Abstract
BACKGROUND:
Fresh frozen plasma (FFP) should be thawed before transfusing to the patient. Prolonged or uncontrolled thawing can denature plasma proteins. The potential risk of contamination by wet thawing had always been a point of concern.
AIMS:
Here, we compared and evaluated the effect of thawing on clotting factor activities by two different methods (wet and dry) and other factors such as risk of bacterial contamination, throughput, turnaround time, and efficacy of thawing.
SUBJECTS AND METHODS:
All FFPs were prepared from Group O donors and stored at −40°C. Twenty-one FFPs were thawed in Plasmatherm II at 45°C for 15 min and another 21 were thawed in thawing bath at 37°C for 20–30 min randomly. Analysis of prothrombin time, activated partial thromboplastin time, fibrinogen, and factor VIII was done in ACL TOP 300 (IL) at the time of preparation and immediately after thawing of FFPs. Volume, duration of thawing, ease of use, accessibility, and equipment maintenance were also compared.
RESULTS:
There was a statistically significant difference in coagulation parameters after thawing in both methods compared to the time of preparation (P < 0.05), but all values were within normal limits. There was no significant difference in coagulation parameters between the two methods (P > 0.05). Mixed bacterial growth was observed from swabs taken from the water bath.
CONCLUSION:
Plasmatherm II can be a good alternative to water bath to rapidly thaw FFPs by preserving coagulation factors and eliminating the risk of bacterial contamination.
Keywords: Bacterial contamination, clotting factor activity, dry thawing, fresh frozen plasma, thawing time
Introduction
Fresh frozen plasmas (FFPs) are prepared from whole blood and stored at −18°C or lower for 1 year according to AABB guidelines[1] and indicated in coagulation factor supplementation in clinical hemotherapy. Labile coagulation factors (Factor V and VIII) in FFP can be lost over storage or thawing,[2] hence, following quality guidelines, Good Manufacturing Practice and manufacturer's instructions help in maintaining the integrity of the product. However, the risk of obtaining transfusion-associated bacterial sepsis is still hovering over the current practice because of the bacterial contamination of the water used in traditional wet water baths, especially by Pseudomonas species.[3,4,5]
The efficacy of thawing devices depends on the speed of thawing and the detection of activated coagulation factors after the thawing procedure.[6] The rationale for comparing traditional water bath and Plasmatherm II was to investigate the efficacy of this newer technology in maintaining coagulation factors of FFP. The primary objective of the study was to compare and evaluate the thawing process on the activity of clotting factors and the secondary aim was to analyze other factors such as risk of bacterial contamination, throughput, turnaround time, and efficacy of thawing by these methods.
Subjects and Methods
This was a prospective observational study conducted for 3 months in the department of transfusion medicine in a tertiary care hospital, Kerala. Forty-two blood group O FFPs were used for the study purpose. All plasmas were prepared by platelet-rich plasma method from 450 ml of whole blood within 1 h of collection. Whole blood was centrifuged in Cryofuge 6000i by Thermo Scientific at 3000 rpm for 10 min. Plasma was expressed using manual plasma extractor (Fenwall). Soon after preparation, all FFPs were stripped, properly mixed, and assessed for prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, and factor VIII. Meanwhile, FFPs were snap frozen at −80°C and shifted to −40°C freezer after transfusion-transmitted infection screening within 24 h for further storage.
All the confounding factors during collection and preparation were removed except for the interunit variability which was nullified by randomized selection of samples. This was achieved by including only blood Group O plasmas, phlebotomy was done by a single person, and separation within 1 h of collection, FFP separation, stripping, and mixing were done by a single technician who was assigned for the job, same freezers for freezing FFPs; coagulation parameters were analyzed within 15 min by a single person.
FFPs were randomly allotted to thaw either in water bath (CB-705, Remi) or in Plasmatherm II (Barkey, LeopoldshÖhe, Germany). Out of the 42 FFPs prepared, 21 were thawed in covered circulating water bath at 37°C for 20–30 min using protective plastic overwraps and visually checked for the completeness of thawing without any manipulation in between the process.
The remaining 21 FFPs were thawed in Plasmatherm II by interposing FFPs in between two silicon cushions which will get filled with water when thawing starts, hence avoiding direct contact with water. Plasmatherm II is equipped with a paddle in between the cushion bags which helps cycle water so as to maintain uniform temperature during thawing. The preset thawing temperature and time were 45°C and 15 min, respectively. After 15 min of thawing, Plasmatherm II automatically switches off with a beeping alarm indicating that thawing is completed and thus prevents uncontrolled thawing.
To study the effect of thawing on coagulation factor activity samples for coagulation assay were prepared from segments of each bag. Every sample was tested for PT and fibrinogen using HemosIL reagent, aPTT using SynthASil reagent, factor VIII using a partially activated thromboplastin, FVIII-deficient plasma. All these coagulation parameters were performed in automated coagulation analyzer ACL TOP 300 (IL) as per manufacturer's recommendations.
Our laboratory reference values of FFP are PT: 9.6–12.6 s, aPTT: 31.6–40.8 s, factor VIII: >0.7 IU/ml, fibrinogen: 200–400 mg/dL (obtained from calculating mean and standard deviation [SD] from the previous values).
For checking the risk of bacterial contamination of FFP bags, multiple swabs were taken from water bath and surface of silicon cushions of Plasmatherm II. The swabs were cultured in brain-heart infusion broth and incubated overnight followed by subculturing in blood agar and MacConkey agar. Volume, throughput, accessibility, and ease of use of both this equipment were also compared.
Inclusion criteria
Only Group O donations during the study tenure
Donor age Group 18–30 years
FFPs prepared within 1 h of collection
Coagulation assays done within 15 min of product preparation.
Exclusion criteria
Donations from outdoor camps
The bags which had prolonged thawing
Damaged bags during the procedure
Tests which showed failed results in coagulation analyzer
Whole-blood collection, component preparation, and coagulation assays done by a staffs who were not assigned for the study.
Statistical tools used
Sample size calculated using nMaster (2.0) based on 95% confidence interval (CI) and the power of the test was 80%. All results were expressed as mean ± SD and P value was compared with alpha (α) at 5% level. If P < 0.05, the results are considered statistically significant. Independent t-test (t-test for equality of mean) was used for between the group comparisons.
Results
The mean volume of 21 bags thawed in water bath was 206.04 ± 3.61 (201–212 ml) and the mean volume of FFPs thawed in Plasmatherm II was 203.62 ± 3.01 (200–209 ml); hence, volumes of FFPs thawed in both methods were comparable.
Mean duration of thawing in water bath was 24.3 ± 2.35 min (20–30) which was significantly higher (P < 0.0001) than the duration in Plasmatherm II (15 min).
In this study, there is a statistically significant difference in coagulation screening tests and coagulation factors after thawing in both methods when compared to the parameters at the time of preparation (P < 0.05), but all values were within normal limits [Tables 1a and b].
Table 1a.
The mean prothrombin time, activated partial thromboplastin time, before freezing, and after thawing and their P values dependent on the thawing procedure
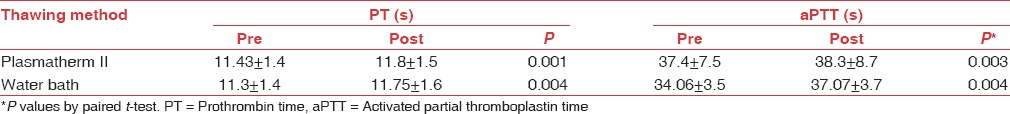
Table 1b.
The mean fibrinogen and factor VIII, before freezing, and after thawing and their P values dependent on the thawing procedure
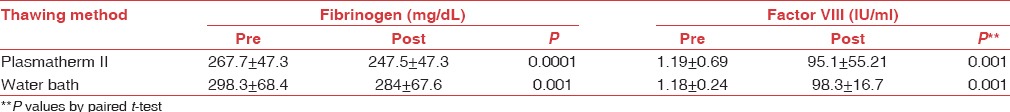
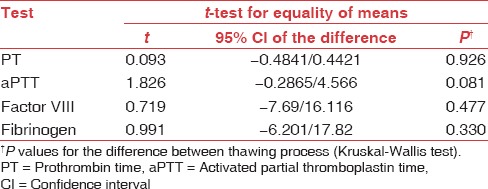
The two methods were compared using Kruskal–Wallis test (t-test for equality of means). There was no statistically significant difference in coagulation parameters in both methods (P > 0.05). The parameters were analyzed after comparing the P values from degrees of freedom and 95% CI [Table 2].
Table 2.
Between the group comparison using Kruskal-Wallis test
Multiple swabs were collected from the water bath for sterility testing and identified mixed bacterial growth predominated by Pseudomonas species; meanwhile, the swabs from Plasmatherm II showed a sterile culture.
Discussion
Denaturation of the plasma protein, especially heat-labile clotting factors are the important factor to be taken care of while thawing. In both methods, we could observe there is a significant reduction in clotting parameters after thawing when compared to parameters at the time of preparation. This can be explained by the influence of temperature, length of storage, freezing methods, and thawing upon coagulation factors. Even though there is a statistically significant reduction in factor VIII and fibrinogen, these values remained within normal limits.
We found that there was no significant difference in coagulation factor activity between two thawing systems. This could be due to shorter exposure of FFP in higher temperature counteracts the prolonged thawing in lower temperature. The current study results were in parallel with Westphal et al.[7] They compared FFP thawing in 37°C and 56°C water bath and reported that there was no significant difference in coagulation parameters between these two temperatures.[8] Plotz and Ciotola[9] also observed same findings using satellite bags where the temperatures they studied were 37°C and 45°C provided immediate removal of FFP from the water bath when they reached a slushy consistency at 45°C.[10] Since they had used satellite bags, their thawing time was markedly reduced unlike the current study.
Duration of thawing was significantly less in Plasmatherm II compared to water bath (P < 0.0001). Advantage of thawing in higher temperature is the reduced thawing time, which is very crucial to secure FFP in emergency scenarios and in massive transfusions. The rapid thawing also facilitates the rational usage of blood component thereby reducing inappropriate transfusions and product discard.
Mixed bacterial growths were observed in the samples taken from the water bath; Pseudomonas was the predominant isolate. The data on the frequency of bacterial contamination of FFP are not available unlike other blood components.[11] There are five reported cases of bacterial contamination of FFP from 2002 to 2003 in Canada and from 1997 to 2007 in Germany.[3,10] McCullough J reported three cases of Pseudomonas septicemia associated with cryoprecipitate thawing.[11] They further addressed that even 0.025 ml of water is capable of causing bacterial contamination of FFPs in their follow-up study.[4] Hence, they recommended the use of plastic overwraps for thawing to reduce the chance of bacterial contamination. Furthermore, micropur tablets, which are commercially available chlorine dioxide tablets, are added onto the distilled water used in Plasmatherm to prevent bacterial growth.
The maintenance of Plasmatherm II is uncomplicated with a weekly cleaning which requires only 5 min and water needs to be changed only yearly unlike water bath which needs frequent water change. Prolonged or uncontrolled thawing can be prevented in Plasmatherm II as the equipment automatically switches off after the process completion which makes it very user-friendly. Since Plasmatherm II is an automated device it is better to warm red blood cell, stem cells, and infusion fluids at 37°C. This is equipped with a leakage sensing alarm and can also connect to local area network, log printer, and barcode scanner which are useful in blood banks where blood bank software is used and traceability of the product will be much easier. Hence, the drawbacks of conventional water bath are taken care by Plasmatherm II and proved to be a reliable alternative to water bath. At a single point of time, we can only thaw maximum of 4 FFPs in Plasmatherm II; however, multiple bags can be thawed in water baths.
Our study limitations include the lack of comparison of labile clotting factor V due to the unavailability of the kit, and we did not study albumin, protein S, protein C, D-dimer, fibrin monomer, ADAMTS13, or any other plasma proteins.
Conclusion
As an alternative to traditional waterbaths, Plasmatherm II can be used to thaw FFPs at a higher temperature without any significant impact on the clotting factors thus reducing the turnaround time which comes to the fore in emergency scenarios. By eliminating the risk of bacterial contamination, it helps to improve the safety and efficacy of transfusion practice and it can also be used as a blood warmer in conditions such as exchange transfusions, stem cell transfusion and cold agglutinin disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dumont LJ, Papari M, Aronson CA, Dumont DF. Whole-blood collection and component processing. In: Fung MK, Grossman BJ, Hillyer DC, Westhoff CM, editors. AABB Technical Manual. 18th ed. Bethesda, Maryland: AABB; 2014. pp. 135–61. [Google Scholar]
- 2.Vazquez R, Larson DF. Plasma protein denaturation with graded heat exposure. Perfusion. 2013;28:557–9. doi: 10.1177/0267659113498921. [DOI] [PubMed] [Google Scholar]
- 3.Keller-Stanislawski B, Lohmann A, Günay S, Heiden M, Funk MB. The German Haemovigilance system – Reports of serious adverse transfusion reactions between 1997 and 2007. Transfus Med. 2009;19:340–9. doi: 10.1111/j.1365-3148.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 4.Rhame FS, McCullough J. Follow-up on nosocomial P. cepacia infection. Morb Mort Weekly Rep. 1979;28:490. [Google Scholar]
- 5.Casewell MW, Slater NG, Cooper JE. Operating theatre water-baths as a cause of Pseudomonas septicaemia. J Hosp Infect. 1981;2:237–47. doi: 10.1016/0195-6701(81)90043-8. [DOI] [PubMed] [Google Scholar]
- 6.von Heymann C, Pruss A, Sander M, Finkeldey A, Ziemer S, Kalus U, et al. Thawing procedures of and the time course of clotting factor activity in fresh frozen plasma: A controlled laboratory investigation. Anaesth and Analg. 2006;103:969–74. doi: 10.1213/01.ANE.0000240416.56803.5B. [DOI] [PubMed] [Google Scholar]
- 7.Westphal RG, Tindle B, Howard PL, Golden EA, Page GA. Rapid thawing of fresh frozen plasma. Am J Clin Pathol. 1982;78:220–2. doi: 10.1093/ajcp/78.2.220. [DOI] [PubMed] [Google Scholar]
- 8.Walther-Wenke G, Däubener W, Heiden M, Hoch J, Hornei B, Volkers P, et al. Effect of safety measures on bacterial contamination rates of blood components in Germany. Transfus Med Hemother. 2011;38:231–5. doi: 10.1159/000330417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plotz RD, Ciotola RT. Thawing of fresh-frozen plasma at 45 degrees C versus 37 degrees C. Comparison using satellite packs of the same donor units. Am J Clin Pathol. 1988;89:381–4. doi: 10.1093/ajcp/89.3.381. [DOI] [PubMed] [Google Scholar]
- 10.Transfusion Transmitted Injuries Surveillance System Program Report. Public Health Agency of Canada. 2002-2003 [Google Scholar]
- 11.Rhame S, McCullough J, Cameron S, Streifel, White K. Pusedomonas cepacia infections caused by thawing cryoprecipitate in a contaminated water bath. Transfusion. 1979;19:653–4. [Google Scholar]


