Abstract
INTRODUCTION:
Hyperleukocytosis (HL) and leukostasis seen in myeloid leukemias are a medical emergency. We present a case series of ten such patients in a 4-year period. Sixteen therapeutic leukocyte reduction (TLR) were done in ten cases along with other supportive measures. The American Society for Apheresis supports the routine implementation of TLR in cases of HL secondary to myeloid leukemias with signs of leukostasis.
MATERIALS AND METHODS:
The procedures were performed on the intermittent flow cell separator after discussion with the treating physician about patient's condition. Clinical, demographic, analytical, and technical variables were reviewed retrospectively and the patients were followed up at the end of 4 years. Descriptive analysis was performed for all variables, and relationships between quantitative variables and categorical variables were determined by applying the Student's t-test.
RESULTS:
The mean age of presentation was 34 years. Priapism was the most common symptom followed by respiratory distress and neurological disturbances. After an average of 1.6 TLR procedures, the mean leukocyte count reduction achieved was 39.9% along with symptomatic relief. The mean survival at 4-year follow-up was 12.8 months and the overall mortality was 20%. Acute myeloid leukemia patients presented with lower mean platelet counts compared to chronic myeloid leukemia patients; however, the platelet loss in the final product was minimized.
CONCLUSION:
TLR is a safe and effective therapy for leukoreduction in hematological malignancies in our experience.
Keywords: Hyperleukocytosis, leukemia, leukocytapheresis, therapeutic leukocyte reduction, tumor lysis syndrome
Introduction
Hyperleukocytosis (HL) is defined as a leukocyte count >100 × 109/L.[1] Cases of acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) may present with HL leading to leukostasis, tumor lysis syndrome (TLS), and/or disseminated intravascular coagulation (DIC).[2] Leukostasis refers to end-organ complications due to microvascular leukoaggregates, hyperviscosity, tissue ischemia, infarction, and hemorrhage that are not attributable to infectious, thromboembolic, or other underlying etiologies.[2] The development of leukostasis depends on the number of leukocytes and on factors such as different morphological, molecular, and plasticity characteristics of the blast cells,[1] as well as the capacity of the endothelial cells to release the cytokines. AML may present with leukostasis at leukocyte counts above 100 × 109/L with severe symptoms. However, symptoms may not appear in CML until the leukocyte count exceeds 500 × 109/L–1000 × 109/L.[1,2] HL mainly affects the central nervous system and respiratory system. In males, it may affect the genito-urinary system presenting as priapism.[1,3,4] Adequate measures for managing this medical emergency include hydration, cytoreduction, management of TLS, and therapeutic leukocyte reduction (TLR), especially in cases complicated by leukostasis and hyperviscosity syndrome.[2] The rationale behind performing TLR lies in the fact that it influences cell differentiation in the bone marrow by increasing the proportion of blast cells in S-phase. This translates into increased efficiency of certain antineoplastic agents such as cytarabine and methotrexate.[1]
The American Society for Apheresis (ASFA) supports the routine implementation of TLR in cases of HL secondary to AML with signs of leukostasis (ASFA indication Level I, evidence Level 1B), whereas the evidence for the efficacy of prophylactic TLR in acute lymphoblastic leukemia (ALL) is controversial (indication Level III, evidence Level 2C).[2] The benefits of TLR in patients with HL have been analyzed in nonrandomized and prospective studies. Retrospective analyses suggest that TLR combined with chemotherapy reduces early mortality; however, the effect on long-term prognosis is unknown.[5,6,7,8] Few studies have not been able to assess the short-term benefits of performing TLR.[9,10,11] We present a retrospective analysis aimed at studying the safety and effectiveness of TLR as a leukoreduction strategy in patients presenting with HL with features of leukostasis, hyperviscosity symptoms, and TLS.
Materials and Methods
We retrospectively reviewed TLR procedures carried out over a period of 4 years (May 2012 to May 2016) for the treatment of HL at the department of transfusion medicine of a 620-bedded tertiary care oncology hospital. The procedures were conducted not only in-house, but also in other departments such as intensive care unit and the casualty unit of the hospital depending on the patient's condition, mobility, and the presence of the treating physician. Patient's clinical condition was discussed with the treating physician, and the relevant clinical data were obtained from patients' electronic medical records (http//:intranet.tmc.gov.in/web/emr). The procedure-related information was documented and maintained by the transfusion medicine department. TLR was considered in patients with a leukocyte count > 100 × 109/L and with symptoms of leukostasis (ASFA indication Level I, evidence Level 1B). Clinical, demographic, analytical, and technical variables were reviewed.[12] We defined the presence of TLS on the basis of the following criteria: hyperkalemia (K+ >6 mmol/L), hyperuricemia (>10 mg/dL), hyperphosphatemia (>10 mg/dL), hypocalcemia (<8.6 mg/dL and symptomatic), and uremia (>42.8 mg/dL).[13] Early mortality was defined as death within the first 14 days after TLR procedure.
As per our center's protocol, TLR was performed through a peripheral venous access (ante-cubital region) or a central venous catheter depending on the status of the peripheral veins. We used peripheral venous access in eight patients and central venous access in two patients. Provision of administration of fluid replacement and medicine was made through a separate peripheral venous access using an intravenous cannula. Haemonetics MCS+ (Haemonetics Corp, Braintree, Massachusetts, USA) intermittent flow system was used for performing TLR. The advantage of this cell separator is its portability, thus making it easier to perform TLR according to the requirement. The cell separator calculated the total blood volume (TBV) based on the parameters such as height and weight entered into the machine, and we assessed the number of cycles to be performed for each patient based on the TBV. The volume to be processed is usually 1.5–2 blood volumes. Acid-citrate-dextrose solution A was used as the anticoagulant, and the blood/anticoagulant ratio ranged between 8:1 and 10:1. MCS + cell separator is provided with a “Centrisurge” option which is kept in the “OFF” mode when platelets are to be extracted along with the leukocytes in the final product. In the case of symptomatic hypocalcemia, calcium gluconate (10% in 100 ml normal saline) was administered intravenously as a slow infusion. The symptoms in the patients observed were paresthesias, tingling numbness, and infrequently carpopedal spasm. As <15% of TBV was removed, normal saline was used to maintain blood pressure during the procedure. Crystalloids were infused for volume replacement.
Statistical methods
Descriptive analysis was performed for all variables, determining the frequencies and percentages for qualitative variables and measures of central tendency (mean ± standard deviation) for quantitative variables. Relationships between quantitative variables and categorical variables were determined by applying the Student's t-test. P < 0.05 was considered statistically significant. We used SPSS for windows software, version 22.0, (IBM Corp. Released 2013. Armonk, NY, USA) for the statistical analyses.
Results
Between May 2012 and May 2016, the TLR procedures were performed on ten patients with a mean age of 34 (range: 12–59 years) years [Table 1]. AML patients presented at a younger age (21.75 years) in comparison to CML patients (42.67 years) [Table 2]. The most frequent diagnosis was CML (6 patients) followed by AML (4 patients). The most frequent symptom was priapism, almost exclusively limited to patients with CML. One case of pediatric AML presented with priapism. Respiratory distress and neurological disturbances were the most common symptoms in patients with AML [Table 1]. Seven patients (five CML and two AML) had biochemical findings suggestive of TLS before undergoing TLR and were put on anti-TLS measures before the procedure [Table 2]. Seven patients were started on cytoreductive therapy with hydroxyurea before initiating TLR [Table 1]. The time interval from initiation of cytoreductive therapy to start of TLR procedure was <24 h in these cases. The mean initial leukocyte count was 312 (±134.56) × 109/L, which reduced to 208.3 (±120.66) × 109/L at the completion of all TLR sessions. A statistically significant drop in initial leukocyte counts was observed in all patients (P < 0.05) after an average of 1.6 TLR sessions; with the average decrease being 107.1 (±32.06) × 109/L (39.9% with respect to the initial counts). There was no significant difference in leukoreduction rates between CML (31.12%) and AML (40.62%) patients [Table 2]. Patients with AML presented with lower mean platelet counts [Table 2], and the cell separator's Centrisurge function was kept “ON” to avoid platelet loss in the final product [Figure 1] and it was kept “OFF” in CML patients to extract platelets along with the leukocytes due to their high counts [Figure 2]. AML patients also had lower mean calcium levels. Hemoglobin values fell by an average of 1 g/dL, hematocrit decreased by 2.31%, and platelet counts dropped on an average of 101.44 × 109/L. Platelet counts reduced by 219.16 × 109/L for each CML patient and by 24.5 × 109/L for each AML patient after performing TLR. Detailed comparison between cases of CML and AML is represented in Table 2.
Table 1.
Clinical and analytical characteristics of AML and CML patients
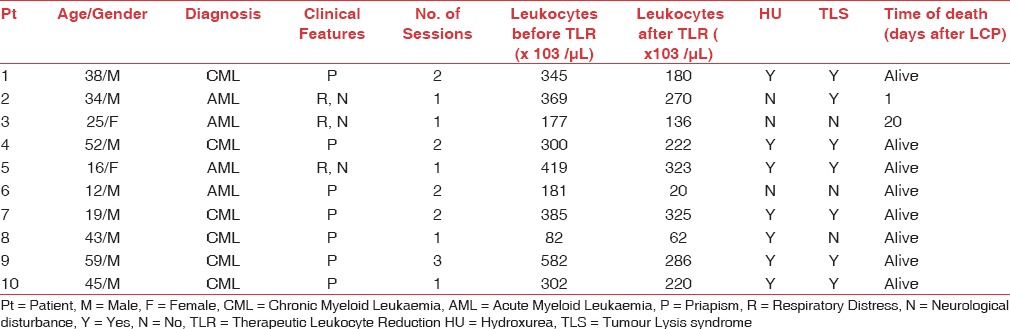
Table 2.
Results in patients with CML vs AML
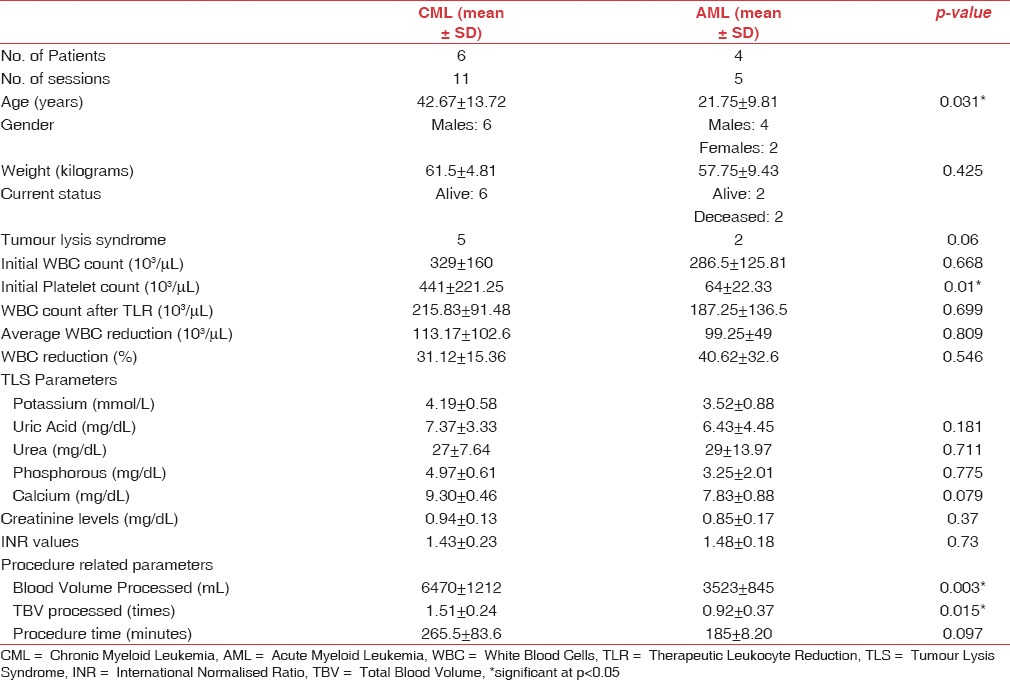
Figure 1.

Layers of red blood cells and leukocytes (buffy coat) after therapeutic leukocyte reduction in an acute myeloid leukemia patient
Figure 2.

Layers of red blood cells, leukocytes, and platelets (buffy coat) after therapeutic leukocyte reduction in a chronic myeloid leukemia patient
The mean follow-up time of the patients was 12.8 ± 10.46 months (range 3–30), with a mortality rate of 20% (2/10) of patients by the end of the study. Almost 80% (8/10) of the patients are alive as assessed on follow-up till August 2016. One patient expired 1 day after the TLR session due to a cardiac event secondary to the underlying disease. Another patient expired on day 20 of performing the procedure due to sepsis related to the underlying condition. Both the deceased were suffering from AML.
A total of 16 sessions were performed on ten patients with each patient undergoing an average of 1.6 (range: 1–3) TLR sessions. The mean weight of patients undergoing TLR was 60 (range: 45–67) kg. In each session, 1.32 times (range: 0.4–1.8) whole blood volumes were processed, equivalent to 5573.75 mL (range: 1483–8512 mL), and the average normal saline replacement volume was 915.62 mL (range: 600–1500 mL). The mean buffy coat volume discarded was 568 mL (range: 180–1000 mL). Patients with CML required 1–3 TLR sessions, whereas AML patients received 1–2 sessions. The TLR procedure was initiated within 6 hours of receiving the request from the treating physician and the sessions were conducted as emergencies on weekends. Procedure-related adverse events were observed in two sessions (12.5%). On both occasions, the event was related to blood flow interruptions. None of these events were severe, but on one occasion, the session was cancelled as no alternate peripheral venous access was available. There was no correlation between the adverse effects and gender, pathology, or age of the patients. Both adverse events occurred in AML patients.
Discussion
HL is managed by intensive supportive care and cytoreduction. Aggressive hydration and allopurinol are given to prevent TLS as supportive care measure. Respiratory support is provided as required. The cytoreduction may be achieved by TLR, induction chemotherapy, and hydroxyurea.[14] A critical problem is that, if the white blood cell (WBC) counts are not reduced before induction therapy, TLS and DIC may be aggravated with the induction treatment.[15]
We performed TLR procedures for ten patients over a period of 4 years from May 2012 to May 2016. Our case series results are comparable to that published in literature in terms of diagnosis, clinical presentation of leukostasis, and causes of death.[5,6,7,8,9,10,11,16,17,18,19] Our patients had a low frequency of neurological (3/10) or hemorrhagic (0/10) symptoms secondary to leukostasis compared to that reported in literature.[6,7,9,10,17,18,19] Priapism was the only symptom in the cases of CML. We performed TLR promptly on the request of the treating physician. Despite the fact that we were treating patients with acute medical emergencies, no important adverse event related to apheresis procedure was reported as we have an effective, trained team to attend in these situations. Central venous catheter insertion was performed in cases where the peripheral venous access was poor.
Our recent survival follow-up shows a mean of 12.8 months which is longer than that in other studies (6.5 months, 10.5 months, and 10 days, respectively).[6,7,16] Five of our CML patients and two of our AML patients were having TLS at the time of presentation and were given anti-TLS measures before TLR. The biochemical values and procedural parameters of our case series are comparable to those published by other centers.[5,6,7,16,17]
The early mortality rate in our case series, i.e., 1/10 (10%) is lower than that reported by Tan et al.[5] (28%) and Bug et al.[6] (25%). The overall mortality in our case series was seen in only AML patients and this incidence was 2/4 (50%). The rates published for AML patients by Chang et al.[9] and De Santis et al.[8] were 33% and 46%, respectively. Hydroxyurea was administered to seven out of the ten patients either prior or at the initiation of TLR. For the remaining three patients, hydroxyurea was not started based on clinical judgment, as these were CML cases in chronic phase without blast crisis. In five of the seven patients who received hydroxyurea therapy, the survival rate was over 360 days, which may be attributable to synergy between TLR and cytoreductive chemotherapy, although the influence of each of these factors on survival rate can be established only through large, well-planned randomized controlled trials.
When comparing results in patients with AML versus CML, we conclude that the former has worse outcomes after TLR procedures, in terms of survival and mortality. HL has been found to be a poor prognostic factor in AML in majority of the studies.[1,15,20] Common arbitrary cutoff suggesting poor prognosis for AML is WBC counts >50,000/μl and >400,000/μl for ALL.[20] Our AML patients presented with HL [Table 2] coupled with a high proportion of blast cells (~80%). As myeloblasts are larger in size than lymphoblasts and lymphocytes, leukostasis is more frequent in AML than in ALL and chronic lymphocytic leukemia.[20] AML patients with HL have demonstrated lower complete remission rates, disease-free survival and overall survival, as well as high rates of early mortality in different studies.[15,20] For CML cases in our study, the blast counts were <1% and the symptoms manifested at counts above 300,000/μL [Table 2]. AML patients presented with lower mean platelet counts as compared to CML patients. Comparable findings have been reported by Gong et al.[15] Centrisurge “ON” feature of the cell separator helped to avoid platelet loss in AML patients.
TLR is not an oft-performed procedure in our setup and the approximate frequency is three cases per year. We performed all the procedures on intermittent flow separator due to ease of performance, availability of staff trained on this cell separator, and the feasibility of avoiding platelet loss during the TLR. There is a possibility of rapid deterioration in the patient's clinical status and a good response can be achieved if the procedure is well timed. TLR may fail to decrease the leukocyte count substantially or may achieve only a transient tumor bulk reduction in some cases. However, it is advisable to try and aim for at least a 20% reduction in leukocyte counts for achieving symptomatic relief.
To summarize, after an average of 1.6 sessions of TLR, we found that the leukocyte reduction was significant in both AML and CML patients, and in conjunct with chemotherapy, it was lifesaving. Even though our case series is of a small number, it was invaluable in terms of the results achieved.
Conclusion
TLR is a safe and effective therapy for leukoreduction in hematological malignancies in our experience.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
All authors fulfill the following three criteria: substantial contributions to research design, or the acquisition, analysis, or interpretation of data, drafting the paper or revising it critically, and approval of the submitted and final versions.
References
- 1.Ganzel C, Becker J, Mintz PD, Lazarus HM, Rowe JM. Hyperleukocytosis, leukostasis and leukapheresis: Practice management. Blood Rev. 2012;26:117–22. doi: 10.1016/j.blre.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing Committee of the American Society for Apheresis: The seventh special issue. J Clin Apher. 2016;31:149–62. doi: 10.1002/jca.21470. [DOI] [PubMed] [Google Scholar]
- 3.Jain R, Bansal D, Marwaha RK. Hyperleukocytosis: Emergency management. Indian J Pediatr. 2013;80:144–8. doi: 10.1007/s12098-012-0917-3. [DOI] [PubMed] [Google Scholar]
- 4.Keoghane SR, Sullivan ME, Miller MA. The aetiology, pathogenesis and management of priapism. BJU Int. 2002;90:149–54. doi: 10.1046/j.1464-410x.2002.02825.x. [DOI] [PubMed] [Google Scholar]
- 5.Tan D, Hwang W, Goh YT. Therapeutic leukapheresis in hyperleukocytic leukaemias – The experience of a tertiary institution in Singapore. Ann Acad Med Singapore. 2005;34:229–34. [PubMed] [Google Scholar]
- 6.Bug G, Anargyrou K, Tonn T, Bialleck H, Seifried E, Hoelzer D, et al. Impact of leukapheresis on early death rate in adult acute myeloid leukemia presenting with hyperleukocytosis. Transfusion. 2007;47:1843–50. doi: 10.1111/j.1537-2995.2007.01406.x. [DOI] [PubMed] [Google Scholar]
- 7.Giles FJ, Shen Y, Kantarjian HM, Korbling MJ, O'Brien S, Anderlini P, et al. Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long-term survival. Leuk Lymphoma. 2001;42:67–73. doi: 10.3109/10428190109097677. [DOI] [PubMed] [Google Scholar]
- 8.De Santis GC, de Oliveira LC, Romano LG, Almeida Prado Bde P, Jr, Simoes BP, Rego EM, et al. Therapeutic leukapheresis in patients with leukostasis secondary to acute myelogenous leukemia. J Clin Apher. 2011;26:181–5. doi: 10.1002/jca.20290. [DOI] [PubMed] [Google Scholar]
- 9.Chang MC, Chen TY, Tang JL, Lan YJ, Chao TY, Chiu CF, et al. Leukapheresis and cranial irradiation in patients with hyperleukocytic acute myeloid leukemia: No impact on early mortality and intracranial hemorrhage. Am J Hematol. 2007;82:976–80. doi: 10.1002/ajh.20939. [DOI] [PubMed] [Google Scholar]
- 10.Porcu P, Danielson CF, Orazi A, Heerema NA, Gabig TG, McCarthy LJ. Therapeutic leukapheresis in hyperleucocytic leukaemias: Lack of correlation between degree of cytoreduction and early mortality rate. Br J Haematol. 1997;98:433–6. doi: 10.1046/j.1365-2141.1997.1943011.x. [DOI] [PubMed] [Google Scholar]
- 11.Thiébaut A, Thomas X, Belhabri A, Anglaret B, Archimbaud E. Impact of pre-induction therapy leukapheresis on treatment outcome in adult acute myelogenous leukemia presenting with hyperleukocytosis. Ann Hematol. 2000;79:501–6. doi: 10.1007/s002770000162. [DOI] [PubMed] [Google Scholar]
- 12.Pham HP, Schwartz J. How we approach a patient with symptoms of leukostasis requiring emergent leukocytapheresis. Transfusion. 2015;55:2306–11. doi: 10.1111/trf.13210. [DOI] [PubMed] [Google Scholar]
- 13.Sans-Sabrafen J, Besses Raebel C, Vives Corrons J. Clinical Hematology. 5th ed. Madrid: Elsevier; 2007. [Google Scholar]
- 14.Ruggiero A, Rizzo D, Amato M, Riccardi R. Management of hyperleukocytosis. Curr Treat Options Oncol. 2016;17:7. doi: 10.1007/s11864-015-0387-8. [DOI] [PubMed] [Google Scholar]
- 15.Gong J, Wu B, Guo T, Zhou S, He B, Peng X. Hyperleukocytosis: A report of five cases and review of the literature. Oncol Lett. 2014;8:1825–7. doi: 10.3892/ol.2014.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson SC, Bruggers CS, Kurtzberg J, Friedman HS. Management of leukemic hyperleukocytosis with hydration, urinary alkalinization, and allopurinol. Are cranial irradiation and invasive cytoreduction necessary? Am J Pediatr Hematol Oncol. 1993;15:351–5. [PubMed] [Google Scholar]
- 17.Chekol SS, Bhatnagar B, Gojo I, Hess JR. Leukopheresis for profound hyperleukocytosis. Transfus Apher Sci. 2012;46:29–31. doi: 10.1016/j.transci.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Shafique S, Bona R, Kaplan AA. A case report of therapeutic leukapheresis in an adult with chronic myelogenous leukemia presenting with hyperleukocytosis and leukostasis. Ther Apher Dial. 2007;11:146–9. doi: 10.1111/j.1744-9987.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 19.Ranganathan S, Sesikeran S, Gupta V, Vanajakshi Emergency therapeutic leukapheresis in a case of acute myeloid leukemia M5. Asian J Transfus Sci. 2008;2:18–9. doi: 10.4103/0973-6247.39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba H, Fan Y, Pounds S, Geiger TL, Rubnitz JE, Ribeiro RC, et al. Clinical and biologic features and treatment outcome of children with newly diagnosed acute myeloid leukemia and hyperleukocytosis. Cancer. 2008;113:522–9. doi: 10.1002/cncr.23581. [DOI] [PubMed] [Google Scholar]


