Abstract
INTRODUCTION:
Repeated blood transfusions can result in the production of alloantibodies against one or more red cell antigens, which complicates subsequent transfusions. Aims: The study was done to find incidence of various red cell alloantibodies; to determine the type of alloantibody; to identify the factors such as frequency of transfusion, splenectomy status, donor ethnicity and gender and their association with the development of antibody in repeatedly transfused patients.
MATERIALS AND METHODS:
This study was carried out in Dept. of IHBT, Shree M. P. Shah Medical College, Jamnagar, Gujarat. Blood was taken from the patients of thalassemia major, sickle cell disease, chronic renal failure, post partum haemorrhage, aplastic anemia, Myelodysplastic syndrome with more than 10 red cell transfusions. The plasma/serum was used for antibody screening and antibody identification test. Three cell antibody screening was performed using antihuman globulin gel cards (ID-Card LISS/Coombs) and three cell panel (ID-DiaCell I, II, III-Asia). Those with positive antibody screening were analyzed further for antibody identification test using eleven cell panel (Set ID-Dia Panel).
RESULTS:
Antibody screening and identification was done in 2 consecutive set of samples (n = 300) which showed, nine (9) patients (3%) were alloimmunized. All repeatedly transfused patients had developed alloantibody before the starting of study period, no patient developed new alloantibody during study period.
CONCLUSIONS:
Alloantibodies should be identified in repeatedly transfused patients and should be given corresponding antigen negative blood unit which will minimize the antibody mediated destruction of transfused red cells.
Keywords: Alloantibodies, antibody identification, antibody screening
Introduction
Alloimmunization to red blood cell (RBC) antigens resulting from the genetic disparities between donor and recipient is one of the risks of blood transfusion. Repeated blood transfusions can result in the production of alloantibodies against one or more red cell (RBC) antigens, which complicate subsequent transfusions. Alloantibodies can interfere in the cross-match testing and therefore can cause delay in obtaining compatible blood and also sometimes associated with delayed type of hemolytic transfusion reaction.
The probability of alloimmunization depends on the number and frequency of transfusions, antigen immunogenicity, and recipient's immune response. The influence of ethnic and antigenic pattern differences between donors and recipients has also been reported.[1]
Various diseases that require repeated red cell transfusions are thalassemia major (THAL), sickle cell diseases (SCDs), aplastic anemia (AA), myelodysplastic syndrome (MDS), chronic myeloproliferative disease and other malignancy, chronic renal failure (CRF).
Antigen matched transfusion would effectively prevent alloimmunization. To do so, the patient's ABO, Rhesus, Kell, Kidd, and Duffy systems should be typed at diagnosis or before the institution of transfusion therapy. Blood to be transfused should always be matched at least with ABO, Rhesus, and Kell system.[2] Further, using of leukocyte filter during transfusion may prevent alloimmunization due to white blood cells.
Management part of red cell alloimmunization cases include detection, identification of antibodies, and finally providing antigen negative blood for further transfusions. This study was designed to determine the prevalence of red cell alloimmunization in repeatedly transfused patients and will contribute to a better comprehension of the problem and will help to find the ideal approach for the future policy in Jamnagar population and our institute.
Aims and objectives
To initiate pretransfusion antibody screening on patient's sample before cross-match to initiate safe transfusion practice
To find out incidence of various RBC alloantibodies as well as autoantibodies in repeatedly transfused patients
To determine the type of antibody present in multiply transfused patients
To identify the factors such as frequency of transfusion, splenectomy status, donor ethnicity, and gender and their association with the development of antibody in repeatedly transfused patients
To identify common RBC alloantibodies.
Subjects and Methods
This prospective study was carried out for duration of 2 years, i.e., August 2011–July 2013 in the Department of Immunohaematology and Blood Transfusion, Shree M. P. Shah Government Medical College and G. G. Government Hospital, Jamnagar, Gujarat. Written consent was taken for each patient. This study was approved by the Ethical Committee of the Shree M. P. Shah Medical College.
Patient selection
Patients of THAL major, SCD, CRF, postpartum hemorrhage, AA, MDS with >10 transfusions
Those who require blood transfusion at 2–4 weeks interval
Repeatedly transfused patients with difficulty in cross-matching to find out matched blood
Those patients having positive direct Coombs test (DCT) and suspected to have alloantibodies, having increased requirement of blood units.
Patients having regular transfusion at our hospital were included in this study. These data were collected from patient's clinical record files.
Patient exclusion criteria
Patients with known connective tissue disorders were excluded
Patients whose clinical history and regular follow-up were not possible.
Clinical transfusion records of the patients who fulfilled the inclusion and exclusion criteria were given a questionnaire and were reviewed for the demographic data, i.e., age, sex, ethnicity, blood group, age at the start of first transfusion, number of packed cell units received, frequency of transfusion, splenectomy status, history of transfusion reactions, if any, use leukocyte filter.
Sample collection
A volume of 3–4 ml of blood was collected in plain and ethylenediaminetetraacetic acid tubes from multiply transfused patients after they met the inclusion and exclusion criteria.
The plasma/serum was used for antibody screening and antibody identification test.
The red cells were used for ABO grouping, Rh grouping, antigen phenotyping, and DCT.
Laboratory methods
Using column agglutination technology, plasma/serum was analyzed for detection of red cell alloantibodies, and again, after 6 months of interval, blood sample was collected for detection of antibody to RBC antigen.
Three-cell antibody screening was performed using antihuman globulin gel cards (ID-Card LISS/Coombs) and three-cell panel (ID-DiaCell I, II, III-Asia). Those with positive antibody screening were analyzed further for antibody identification test using eleven-cell panel (Set ID-Dia Panel).
The red cells were used for ABO grouping, Rh grouping, and DCT during antibody screening.
The entire test was done using Bio-Rad ID microtyping system.
Criteria for completion of test/further testing
Antibody identification is considered complete if:
The panel results are consistent with one or more clear-cut antibody specificities satisfying the conditions for identification
The patient types negative for corresponding antigen
The direct antiglobulin test (DAT) is negative.
Results
Out of total 300 patients, there were 178 males and 122 females who received regular blood transfusions. The age range of the patients was from 1 year to 80 years. There were 223 Hindu, 52 Muslim, 25 Sindhi patients.
Distribution of patients according to diagnosis is demonstrated in Table 1.
Table 1.
Distribution of patients by diagnosis

The packed cell units transfused were between 10 and 549 units (mean – 101.98 units). Only 4 (1.33%) patients were using leukocyte filter during blood transfusion. The low rate of using leukocyte filter during blood transfusion can be due to lack of awareness and cost-effectiveness. Sixteen (5.33%) patients had transfusion reactions in the form of mild itching, fall in blood pressure, fever, chills, rigors, and breathlessness. 10–550 transfusion episodes were tracked to capture transfusion reaction. In the present study, 12 (4%) out of total 300 patients had splenectomy, but no one was alloimmunized.
Result of antibody screening and identification
A total of nine RBC alloantibodies were detected in 9 out of total 300 patients, and none developed red cell alloantibody during the study period.
The incidence of red cell alloantibodies in two consecutive sets of samples of repeatedly transfused patients was 3% and 3% respectively. Interval between two tests was 6 months, and the patients were transfused between two intervals.
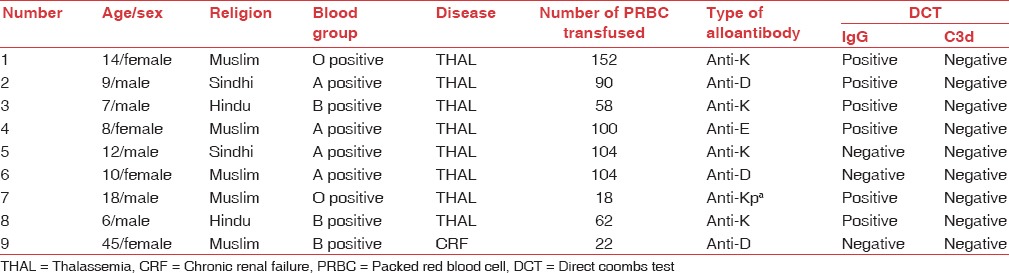
A total of nine samples showed to have alloantibody, 5 (55.55%) belonged to Kell blood group and 4 (44.44%) belonged to Rhesus system. Out of five Kell alloantibodies, four were anti-K and one was anti-Kpa. Out of four Rhesus alloantibodies, three were anti-D and one was anti-E [Table 2].
Table 2.
Data of patients with alloantibodies (n=9)
Out of 178 males, 5 (2.81%) developed alloantibodies, and out of 122 females, 4 (3.28%) developed alloantibodies [Graph 1].
Graph 1.
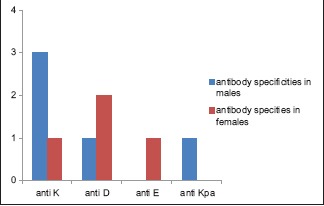
Alloantibody specificities in males and females
Out of 52 Muslims, 5 (9.61%); of 25 Sindhis, 2 (8%); of 223 Hindus, 2 (0.9%) developed alloantibodies [Graph 2].
Graph 2.
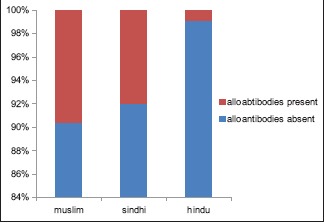
Alloimmunization rate in different caste
Rate of alloimmunization in THAL major patients was 3.2% and in CRF was 5%. Other patients did not produce alloantibody.
Number of packed cells transfused in alloimmunized patients was between 18 and 152 units (mean – 86 units).
Out of nine alloimmunized patients, six patients (66.67%) were DCT positive for IgG only while three were negative both for IgG and C3d [Table 2].
Discussion
The red cell alloantibodies develop in a variable number of multiply transfused patients. In such circumstances, transfusion therapy may become significantly complicated. Effects of alloimmunization may include difficulty in finding compatible RBC units because of the presence of clinically significant RBC antibodies, transfusion reactions, or platelet refractoriness. The present study is an effort to characterize blood group alloantibody formation in the patient population.
Rate of alloimmunization
The present study showed low rate of alloimmunization 3% which can be explained by homogeneity between the donor and the recipient population.[3]
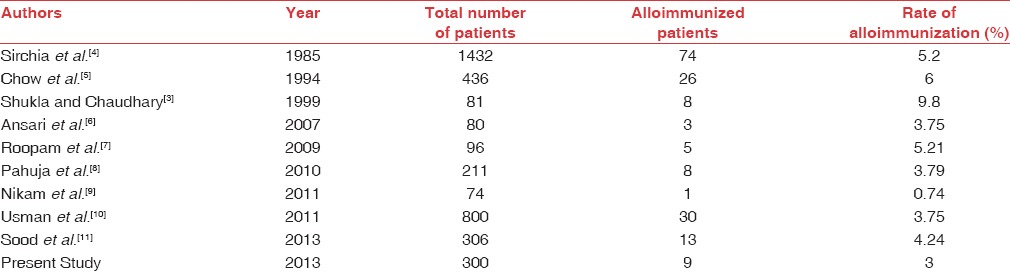
In the present study, rate of alloimmunization was consistent with other studies done internationally[4,5,6] and various studies from India [Table 3].[3,7,9,11]
Table 3.
Rate of alloimmunization in various studies
Antibody specificity (type of antibody)
In the present study, a total of nine samples showed presence of alloantibodies, four anti-K, three anti-D, one anti-Kpa, and one anti-E. Kell blood group consisted of majority of alloantibodies with 55.55% followed by Rhesus blood group with 44.44%.
The present study showed alloantibodies against Kell blood group are most common, i.e., 55.55%, which showed similar results, i.e. Khalid et al.[12] (47.06%), Bilwani et al.[13] (38.85%), and Roopam et al.[7] (80%).
Rate of red cell autoantibodies
Red cell autoantibodies are antibodies that are directed against patient's own RBC antigen. Patients may show persistent positive DCT for both IgG and C3d and can result in significant hemolysis and increased requirement for transfusions.
Out of total 300 patients, 10 patients (3.33%) were DCT positive for IgG but not for C3d, and out of nine alloimmunized patients, six were positive for DCT for IgG only. There was no evidence of autoimmune hemolytic anemia in these patients. Positive DAT did not interfere in finding compatible blood. Positive DAT may indicate alloantibodies in a recipient's circulation, reacting with antigens on recently transfused donor red cells.
The present study showed 3.33% rate of autoantibodies which shows similar to other studies reported.[11]
Gender
In the present study, out of 300 patients of repeatedly transfused, there were 178 males and 122 females. It was observed that there was increased incidence of THAL.
In the present study, the rate of alloimmunization was higher in females (3.28%) comparable with males (2.81%) which is similar to other studies.[3,7,11]
There was no alloimmunization in multiparous women.
The association between sex and alloimmunization was statistically not significant (P = 0.9122).
Age
In the present study, the age range of the patients was from 1 year to 80 years with mean age of 12.76 years. Majority of the patients in this study were more than 8 years – 55.7%.
In the present study showed that out of 300 patients of repeatedly transfused, nine showed presence of alloantibody. The age of alloimmunized individuals ranged between 6 and 45 years (mean – 14.33 years) showed results similar to other studies.[7,13]
Religion and caste
In the present study, out of 300 cases, 223 were Hindus, 52 were Muslims, and 25 were Sindhis.
In the present study, nine patients were alloimmunized; two Hindus (one Rajput, one Koli), two Sindhis, and five Muslims. Hence, alloimmunization rate was more in Muslims and Sindhis as compared to Hindu patients.
Till now, to the best of our knowledge, there has not been any study in alloimmunization regarding religion and caste in India.
Age at the first transfusion
In case of THAL major, early detection in a patient may help in treatment and preventing subsequent allo and autoantibody formation in repeatedly transfused patients.
Immune response may be affected by patient's age at the start of transfusion. Transfusion at early age (<3 years) may offer some immune tolerance and protection against alloimmunization in THAL patients.[5]
In the present study, alloimmunized patients received the first transfusion ranged from 4 months to 44 years (mean – 7.85 years), and results were similar with other studies.[15,16]
Number of packed cells transfused
In the present study, number of packed cell units transfused ranged from 18 to 152 units (mean – 78.89 units). All alloimmunized patients received more than ten transfusions correlating with other studies.[7,12,13,14,16]
Bhatti et al.[17] and Chaudhary et al.[3] showed no correlation between the number of transfusions and the alloimmunization rate. In the present study, statistical association between number of packed cell units transfused and alloimmunization was not significant (P = 0.7894). The result of the present study for association of number of packed cell units transfused as a risk factor for alloimmunization was not established.
Splenectomy
The absence of spleen may enhance the immune response to the infused foreign antigens, which are not effectively filtered. Splenectomy may enhance or promote immune reactions as there is absence of an efficient filtering system for removal for damaged RBC.
Singer et al.[18] found from their study that patients who had a splenectomy had a higher alloimmunization rate.
In the present study, nine patients were alloimmunized, but none had splenectomy. Out of total 300 patients, 12 had splenectomy. No association between alloimmunization and splenectomy (P = 0.8089) was found out in the present study similar to other studies.[9,16]
Leukocyte filter
Another important aspect that has emerged is the role of contaminating leukocytes of the allogeneic blood transfusion in causing immunomodulatory effects in the recipient. Contaminating leukocytes downregulate T-helper cell Type 1 immune responses and drive the recipient toward T-helper cell Type 2 responses. Such skewing toward Type 2 immunity may enhance alloantibody formation.[19]
In the present study, only 4 (4–1.33%) patients were using leukocyte filter, none of them were alloimmunized, the difference was not statistically significant (P = 0.2622).
In the present study, however, it was not established that leukocyte filter would prevent alloimmunization probably due to a small number of patients, in whom leukocyte filters were used during transfusions.
Conclusion
It is concluded here that red cell alloimmunization should not be overlooked in repeatedly transfused patients. It should always be considered if the patient repeatedly suffers from hemolytic transfusion reactions, difficulty in finding compatible blood during cross–match, or patients not able to maintain hemoglobin at desired level in spite of regular transfusions.
It is also concluded here that regular screening for development of alloantibodies in repeatedly transfused patients would add toward better management of these patients. With the screening and identification technique, the alloantibodies should be identified and patients should be given corresponding antigen negative blood unit which will minimize the antibody-mediated destruction of transfused red cells.
Several factors might contribute to red cell alloimmunization such as heterogeneity of population, difference in age at first transfusion, antigenic difference between the donor and the recipients, recipient's immune status, immunomodulatory effects of allogenic blood transfusion on recipient's immune status, and splenectomy.
Obtaining RBC antigenic phenotype on all repeatedly transfused patients, providing leukodepleted blood-matched for antigens of ABO, Rhesus, and Kell systems in patients who have a lifelong transfusion dependency could be effective against RBC alloimmunization.
The present study also recommended extended phenotype-matched blood transfusion for antigen against Kell and Rhesus blood group system. This will minimize the antibody-mediated destruction of transfused red cells that result in reduction of transfusion needs for the patients. Less number of transfusions reduces the psychological and financial burden on the family and will increase the patient compliance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–21. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 2.Hoffbrand AV. Postgraduate Hematology. New York: Oxford University Press Inc; 2001. Genetic disorders of haemoglobin; pp. 91–119. [Google Scholar]
- 3.Shukla JS, Chaudhary RK. Red cell alloimmunization in multi-transfused chronic renal failure patients undergoing hemodialysis. Indian J Pathol Microbiol. 1999;42:299–302. [PubMed] [Google Scholar]
- 4.Sirchia G, Zanella A, Parravicini A, Morelati F, Rebulla P, Masera G. Red cell alloantibodies in thalassemia major. Results of an Italian cooperative study. Transfusion. 1985;25:110–2. doi: 10.1046/j.1537-2995.1985.25285169198.x. [DOI] [PubMed] [Google Scholar]
- 5.Chow MP, Hu HY, Lyou JY, Lin JS, Yung CH, Lee A. Red cells, HLA and platelet antibody formation in patients with multiple transfusions. Acta Haematol. 1994;92:57–60. doi: 10.1159/000204175. [DOI] [PubMed] [Google Scholar]
- 6.Ansari S, Voosogh P, Mosthaghian S. Assessment of frequency of allo immunization and erythrocyte alloimmunisation in transfusion dependent thalassemia patients. Acta Med Iran. 2008;46:137–40. [Google Scholar]
- 7.Roopam J, Perkins J, Susan JT, Choudhury NA. A prospective study for detection and identification of red cell allo-antibodies in multiply transfused thalassemia major patients: 34th National Congress of Indian Society of Blood Transfusion and Immunohaematology. 2009:20–2. doi: 10.1007/s12288-013-0282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pahuja S, Pujani M, Gupta SK, Chandra J, Jain M. Alloimmunization and red cell autoimmunization in multitransfused thalassemics of Indian origin. Hematology. 2010;15:174–7. doi: 10.1179/102453309X12583347114013. [DOI] [PubMed] [Google Scholar]
- 9.Nikam SA, Dama SB, Saraf SA, Jawale CJ, Kirdak RV, Chondekar RP. Prevalence of red cell allo-immunization in repeatedly transfused patients with B-thalassemia in Solapur district, Maharashtra state, India. UGC-Sponsored National Level Workshop cum Seminar on “Bio-Resources for Bio-Industries and Economic Zoology” Organized by Department of Zoology, D.B.F. Dayanand College of Arts and Science, Solapur (M.S.) 2011:24–5. [Google Scholar]
- 10.Usman M, Moin S, Moinuddin M, Saeed A, Perveen R, Azmi MA. Frequency of red cell alloimmunization among patients with transfusion dependent beta thalassemia in Pakistan. Int J Hematol Oncol. 2011;21:166–9. [Google Scholar]
- 11.Sood R, Makroo RN, Riana V, Rosamma NL. Detection of alloimmunization to ensure safer transfusion practice. Asian J Transfus Sci. 2013;7:135–9. doi: 10.4103/0973-6247.115577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalid H, Younus M, Ikran N, Naseem L, Zaheer HA. Red cell alloimmunization in repeatedly transfused thalassemia major patients. Int J Pathol. 2004;2:16–9. [Google Scholar]
- 13.Bilwani F, Kakepoto GN, Adil SN, Usman M, Hassan F, Khurshid M. Frequency of irregular red cell alloantibodies in patients with thalassemia major: a bicenter study. J Pak Med Assoc. 2005;55:563–5. [PubMed] [Google Scholar]
- 14.Gupta R, Singh B, Rusia U, Goyal S. Alloimmunization to red cells in thalassemics: Emerging problem and future strategies. Transfus Apheresis Sci. 2011;45:167–70. doi: 10.1016/j.transci.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Bashawri LA, Ahmed MS, AL-Qatary AA, Ahmed MA. Red cell alloimmunization in thalassemia patients. Bahrain Med Bull. 2005;27:1–5. [Google Scholar]
- 16.Chaudhari CN. Red cell alloantibodies in multiple transfused thalassaemia patients. Med J Armed Forces India. 2011;67:34–7. doi: 10.1016/S0377-1237(11)80008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatti FA, Salamat N, Nadeem A, Shabbir N. Red cell immunization in beta thalassaemia major. J Coll Physicians Surg Pak. 2004;14:657–60. [PubMed] [Google Scholar]
- 18.Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly asian descent. Blood. 2000;96:3369–73. [PubMed] [Google Scholar]
- 19.Clark JA, Tanley PC, Wallace CH. Evaluation of patients with positive direct antiglobulin tests and nonreactive eluates discovered during pretransfusion testing. Immunohematology. 1992;8:9–12. [PubMed] [Google Scholar]


