Abstract
BACKGROUND:
Neuromyelitis optica (NMO) is an autoimmune demyelinating disease preferentially targeting the optic nerves and spinal cord. Plasmapheresis (PP) is an effective adjunct therapy in severe NMO attacks. The recommended minimum plasma volume to be treated per session of PP is equivalent to total plasma volume (TPV) of the patient.
AIM:
To study the effect of lower plasma volume treated in patients with NMO on clinical efficacy of plasmapheresis in comparison to minimum recommended volume.
METHODS:
This retrospective study was done on acute NMO patients who were managed with PP at our center. Patients who had 5 sessions of PP, spread over 10 days, were included. Clinical outcome was defined as per predefined criteria.
RESULTS:
24 patients who underwent PP for acute NMO met our inclusion criteria. Females (age; mean (SD) 33.7 (11.2) years) were more common (n = 18). The minimum recommended plasma volume (PV) that was supposed to be treated per patient during entire acute therapeutic period was 195.5 (14.6) mL per kilogram-body-weight (kg-bw). We treated lower plasma volume (mean (SD) 112.7 (17.0) mL per kg-bw); the difference was significant (P < 0.05). The volume treated was same across the gender (P > 0.05). Significant clinical improvement was observed in 79% of patients (n = 19) after 6 months. There were no significant differences in volume of plasma treated, between patients who had moderate and marked improvement; also, who did, and did not have significant clinical improvement (P > 0.05; for both).
CONCLUSIONS:
Plasmapheresis is a safe and efficient add-on therapy in NMO, especially in steroid-resistant cases. Although the volumes of plasma treated during acute plasmapheresis were less than recommended minimum volumes, majority of patients had significant clinical improvement.
Keywords: Low plasma volume, neuromyelitis optica, plasmapheresis, transfusion
Introduction
Neuromyelitis optica (NMO) is an idiopathic inflammatory disease of the central nervous system (CNS) with predilection for the optic nerves and spinal cord[1] and which, unlike multiple sclerosis (MS), commonly spares the brain in the early stages. The disease is associated with paraplegia/paraparesis, due to severe transverse myelitis (TM) and blindness, due to severe optic neuritis (ON). NMO can have either a monophasic or relapsing course.[2] Monophasic course is associated with younger age at disease onset, equal male to female predominance, and 5-year survival rate is 90%. Approximately 80% of patients with NMO have relapsing course,[3,4] which has a poor prognosis: 50% of patients become legally blind or wheelchair bound and 30% die with respiratory failure within 5 years. There is not a progressive phase like MS; the disease worsens by incomplete recovery with each acute attack.
NMO patients with myelitis attacks present with severe clinical disability (para/tetraparesis and sphincter disturbances). Most disabilities arise from the discrete acute attacks.[5] These significantly impact the patients' quality of life.[6] In 2004, Lennon et al. detected NMO-IgG in 50%–70% of patients with NMO.[7] This antibody is targeted against the aquaporin-4 (AQP4) water channel widely expressed in the optic nerves, the spinal cord, and the periventricular regions.[8,9]
Revised diagnostic criteria for NMO proposed, in addition to the two major symptoms (myelitis and ON), any two of the following three criteria: extended myelitis on spinal cord magnetic resonance imaging (MRI), normal brain MRI at onset, and positive anti-AQP4 antibodies.[10,11]
Various studies suggest that NMO is more severe than MS, and the most effective therapeutic option in NMO is immunosuppressive (IS) rather than immunomodulatory (IM) drugs.[12,13,14]
There are four aspects of the treatment of NMO: acute treatment of relapses, prevention of relapses, symptom management, and rehabilitation.[5] After establishment of diagnosis, acute management of relapse is of prime importance. Despite the absence of evidence-based medicine studies, administration of high-dose intravenous methylprednisolone (IVMP) is typically the first treatment given to patients with NMO to reduce disease activity and further progression and restore neurologic function.[15] However, in some cases, this first-line treatment is not sufficient to reduce the inflammatory process and another strategy needs to be used, most notably therapeutic plasmapheresis (TP) with exchange transfusion. As per the American Society for Apheresis (ASFA) (Category 2, Grade 1B),[2] it is defined as a procedure in which blood of the patient is passed through a medical device which separates out plasma from other components of blood, and the plasma is removed (i.e., <15% of total plasma volume [PV]) without the use of replacement solution. This is done typically in 5–7 sessions over a period of 10–14 days.[16,17] Early initiation of PV treatment (in addition to corticosteroids and other immunosuppressant medications) is considered standard practice.
Anti-AQP4 antibody-mediated autoimmunity is implicated in the pathogenesis of NMO.[18,19,20,21,22,23] Plasmapheresis is effective in suppressing acute attacks in 50%–89% of patients with NMO.[17,24,25,26,27]
TP is effective in patients with CNS inflammatory demyelinating diseases (IDDs) who do not respond to IVMP treatment. The efficacy of plasmapheresis is due to the removal of circulating autoantibodies from the blood.[7] The exact efficacy of TP for NMO attacks may have been underestimated.[17,25] Plasmapheresis is becoming the preferred standard rescue therapy for NMO when high-dose IVMP treatment elicits only a weak response.[15,28,29] However, evidence for the therapeutic efficacy of plasmapheresis for acute attacks of NMO is still limited. Recommended technical considerations regarding TP are given in [Table 1].[2] This study assesses the clinical efficacy and safety of plasmapheresis in patients with NMO.
Table 1.
Describing technical considerations regarding therapeutic plasmapheresis

Materials and Methods
This retrospective study of a prospective cohort was conducted in a tertiary neurological care center in between January 2015 and December 2015 with following inclusion criteria: patients with clinically definite or laboratory-supported definite NMO who have undergone plasma treatment, age 14–70 years; acute neurological deficit of major proportion, affecting spinal cord function, resulting in marked impairment in activities of daily living by virtue of hemiplegia, paraplegia, or quadriplegia; no preattack neurological deficit; treatment with IVMP with trivial improvement; and patients who received PV treatment for a total of five sessions every alternate day over 10 days.
Our exclusion criteria were as follows: (1) clinical, radiological, or pathological findings that suggested diseases other than NMO, (2) recurrence or relapse of the disease, (3) inability to establish peripheral or central IV access, (4) cardiac or other conditions with increased risk from hypovolemia.
We did following observations: patients had five sessions of plasmapheresis beginning on day 1, using acid citrate dextrose for anticoagulation. Plasma was separated by discontinuous flow centrifugation on a Haemonetics® MCS® Plus 9000 Apheresis System (Braintree, MA, USA) with crystalloids and hydroxyethyl starch as replacement. Plasma replacement was done by transfusing fresh frozen plasma (FFP) after the procedure. On day 10, clinical outcome was defined as per criteria of Keegan et al. (Neurology, 2002), whether no improvement, mild improvement (slight but definite change or no impact on function), moderate improvement (obvious improvement that impacts function), and marked improvement (important difference from baseline with major functional improvement) had occurred. Patients were evaluated at follow-up visit at 6 months to determine durability of improvement and recurrent disease activity. Guidelines for mild, moderate, or marked improvement are based on scales developed by Weinshenker et al.[16]
Our statistical analysis was as follows: the outcome was based on whether moderate or marked improvement occurred. The primary analysis compared the PV treated in patients at our center, with the minimum recommended PV that should be treated as per the ASFA guidelines.
Results
A total of 429 patients had plasma treatment in 1 year. About 58 (13.5%) patients were diagnosed with NMO. Of them, 24 (41.4%) patients met our exclusion and inclusion criteria. The most common reasons for ineligibility were different diagnoses; plasmapheresis sessions less or more than 5; and patients on maintenance therapy for NMO. These 24 patients underwent plasmapheresis for acute attack of NMO. Females (age; mean [standard deviation (SD)] 33.7 [11.2] years) were more common (75.0%; n = 18). About 33.3% (n = 8) of patients tested positive for anti-AQP4 (NMO) IgG antibodies (NMO [AQP4]). Of note, 16.7% (n = 4) of patients were clinically and radiologically diagnosed with NMO though laboratory confirmation for anti-AQP4 (NMO) IgG antibodies was not done.
Against the minimum recommended PV that was supposed to be treated during entire acute therapeutic period (mean [SD] 195.5 [14.6] mL/kg bw), ([kg bw] - kilogram-body-weight) lower PV (112.7 [17.0] mL/kg bw) was treated per patients (n = 24); the difference was significant (P < 0.05). The volume treated was same across the gender (P > 0.05). There was no significant difference between volume of plasma treated in patients who were positive and negative for anti-NMO (AQP4) antibodies, respectively (P > 0.05). About 83.3% (n = 20) of patients were transfused FFP as replacement fluid. The rest were transfused with 20% human albumin. In 91.7% (n = 22) of patients, plasmapheresis was done through peripheral venous access.
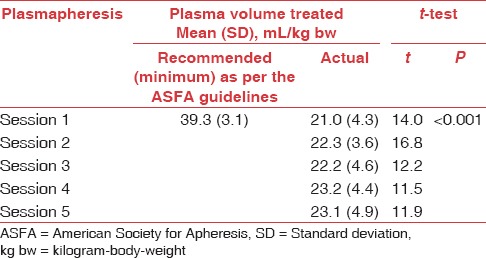
Significant clinical improvement (in neurological status and function) was observed in 79% of patients (n = 19) (13 with moderate improvement and 6 with marked improvement), 6 months after plasmapheresis. Five of 24 patients who received a course of active treatment had mild to negligible improvement. Nonresponders may have sustained severe, irreversible axonal injury. The interval required from onset to enrollment may have resulted in irreversible damage in some patients. Those who improved on active treatment tended to be younger and male although these trends were not significant. There were no significant differences in volume of plasma treated between patients who had moderate improvement and patients who had marked improvement and between patients who did and did not have significant clinical improvement (P > 0.05; for both) [Tables 2 and 3].
Table 2.
Comparison of recommended and actual plasma volume treated per session in patients with neuromyelitis optica at our center (n=24)
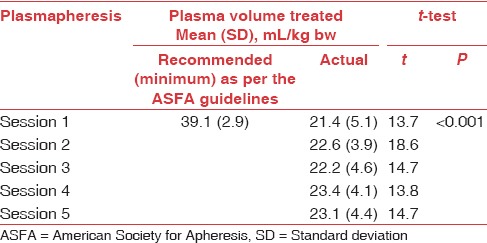
Table 3.
Comparison of recommended and actual plasma volume treated per session in patients with neuromyelitis optica that had significant clinical improvement (n=19)
Discussion
Our study was aimed to compare the low PV treatment, with conventional minimum recommended PV treatment, among NMO patients (who had acute attack) (n = 24). The clinical outcome of these patients was then assessed to ascertain the therapeutic benefit of low PV treatment. We analyzed all patients with NMO diagnosed according to the current criteria. The following elements were critical in our study: enrollment of patients unlikely to recover or going to have a long relapsing course; an early response on active treatment, which allowed us to attribute benefit to the plasma treatment being given; primary outcome, that is, clinical improvement; and concomitant immunosuppressive treatment was not administered so that we would be able to evaluate the specific contribution of plasmapheresis to neurological improvement. At our center, the decision to treat plasma with TP in severe attacks was driven by the availability and safety of this technique. As the retrospective observational nature of the study might introduce some bias (i.e., lack of follow-up data for a given attack), we designed stringent inclusion criteria to overcome these limitations. We analyzed the clinical outcome of each well-described acute attacks of NMO in patients who underwent plasmapheresis as a part of the therapeutic protocol.
Jacob et al.[5] reported age of onset of NMO around the fourth decade of life though the authors noted that the first attack may occur at any age from early childhood to elderly patients.[5] Bonnan et al.[30] in their study observed mean (SD) age 34 (14) years at disease onset, which is similar to what we observed in our study (mean [SD] age 34[11] years). Collongues and Nagaishi et al.[3,31] observed female predominance in NMO, with a female to male ratio ranging from 3:1 in France to 10:1 in Japan. Our study also noted 75.0% of female patients. Bonnan et al.[30] found 93% (40/43) of females in their study. Furthermore, in concordance with our study, Jarius et al. reported female predominance in AQP4 antibody-positive patients.[32]
Jacob et al. initiated plasmapheresis in the 2nd week (after high-dose steroids)[5] if no recovery is seen and if deficits are severe. Bonnan et al.[30] started plasmapheresis at a mean (SD) of 9.4 (10.1) days after attack onset. This was again in concordance with our data.[5]
Plasmapheresis-treated attacks achieved a significantly better outcome after a spinal attack in our study patients. Bonnan et al. also reported dramatic clinical improvement when plasmapheresis was initiated during the first attack.[30] We have observed initiation of plasmapheresis in NMO within a week of acute attack. Weinshenker et al.[16] gave a possible explanation for the variation in response to plasmapheresis. They reported the presence of a critical humoral factor in the plasma of responders.[16] The pathologic mechanisms in IDDs are heterogeneous.[32] The factor (s) could be antibodies, complement or complement components, circulating immune complexes, or cytokines.[33,34]
Genain et al. demonstrated antibodies that are directed against some specific epitope. Similar observations were made by Storch et al.[35,36] These observations are relevant to the mechanism of action of plasmapheresis in NMO.
For patients with acute, severe attacks of NMO who fail to improve after high-dose corticosteroid treatment, Weinshenker et al. have recommended seven courses of plasmapheresis.[16] Keegan et al. also reported seven sessions of plasmapheresis administered every other day for 14 days.[24] In our patients, we followed the protocol of five courses of plasmapheresis with similar clinical picture. Those who fail to respond or had mild clinical improvement; a few of them were treated with two more sessions of plasmapheresis. We processed and treated 59% less PV (22 mL/kg [0.6 PV]) than reported by Keegan et al. 16 (54 mL/kg [1.1 PV]); and Keegan et al. (55 mL/kg), per session of plasmapheresis.[24]
Keegan et al. reviewed the clinical data from 59 patients who received TP for IDDs, including 10 NMO and 6 acute transverse myelitis (ATM) cases.[24] A moderate or marked improvement was seen in 50% of NMO and ATM patient groups. They did rescue therapy with TP in patients who were on corticosteroids without prompt clinical improvement or who had myelitis attacks despite corticosteroid therapy. Our study showed moderate or marked improvement in 79% of NMO patients. Keegan et al. also noted that male sex was associated with a moderate or marked improvement.[24] Our study, in contrast, showed that female sex was associated with significant improvement. This may be explained by female predominance in our study population. This observation in our study was further collaborated by Watanabe et al.[26] They reported treatment in six female NMO-IgG-positive patients with 3–5 TP sessions, each session treating 2–3 L of plasma. One with ON and two with myelitis showed definite functional improvement following the procedure.[26]
As the volume treatment concerns patients on the whole, the late outcome at 1 year was more or less obtained by Keegan et al.[24] during the 1st month in the case of both success or failure of the treatment. We analyzed outcome of treatment at the end of 6 months, which gave us more time to definitively access clinically the success or failure of the treatment. A small number of case reports and a study of three cases were reported with variable improvement.[11,26,37,38,39,40,41,42]
As TP proved to be a promising treatment, it would now be unethical to design a study with a sham-treated group. In clinical trials of therapeutic interventions, sham treatment is an important scientific control.[43] However, we could study effect of low volume treatment on the clinical outcome of NMO patients.
Conclusion
Plasmapheresis is a safe and efficient add-on therapy in NMO, especially in steroid-resistant cases. Although the volumes of plasma treated during acute plasmapheresis were less than recommended minimum volumes, majority of patients had a significant clinical improvement. We emphasized the need and importance of plasmapheresis for NMO as a part of acute therapy, besides its role in severe relapse resistant to a high dose of corticosteroids. Owing to the low frequency of the disease, collaborative efforts will be needed for therapeutic trials in NMO. We could have been more certain of the therapeutic efficacy of low PV treatment, if we had randomly compared low-volume treatment with conventional minimal recommended PV treatment. Further randomized therapeutic trial that compares clinical improvement in patients with NMO who would and would not have their PV treated as per the ASFA recommendation is required though the pathology of NMO remains rare and randomized trials are difficult to manage.
Financial support and sponsorship
This study was financially supported by the Department of Transfusion Medicine and Hematology, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I feel privileged to offer my deepest sense of gratitude and respect to my guide, Dr. Sundar Periyavan, MD, professor and head, Department of Transfusion Medicine and Hematology, NIMHANS, for his invaluable guidance, supervision, and blessings, without which these works would not have seen the light of the day.
References
- 1.Kim SH, Kim W, Huh SY, Lee KY, Jung IJ, Kim HJ. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti-aquaporin-4 antibody levels. J Clin Neurol. 2013;9:36–42. doi: 10.3988/jcn.2013.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice. J Clin Apher. 2013;28:145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 3.Collongues N, Marignier R, Zéphir H, Papeix C, Blanc F, Ritleng C, et al. Neuromyelitis optica in France: A multicenter study of 125 patients. Neurology. 2010;74:736–42. doi: 10.1212/WNL.0b013e3181d31e35. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 5.Jacob A, McKeon A, Nakashima I, Sato DK, Elsone L, Fujihara K, et al. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2013;84:922–30. doi: 10.1136/jnnp-2012-302310. [DOI] [PubMed] [Google Scholar]
- 6.Kanamori Y, Nakashima I, Takai Y, Nishiyama S, Kuroda H, Takahashi T, et al. Pain in neuromyelitis optica and its effect on quality of life: A cross-sectional study. Neurology. 2011;77:652–8. doi: 10.1212/WNL.0b013e318229e694. [DOI] [PubMed] [Google Scholar]
- 7.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet. 2004;364:2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 8.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964–8. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 9.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–7. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet F, Mercié P, Morlat P, Hocke C, Vergnes C, Ellie E, et al. Devic's neuromyelitis optica during pregnancy in a patient with systemic lupus erythematosus. Lupus. 1999;8:244–7. doi: 10.1191/096120399678847696. [DOI] [PubMed] [Google Scholar]
- 12.Cabre P, González-Quevedo A, Bonnan M, Saiz A, Olindo S, Graus F, et al. Relapsing neuromyelitis optica: Long term history and clinical predictors of death. J Neurol Neurosurg Psychiatry. 2009;80:1162–4. doi: 10.1136/jnnp.2007.143529. [DOI] [PubMed] [Google Scholar]
- 13.de Seze J, Lebrun C, Stojkovic T, Ferriby D, Chatel M, Vermersch P. Is Devic's neuromyelitis optica a separate disease? A comparative study with multiple sclerosis. Mult Scler. 2003;9:521–5. doi: 10.1191/1352458503ms947oa. [DOI] [PubMed] [Google Scholar]
- 14.Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B, et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler. 2007;13:256–9. doi: 10.1177/1352458506070732. [DOI] [PubMed] [Google Scholar]
- 15.Collongues N, de Seze J. Current and future treatment approaches for neuromyelitis optica. Ther Adv Neurol Disord. 2011;4:111–21. doi: 10.1177/1756285611398939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinshenker BG, O'Brien PC, Petterson TM, Noseworthy JH, Lucchinetti CF, Dodick DW, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878–86. doi: 10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Magaña SM, Keegan BM, Weinshenker BG, Erickson BJ, Pittock SJ, Lennon VA, et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011;68:870–8. doi: 10.1001/archneurol.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133(Pt 2):349–61. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinshenker BG, Wingerchuk DM, Vukusic S, Linbo L, Pittock SJ, Lucchinetti CF, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol. 2006;59:566–9. doi: 10.1002/ana.20770. [DOI] [PubMed] [Google Scholar]
- 20.Matiello M, Lennon VA, Jacob A, Pittock SJ, Lucchinetti CF, Wingerchuk DM, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology. 2008;70:2197–200. doi: 10.1212/01.wnl.0000303817.82134.da. [DOI] [PubMed] [Google Scholar]
- 21.Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–29. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, et al. Neuromyelitis optica: Pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–43. doi: 10.1002/ana.21837. [DOI] [PubMed] [Google Scholar]
- 23.Kim W, Kim SH, Kim HJ. New insights into neuromyelitis optica. J Clin Neurol. 2011;7:115–27. doi: 10.3988/jcn.2011.7.3.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: Predictors of response. Neurology. 2002;58:143–6. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 25.Llufriu S, Castillo J, Blanco Y, Ramió-Torrentà L, Río J, Vallès M, et al. Plasma exchange for acute attacks of CNS demyelination: Predictors of improvement at 6 months. Neurology. 2009;73:949–53. doi: 10.1212/WNL.0b013e3181b879be. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe S, Nakashima I, Misu T, Miyazawa I, Shiga Y, Fujihara K, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128–32. doi: 10.1177/1352458506071174. [DOI] [PubMed] [Google Scholar]
- 27.Wang KC, Wang SJ, Lee CL, Chen SY, Tsai CP. The rescue effect of plasma exchange for neuromyelitis optica. J Clin Neurosci. 2011;18:43–6. doi: 10.1016/j.jocn.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019–32. doi: 10.1111/j.1468-1331.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 29.Szczepiorkowski ZM, Winters JL, Bandarenko N, Kim HC, Linenberger ML, Marques MB, et al. Guidelines on the use of therapeutic apheresis in clinical practice – Evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher. 2010;25:83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]
- 30.Bonnan M, Valentino R, Olindo S, Mehdaoui H, Smadja D, Cabre P. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler. 2009;15:487–92. doi: 10.1177/1352458508100837. [DOI] [PubMed] [Google Scholar]
- 31.Nagaishi A, Takagi M, Umemura A, Tanaka M, Kitagawa Y, Matsui M, et al. Clinical features of neuromyelitis optica in a large Japanese cohort: Comparison between phenotypes. J Neurol Neurosurg Psychiatry. 2011;82:1360–4. doi: 10.1136/jnnp-2011-300403. [DOI] [PubMed] [Google Scholar]
- 32.Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucchinetti CF, Brück W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 1996;6:259–74. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dau PC. Plasmapheresis in acute multiple sclerosis: Rationale and results. J Clin Apher. 1991;6:200–4. doi: 10.1002/jca.2920060405. [DOI] [PubMed] [Google Scholar]
- 35.Storch MK, Piddlesden S, Haltia M, et al. Multiple sclerosis: In situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–71. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 36.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–5. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 37.Okai AF, Muppidi S, Bagla R, Leist TP. Progressive necrotizing myelopathy: Part of the spectrum of neuromyelitis optica. Neurol Res. 2006;28:354–9. doi: 10.1179/016164106X98279. [DOI] [PubMed] [Google Scholar]
- 38.Nozaki I, Hamaguchi T, Komai K, Yamada M. Fulminant Devic disease successfully treated by lymphocytapheresis. J Neurol Neurosurg Psychiatry. 2006;77:1094–5. doi: 10.1136/jnnp.2005.086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguilera AJ, Carlow TJ, Smith KJ, Simon TL. Lymphocytaplasmapheresis in Devic's syndrome. Transfusion. 1985;25:54–6. doi: 10.1046/j.1537-2995.1985.25185116504.x. [DOI] [PubMed] [Google Scholar]
- 40.Flechtner KM, Baum K. Mixed connective tissue disease: Recurrent episodes of optic neuropathy and transverse myelopathy. Successful treatment with plasmapheresis. J Neurol Sci. 1994;126:146–8. doi: 10.1016/0022-510x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 41.Mitwalli AH, Memon NA, Abu-Aisha H, Al-Wakeel JS, Tarif N, Askar A, et al. Transverse myelitis in a patient with severe lupus nephritis: A case report. Saudi J Kidney Dis Transpl. 2002;13:492–7. [PubMed] [Google Scholar]
- 42.Schilling S, Linker RA, König FB, Koziolek M, Bähr M, Müller GA, et al. Plasma exchange therapy for steroid-unresponsive multiple sclerosis relapses: Clinical experience with 16 patients. Nervenarzt. 2006;77:430–8. doi: 10.1007/s00115-005-2019-1. [DOI] [PubMed] [Google Scholar]
- 43.Miller FG, Kaptchuk TJ. Sham procedures and the ethics of clinical trials. J R Soc Med. 2004;97:576–8. doi: 10.1258/jrsm.97.12.576. [DOI] [PMC free article] [PubMed] [Google Scholar]


