows the partially purified protein preparation obtained after separating the SUMO moiety and the His-tag using SUMO protease. Three bands can be appreciated on the gel: the two most prominent ones correspond to PCK2 and Cpn60. The latter was identified by mass spectrometry (data not shown) to determine that it was not a proteolysis fragment of the former. The third band has a molecular weight of about 10 kDa, corresponding to the SUMO moiety. The specific activity of this PCK2 preparation, calculated based on the content of just PCK2 and excluding therefore Cpn60 and SUMO, was similar to the specific activity of other PCK2 from different species [17], [18].
Fig. 1.
SDS-PAGE analysis of partially purified PCK2 protein and Cpn60 after separating the SUMO moiety. A Coomassie blue-stained gel is shown. MW: Molecular weight markers (in kDa) run alongside.
ICP-AES analysis showed that the PCK2 preparation without the chelating resin had manganese levels below the detection limit (<1 µg/L). Magnesium concentration was 21±2 µg/L. Therefore, the kinetic assay had only around 0.01 µM final concentration of magnesium. Manganese and magnesium levels were below the detection limit when the chelating resin was used. PCK2 preparation without the chelating resin was used in the kinetic assays because PCK2 precipitated in its presence.
3.3. Ion requirements for activity
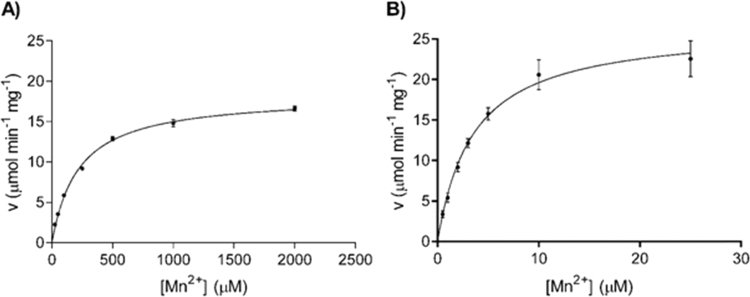
PCK isoenzymes of diverse origins need divalent cations for activity. Two cations play a role in their catalytic mechanism: one binds directly to amino acids of the active site and the other binds and stabilizes the negative charged phosphates of the nucleotide substrate [1]. Whereas there exist an almost absolute requirement for Mn2+ to fulfill the first role, nucleotide stabilization can be achieved by either Mg2+ or Mn2+, although both ions are not equally efficient in this task. Human PCK2 is no exception to this behavior (see Fig. 2), since no activity can be observed if only Mg2+ is present in the assay medium. However if only Mn2+ is added (the assay contains also 0.01 μM Mg2+ due to the ion content of the PCK2 preparation) the activity reaches values of kcat up to 21.3 s−1 (Table 1). The addition of 2 mM Mg2+ to Mn2+-containing assays has two effects: it enhances the maximal activity up to kcat values of 31.3 s−1 and it reduces drastically Km values from 200 to 3.5 µM. These changes result in an increase in the specificity constant of almost 200-fold (see Table 1).
Fig. 2.
Effect of concentration of divalent cations on PCK2 activity. (A) Effect of variable Mn2+ (25–2000 µM) and 0.01 µM of Mg2+. (B) Effect of variable Mn2+ (0.5–25 µM) in the presence of fixed Mg2+ concentration (2 mM). All reactions were performed in the PEP carboxylation direction as described in Materials and Methods. Mean±SD were adjusted to the Michaelis-Menten equation.
Table 1.
PCK2 kinetic parameters for divalent cations.
| Substrate | Km(µM) | kcat(s−1) | kcat/Km(M−1 s−1) | Figure |
|---|---|---|---|---|
| Mg2+ | Not detecteda | |||
| Mn2+ | 200±11 | 21.3±0.3 | 1.1×105 | 2A |
| Mn2++Mg2+ | 3.5±0.4 | 31.3±1.2 | 8.9×106 | 2B |
See Section 2.
3.4. Kinetic parameters of PCK2
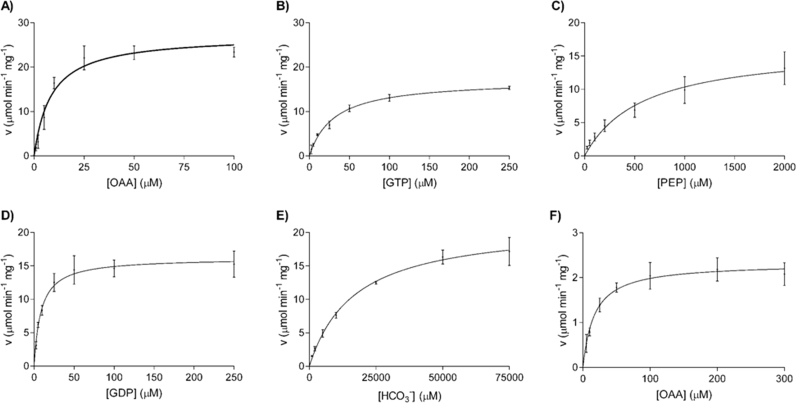
We calculated Km, kcat and kcat/Km values for the five PCK substrates. The divalent cation concentration in the assays was optimized to reach maximal activity. Assays in the direction of OAA decarboxylation were performed at 0.2 mM Mn2+ because higher Mn2+ concentrations resulted in high rates of spontaneous OAA decarboxylation (data not shown) whereas assays performed in the direction of OAA synthesis were performed at 2 mM Mn2+ concentration. Mg2+ was set at 2 mM in all the assays. PCK2 followed Michaelis-Menten kinetics for the five substrates (see Fig. 3A–E). The values of Km, kcat and kcat/Km are presented in Table 2. The specificity constant (kcat/Km) is a useful parameter for comparing alternative and competing substrates for the same enzyme [19]. Specificity constants are similar for nucleotides in both reaction directions but about 100-fold larger for OAA than for PEP. All kcat values are slightly lower than those of human PCK1. Most Km values are also of the same order of magnitude than those of human PCK1. Only the Km for GDP is an order of magnitude lower in PCK2 than in PCK1 [4]. OAA and GTP Km values are in the same range of OAA and GTP physiological concentrations [2], [3], however the PEP Km value is higher than the PEP physiological concentration [5]. The difference in Km values and specificity constants shows that the enzyme favors the reaction in the direction of PEP synthesis (or OAA decarboxylation) as it happens with other PCK isoenzymes [4], [18], [20], [21].
Fig. 3.
Kinetic characterization of human PCK2. Effect of OAA (A), GTP (B), PEP (C), GDP (D) and HCO3− (E) concentrations on PCK2 activity. (F) Effect of OAA concentration on pyruvate kinase-like activity of PCK2. Mean±SD were adjusted to the Michaelis-Menten equation.
Table 2.
Kinetic parameters of human PCK2.
| Substrate | Km(µM) | kcat(s−1) | kcat/Km(M−1 s−1) | Figure |
|---|---|---|---|---|
| OAA decarboxylation: OAA+GTP→PEP+CO2+GDP | ||||
| OAA | 8.7±2.5 | 32.0±2.5 | 3.7×106 | 3A |
| GTP | 29±7 | 19.8±1.5 | 6.9×105 | 3B |
| PEP carboxylation. PEP+GDP+CO2→OAA+GTP | ||||
| PEP | 585±120 | 18.8±1.7 | 3.2×104 | 3C |
| GDP | 8.6±1.0 | 19.1±0.5 | 2.2×105 | 3D |
| HCO3− | 17,454±2529 | 25.3±1.3 | 1.4×103 | 3E |
| Pyruvate formation. OAA+GTP→Pyruvate+CO2+GDP | ||||
| Not detecteda | ||||
| Pyruvate formation. OAA+GDP→Pyruvate+CO2+GDP | ||||
| OAA | 17±2.7 | 2.7±0.1 | 1.6×105 | 3F |
See Section 2.
Some PCK isoenzymes show pyruvate kinase-like activity [21], [22]. This activity derives from the instability of the enolate reaction intermediate and its tendency to yield pyruvate. To avoid pyruvate formation, the active site of PCK is closed during catalysis by an Ω-loop that helps to position the substrates correctly in the active site and that stabilizes the enolate reaction intermediate protecting it against protonation [1]. To check if human PCK2 was able to accelerate the transformation of OAA into pyruvate we excluded the auxiliary enzyme pyruvate kinase and its substrate ADP from the assays. No pyruvate formation was detected if GTP was present, but in the presence of GDP pyruvate was readily formed (Fig. 3F). The Km and kcat values for OAA in this reaction were 17 µM and 2.7 s−1, respectively. The resulting kcat/Km value is 1.6×105 M−1 s−1 (Table 2). These results are similar to those of rat [21] and human [22] PCK1 in terms of kcat. However, there are differences in Km values; rat and human values were 10-fold higher and 40% lower than the PCK2 value, respectively. The pyruvate kinase-like activity of human PCK2 in the presence of GDP, together with its larger affinity for GDP than PCK1, could, at least theoretically, enhance mitochondrial pyruvate formation, and its subsequent transformation into oxaloacetate, when energy levels in the mitochondria (GTP/GDP ratio) are low, contributing in this way to the Krebs cycle. This is an unlikely scenario and perhaps only possible under extreme exercise or long fasting conditions. However, to the best of our knowledge, there is no interpretation in the literature of the biological role, if any, of the PCK pyruvate kinase-like activity.
3.5. Gluconeogenic and glyceroneogenic activity of PCK2 in cell cultures
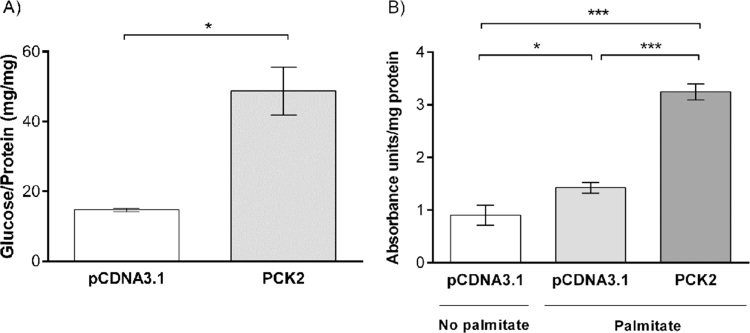
PCK2 was transiently overexpressed in HEK293T cells in a glucose-free medium. Cells overexpressing PCK2 yielded 3.3-fold more glucose (48.1±6.9 mg glucose/mg protein) than cells that did not express PCK2 (14.7±0.5 mg glucose/mg protein). To analyze its effects on lipid production, PCK2 was transiently overexpressed in HEK293T cells in complete DMEM supplemented with palmitic acid to enhance lipid synthesis. Cells overexpressing PCK2 produced 2.3-fold more lipids (3.25±0.2 absorbance units/mg protein) in the presence of palmitate than cells transfected with an empty pCDNA3.1 plasmid (1.42±0.1 absorbance units/mg protein). In both experiments, differences between PCK2-overexpressing cells and controls were statistically significant (Fig. 4).
Fig. 4.
Effect of PCK2 overexpression on glucose and lipid production. (A) Glucose production in HEK293T cells using an empty pCDNA3.1 plasmid as control (14.7±0.5 mg glucose/mg protein) or overexpressing PCK2 (48.1±6.9 mg glucose/mg protein). Shapiro-Wilk test was performed to test normality and a two-sample t-test, to analyze glucose production (*p<0.05). (B) Lipid production in HEK293T cells using an empty pCDNA3.1 plasmid as control in absence (0.9±0.2 absorbance units/mg protein) or presence (1.42±0.1 absorbance units/mg protein) of palmitate or overexpressing PCK2 in presence of palmitate (3.25±0.2 absorbance units/mg protein). Shapiro-Wilk test was performed to test normality. One-way ANOVA following a Tukey post-hoc test was performed to analyze lipid production (*p<0.05, **p<0.01, ***p<0.001).
PCK2 has arisen as an important regulatory protein in cell energy metabolism that is also linked to pathological processes such as cancer [23]. In particular, PCK2 is a pro-survivor protein because it enables tumor cells to adapt to low-glucose environments [12]. Although the capacity of PCK1 to produce glucose has been previously reported [24], [25], this is the first time that PCK2 is overexpressed in cell culture and tested for glucose production. Our results indicate that PCK2 behaves as a gluconeogenic enzyme, similar to PCK1. PCK2 is the only isoform that provides PCK activity in pancreas due to the involvement of PCK2 in glucose-stimulated insulin secretion, linking the production of mitochondrial GTP by succinyl-CoA synthetase to anaplerotic PEP cycling [26] and it is responsible for half of PCK activity in human liver. However, to date only PCK1 has been a drug target for diabetes. The gluconeogenic activity of PCK2 suggests that this protein could play an important role in diabetes [14], and this is further supported by the results obtained when testing the ability of PCK2 to increase lipid synthesis. In the case of PCK1, its overexpression is responsible for lipid deposition in diabetic liver and its silencing improves dyslipidemia in mice [27]. These results in PCK1 agree with our findings showing that PCK2 overexpression can lead to lipid deposition in cells and support the potential role of PCK2 in diabetes.
Acknowledgements
This study was supported by research grants AGL2015–66177-R to P.L.B. and J.A.C and UZ2015-BIO01 to J.A.C. P.L. was supported by a predoctoral fellowship of Fundación La Caixa. Thanks are also given to the Regional Government of Aragón (Grant no. A51) (DGA) for financial help to the research group A51. Authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación – SAI, Universidad de Zaragoza for ICP-AES analysis.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.06.007.
Contributor Information
José Alberto Carrodeguas, Email: carrode@unizar.es.
Pascual López-Buesa, Email: plopezbu@unizar.es.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Carlson G.M., Holyoak T. Structural insights into the mechanism of phosphoenolpyruvate carboxykinase catalysis. J. Biol. Chem. 2009;284:27037–27041. doi: 10.1074/jbc.R109.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siess E.A., Kientsch-Engel R., Wieland O.H. Concentration of free oxaloacetate in the mitochondrial compartment of isolated liver cells. Biochem. J. 1984;218:171–176. doi: 10.1042/bj2180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traut T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 4.Case C.L., Mukhopadhyay B. Kinetic characterization of recombinant human cytosolic phosphoenolpyruvate carboxykinase with and without a His10-tag. Biochim. Biophys. Acta Gen. Subj. 2007;1770:1576–1584. doi: 10.1016/j.bbagen.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Lagunas R., Gancedo C. Role of phosphate in the regulation of the pasteur effect in Saccharomyces cerevisiae. Eur. J. Biochem. 1983;137:479–483. doi: 10.1111/j.1432-1033.1983.tb07851.x. [DOI] [PubMed] [Google Scholar]
- 6.Hlavaty J.J., Nowak T. Characterization of the second metal site on avian phosphoenolpyruvate carboxykinase. Biochemistry. 2000;39:1373–1388. doi: 10.1021/bi991692a. [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Kalhan S.C., Hanson R.W. What is the metabolic role of phosphoenolpyruvate carboxykinase? J. Biol. Chem. 2009;284:27025–27029. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordlie R.C., Lardy H.A. Mammalian liver phosphoenolpyruvate carboxykinase activities. J. Biol. Chem. 1963;238:2259–2263. [PubMed] [Google Scholar]
- 9.Hanson R.W., Garber A.J. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am. J. Clin. Nutr. 1972;25:1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- 10.Yang J., Reshef L., Cassuto H., Aleman G., Hanson R.W. Aspects of the control of phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 2009;284:27031–27035. doi: 10.1074/jbc.R109.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leithner K., Hrzenjak A., Trotzmuller M., Moustafa T., Kofeler H.C., Wohlkoenig C., Stacher E., Lindenmann J., Harris A.L., Olschewski A. PCK2 activation mediates an adaptive response to glucose depletion in lung cancer. Oncogene. 2015;34:1044–1050. doi: 10.1038/onc.2014.47. [DOI] [PubMed] [Google Scholar]
- 12.Méndez-Lucas A., Hyroššová P., Novellasdemunt L., Viñals F., Perales J.C. Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability. J. Biol. Chem. 2014;289:22090–22102. doi: 10.1074/jbc.M114.566927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beale E., Harvey B., Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem. Biophys. 2007;48:89–95. doi: 10.1007/s12013-007-0025-6. [DOI] [PubMed] [Google Scholar]
- 14.Stark R., Guebre-Egziabher F., Zhao X., Feriod C., Dong J., Alves T.C., Ioja S., Pongratz R.L., Bhanot S., Roden M. A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J. Biol. Chem. 2014;289:7257–7263. doi: 10.1074/jbc.C113.544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modaressi S., Brechtel K., Christ B., Jungermann K. Human mitochondrial phosphoenolpyruvate carboxykinase 2 gene. Structure, chromosomal localization and tissue-specific expression. Biochem. J. 1998;333:359–366. doi: 10.1042/bj3330359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kube M., Chernikova T.N., Al-Ramahi Y., Beloqui A., Lopez-Cortez N., Guazzaroni M., Heipieper H.J., Klages S., Kotsyurbenko O.R., Langer I. Genome sequence and functional genomic analysis of the oil-degrading bacterium oleispira antarctica. Nat. Commun. 2013;4 doi: 10.1038/ncomms3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond K.D., Balinsky D. Kinetic studies on phosphoenolpyruvate carboxykinase purified from the mitochondrial and cytosol fractions of monkey liver. Int. J. Biochem. 1978;9:199–211. doi: 10.1016/0020-711x(78)90150-7. [DOI] [PubMed] [Google Scholar]
- 18.Sato A., Suzuki T., Kochi H. Purification and characterization of cytosol-specific phosphoenolpyruvate carboxykinase from chicken liver. J. Biochem. 1986;100:671–678. doi: 10.1093/oxfordjournals.jbchem.a121759. [DOI] [PubMed] [Google Scholar]
- 19.Eisenthal R., Danson M.J., Hough D.W. Catalytic efficiency and kcat/KM: a useful comparator? Trends Biotechnol. 2007;25:247–249. doi: 10.1016/j.tibtech.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Latorre P., Burgos C., Hidalgo J., Varona L., Carrodeguas J.A., López-Buesa P. A2456C substitution in PCK1 gene changes the enzyme kinetic and functional properties modifying fat distribution in pigs. Sci. Rep. 2016;6 doi: 10.1038/srep19617. 19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson T.A., Holyoak T. Increasing the conformational entropy of the Ω-loop lid domain in phosphoenolpyruvate carboxykinase impairs catalysis and decreases catalytic fidelity. Biochemistry. 2010;49:5176–5187. doi: 10.1021/bi100399e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharmarajan L., Case C.L., Dunten P., Mukhopadhyay B. Tyr235 of human cytosolic phosphoenolpyruvate carboxykinase influences catalysis through an anion–quadrupole interaction with phosphoenolpyruvate carboxylate. FEBS J. 2008;275:5810–5819. doi: 10.1111/j.1742-4658.2008.06702.x. [DOI] [PubMed] [Google Scholar]
- 23.Vincent E., Sergushichev A., Griss T., Gingras M., Samborska B., Ntimbane T., Coelho P., Blagih J., Raissi T., Choinière L. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol. Cell. 2015;60:195–207. doi: 10.1016/j.molcel.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C.R., Granner D.K. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W., Wang S., Xiao M., Lin Y., Zhou L., Lei Q., Xiong Y., Guan K., Zhao S. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 Ubiquitin ligase. Mol. Cell. 2011;43:33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark R., Pasquel F., Turcu A., Pongratz R.L., Roden M., Cline G.W., Shulman G.I., Kibbey R.G. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J. Biol. Chem. 2009;284:26578–26590. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez-Valadés A.G., Méndez-Lucas A., Vidal-Alabró A., Blasco F.X., Chillon M., Bartrons R., Bermúdez J., Perales J.C. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes. 2008;57:2199–2210. doi: 10.2337/db07-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material