Abstract
BACKGROUND:
Use of blood and its components is lifesaving. However, their use is often associated with adverse events.
OBJECTIVE:
To analyze the pattern of adverse reactions associated with transfusion of blood and its components in pediatric and surgical patients at a tertiary care teaching hospital.
MATERIALS AND METHODS:
Patients receiving transfusion of blood or its components in a randomly selected unit each from Departments of Pediatrics, including thalassemia OPD and surgery, were monitored intensively for a period of 6 months. Clinical course, management, outcome, causality, severity, seriousness, and preventability of observed transfusion reactions (TRs) were analyzed.
RESULTS:
A total of 411 pediatric and 433 surgical patients received 594 and 745 transfusions respectively during the study period. Of these, TRs were observed in 69 (11.6%) children and 63 (8.4%) surgical patients. Majority of reactions in children (48, 69.5%) and surgical patients (51, 80.9%) were acute, developing within 24 h of transfusion. TRs were observed with packed cells (13.2%), cryoprecipitate (10%), platelet concentrate (14.3%) and fresh frozen plasma (1.3%) in pediatric patients and with packed cells (7.2%), whole blood (25%) and platelet concentrate (62.5%) in surgical patients. Most common TRs included febrile nonhemolytic TRs (FNHTRs) and allergic reactions. Reactions were more frequent in patients with a previous history of transfusion or those receiving more than one transfusion and in children, when transfusion was initiated after 30 min of issue of blood component. Majority of reactions were managed with symptomatic treatment, were nonserious, moderately severe, probably preventable and probably associated with the suspect blood component in both populations.
CONCLUSION:
Transfusion reactions in children and surgical patients are commonly observed with cellular blood components. Majority of reactions are acute and nonserious. FNHTRs and allergic reactions are the most common transfusion reactions. Risk of transfusion reactions is more in patients receiving multiple transfusions.
Keywords: Allergic reaction, febrile nonhemolytic transfusion reaction, intensive monitoring, pediatric patients, surgical patients, transfusion reactions
Introduction
Blood and blood components are indispensable. Use of these components, however, is often associated with adverse events, ranging from minor chills and rigors to life-threatening anaphylaxis.[1] Incidence of transfusion reactions (TRs) is estimated at 0.001%–10%.[2,3,4] Although the incidence has declined substantially with modern facilities such as improved screening and transfusion practices, use of leukofilters and modified blood components, a significant number of cases are still observed due to human errors, alloimmunization, bacterial contamination and immunomodulation.[5]
Hemovigilance is “a set of surveillance procedures covering the entire transfusion chain from collection of blood and its components to the follow-up of its recipients, intended to collect and assess information on unexpected or undesirable effects resulting from the therapeutic use of labile blood products, and to prevent its occurrence and recurrence.”[6] It includes monitoring, identifying, reporting, investigating and analyzing adverse events associated with transfusion of blood and blood components.[7] Information gathered from such monitoring is useful for early identification, management and prevention of TRs.[8]
Since relatively less amount of data are available in this regard from the Indian population at present, present study was conducted to detect and analyze transfusion-related adverse reactions in two populations, i.e., children and surgical patients, using an intensive monitoring method.
Materials and Methods
This observational, prospective study was conducted in a tertiary care teaching hospital in Gujarat, India, over a period of 18 months, i.e., December 2013 to May 2015. Permission to conduct the study was obtained from the Institutional Ethics Committee (IEC Approval No. 124/14) and heads of Departments of Pediatrics, Surgery and Immunohematology and Blood Transfusion (IHBT). To monitor TRs, one unit each from Departments of Pediatrics and Surgery with maximum patient inflow as detected from the past admission records were selected for patient enrollment. Further, all children receiving transfusion in thalassemia OPD were eligible for enrollment irrespective of their units of admission. The study was conducted in patients receiving transfusion of blood or blood components in the selected units and thalassemia OPD. Each unit of blood or blood component transfused was considered as a separate transfusion. Patients with a previous history of transfusion or those receiving transfusion with more than one unit of blood or blood components were considered as having received multiple transfusions. TRs were defined according to the World Health Organization (WHO) guidelines for clinical use of blood.[9] Information regarding issue of blood components to these patients was obtained from the Department of IHBT to trace the patients. Leukodepleted components or bedside leukodepletion were not used in current setup at the time of study. A written informed consent was obtained from patients or guardians before enrollment. Detailed information of enrolled patients, transfusions including time of onset of transfusion since issue of blood component from the blood bank and TRs was collected in a pretested case record form. Each patient was followed up daily till discharge to monitor transfusion-related adverse reactions. Subsequently, telephonic follow-up was done, once every week for 6 months. Patients were also asked to report adverse reactions following discharge using telephonic communication. Data were collected for a period of 6 months in each department. Causality of TRs was assessed using WHO-Uppsala Monitoring Centre scale[10] and Naranjo score.[11] Preventability of TRs was assessed using Modified Schumock and Thornton criteria.[12] Severity and seriousness of TRs were assessed using Modified Hartwig and Siegel scale[13] and Central Drugs Standard Control Organization criteria,[14] respectively. Data were entered in MS Excel Worksheet 2007 to analyze the frequency, percentage, mean and standard deviation. GraphPad InStat Demo version 3.06 (Graph pad Software, Inc., San Diego, California, USA) was used to apply Fisher's exact test. The test was used to detect a difference in frequency of TRs in patients receiving single versus multiple transfusions and that in patients receiving transfusion within 30 mins of issue of blood components versus those receiving transfusion after 30 mins. P < 0.05 was considered statistically significant.
Results
A total of 411 pediatric patients, including 249 boys and 162 girls, were transfused with 594 units of blood components (1.4 transfusion per patient), whereas 433 surgical patients, including 271 men and 162 women, were transfused with 745 units of blood or its components (1.7 transfusions per patient) during the study period [Tables 1 and 2]. All units issued were transfused to respective patients. Mean age and hemoglobin of children receiving transfusions were 5.4 ± 3.5 years and 7.8 ± 1.7 g/dL respectively, whereas those of surgical patients were 43.1 ± 18.5 years and 9.3 ± 2.1 g/dL respectively [Figure 1]. A previous history of transfusion was present in 35 children and 49 surgical patients, whereas a history of febrile nonhemolytic TR (FNHTR) was present in one child. Premedication was not used in any of patients receiving transfusion.
Table 1.
Details of blood components and frequency of transfusion reactions in pediatric patients in the study (n=411)
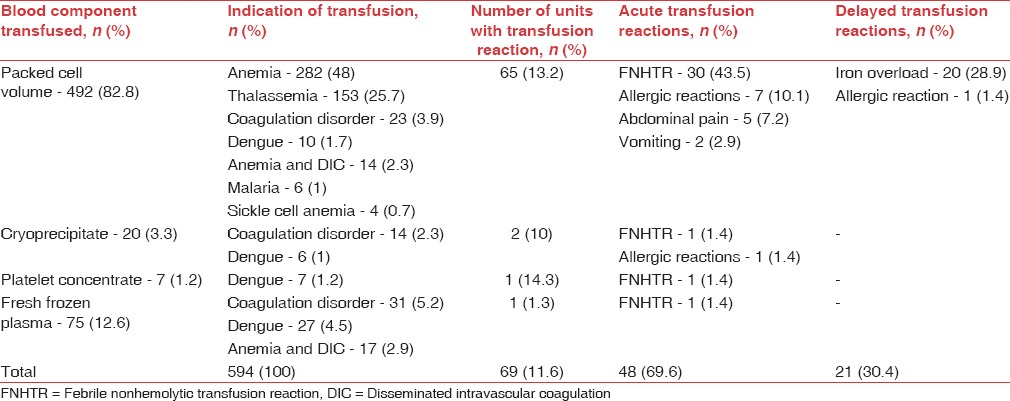
Table 2.
Details of blood components and frequency of transfusion reactions in surgical patients in the study (n=433)
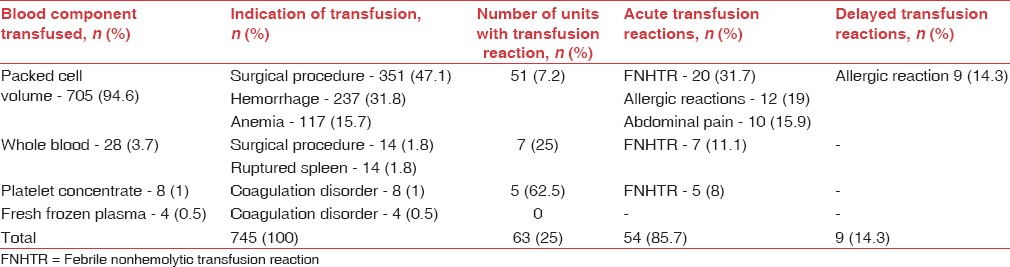
Figure 1.
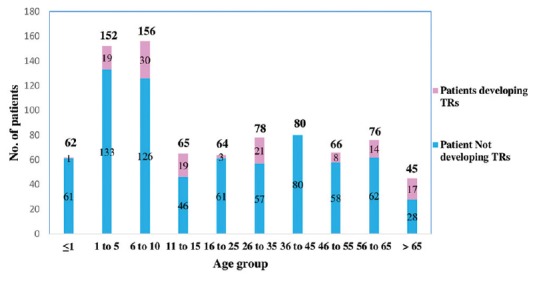
Age distribution of patients receiving blood transfusion and those developing transfusion reactions (TRs)
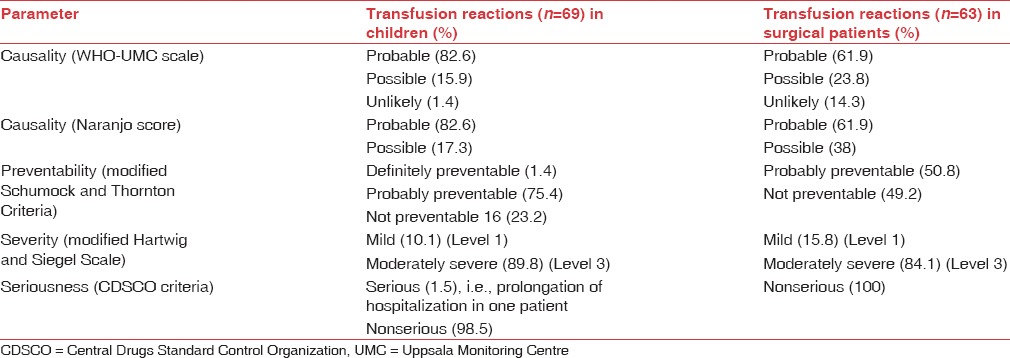
TRs were observed with 69 out of 594 transfusions (11.6%) in children and 63 out of 745 transfusions (8.4%) in surgical patients, affecting males and females similarly (P: 0.8927 in children and P: 0.0675 in surgical patients). Majority of reactions in children (48, 69.5%) and surgical patients (51, 80.9%) were acute i.e. developing within 24 h of transfusion [Tables 1 and 2] and included FNHTRs, allergic reactions, abdominal pain and vomiting. Delayed TRs included iron overload in children and allergic reactions [Tables 3 and 4]. Reactions were most frequent with packed cells in both children (65, 94.2%) and surgical patients (51, 80.9%), followed by whole blood, platelet concentrate, cryoprecipitate and fresh frozen plasma (FFP) Tables 1 and 2. Mean volume of blood or blood components transfused to pediatric and surgical patients developing TRs was 223 ± 71.4 mL and 236 ± 98.8 mL respectively. Clinical presentation and management of TRs are described in [Tables 3 and 4]. Medicines used to treat TRs included chlorpheniramine (92), paracetamol (63), deferasirox (20), ondansetron (2) and prednisolone (1). All reactions, except iron overload in children, recovered completely. Causality, preventability, severity and seriousness of TRs as assessed by various scales are mentioned in Table 5.
Table 3.
Clinical presentation and management of transfusion reactions (n=69) in pediatric patients
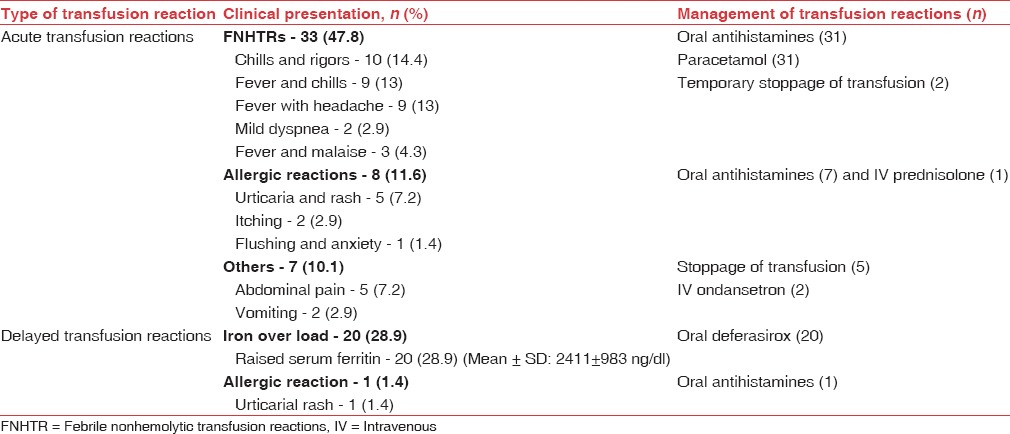
Table 4.
Clinical presentation and management of transfusion reactions (n=63) in surgical patients
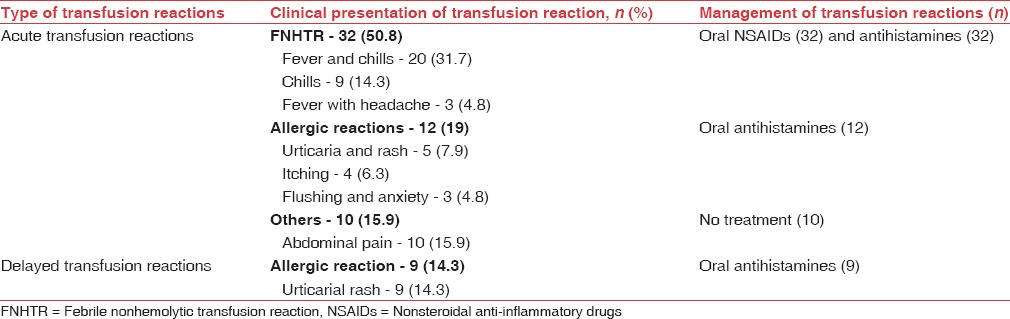
Table 5.
Causality, preventability, severity and seriousness of transfusion reactions in pediatric and surgical patients
Transfusion reactions were more frequent in patients with multiple transfusions compared to those receiving single transfusion (P = 0.0401 in children and P < 0.0001 in surgical patients) and in children, when transfusion was initiated after 30 min of issue of blood component (416, 70%) as compared to when initiated within 30 min (178, 30%) (P = 0.0021).
Suspected blood component was probably associated with adverse reaction in majority of children (57, 82.6%) and surgical patients (39, 61.9%) [Table 5]. A total of 68 (98.5%) reactions in children and all reactions in surgical patients were not serious in nature, while prolongation of hospitalization in a child with allergic reaction was serious in nature. TRs in children were probably preventable (52, 75.4%), not preventable (16, 23.2%) and definitely preventable (1, 1.4%). Whereas in surgical patients, these were probably (32, 50.8%) or not (31, 49.2%) preventable. Majority of TRs in children (62, 89.8%) and surgical patients (53, 84.1%) were moderately severe (Level 3), followed by mild reactions (Level 1) in children (7, 10.1%) and surgical patients (10, 15.8%).
Discussion
The present study was conducted to evaluate the pattern of TRs in pediatric and surgical patients at a tertiary care teaching hospital in India. Data were collected from each study population over a period of 6 months. Clinical course, management, outcome, causality, severity, seriousness and preventability of TRs were assessed.
In the present study, number of transfusions exceeded the number of patients in both populations. This was attributed to the requirement of multiple transfusion due to diseases, such as thalassemia, coagulation disorders and anemia in children and intraoperative transfusion requirement, hemorrhage and anemia in surgical patients. Multiple transfusions were also reported by Venkatachalapathy and Bhattacharya et al. in patients with anemia, hemato-oncology disorders and elective surgery.[15,16] Multiple transfusions increase the risk of TRs[17] and use of modified blood components can help reduce the risk in such patients.
In the present study, more than 70% children receiving transfusions were 1–5 years or 5–10 years (5.4 ± 3.5 years) of age. This was attributed to an increased prevalence of severe anemia and thalassemia in these age groups. Severe anemia in young malnourished children usually manifests during the period of rapid growth.[18] Further, thalassemia is also known to manifest early and require multiple transfusions. The findings, however, could not be adequately compared due to lack of adequate studies on TRs in children. A similar number of surgical patients in different age groups received transfusions, owing to intraoperative requirement (58.5%) and treatment of hemorrhage (23.5%). This contributed to a mean age of 43.1 ± 18.5 years. Mean age of patients receiving transfusions in studies conducted at Delhi and Kashmir had been reported at 34.1 and 32.1 years, respectively.[4,19] However, these studies involved patients of all age groups admitted to various specialties.
Mean Hb level in children receiving transfusion (7.8 ± 1.7 g/dL) was less than that (9.3 ± 2.17 g/dl) in surgical patients in the present study. This reflected in the fact that anemia was a more common indication of transfusion in children as compared to surgical patients. Mean Hb also indicates a rational use of transfusions in both populations in view of anemia or anticipated blood loss during surgery. In a study conducted in South India,[15] majority patients had a Hb >10 g/dl. Since the said study involved patients of all specialties, further studies are required to detect any significant difference in this parameter.
Anemia and thalassemia were the most common indications of transfusion in children in the present study. Prevalence of anemia is high among children in developing countries[20] and an association between occurrence of anemia and requirement of transfusion has been reported.[21] Thalassemia and coagulation disorders commonly manifest at an early age and require repeated transfusions. Surgical procedures, due to associated blood loss, were the most common indication of transfusion in surgical patients followed by hemorrhage. Elective surgery and anemia have been reported as most common indications of transfusion in studies conducted at AIIMS, Delhi and South India,[15,19] similar to our observations. In the present study, other indications i.e. dengue, disseminated intravascular coagulation (DIC), malaria and sickle cell anemia accounted for <10% of total cases. This can be attributed to less incidence of sickle cell anemia and DIC and less occurrence of dengue and malaria during periods of data collection, i.e., winter and summer.
Packed cells (PCV) were the most frequently transfused blood component in both populations in the present study, owing to high incidence of anemia, thalassemia and coagulation disorders in children and elective surgery, hemorrhage and anemia in surgical patients. Together these indications accounted for transfusions in 91% children and 86% surgical patients. Although whole blood can be used for these conditions, use of whole blood has declined[22] due to a higher incidence of TRs. Whole blood was employed only in few surgical patients in the present study. Similarly, PCV was the most frequently transfused blood component in studies at Kashmir (52.7%), Namibia (74%) and Zimbabwe (75.4%).[4,23,24] Other blood components i.e. cryoprecipitate (3.4%), platelet concentrate (2.2%) and FFP (13.1%) were transfused to patients suffering from dengue and coagulopathies, primarily to correct the underlying deficit of clotting factors or platelets.
In the present study, TRs were observed in 11.6% children and 8.4% surgical patients. This incidence is higher as compared to that reported from other parts of the country, i.e., 0.05%–3.3%.[15,19,25] This may be attributed to intensive method of monitoring employed in the present work as compared to conventional reporting or retrospective analysis of records employed in other studies. Intensive monitoring can detect a larger number of adverse reactions since minor, less severe reactions are often not reported to blood bank by conventional reporting.[4,26] Furthermore, modified blood components were not employed for transfusion in the present setup, which could have contributed to a higher incidence of TRs.
In the present study, mean age of children developing TRs was 7.4 years owing to the fact that reactions were frequent (43.5%) in children of 6–10 years. Among surgical patients, TRs affected all age groups except patients of 36–45 years. The finding could not be explained and needs further evaluation. Results could not be adequately compared since studies focusing on TRs in these populations could not be found on literature search.
Majority of TRs in children (69.5%) and surgical patients (~81%) in the present study were acute i.e. developing within 24 h of transfusion. Iron overload, a delayed reaction, was not observed in surgical patients and hence, the population showed a higher proportion of acute reactions. Acute TRs are easily recognized since majority appear soon after initiation of transfusion.[4,16] Longer the time of onset, more is the likelihood of reaction getting missed, especially if it is mild or nonspecific.[16] Most studies report acute reactions to be more frequent than delayed reactions.[4,16,24]
Packed cells were most frequently associated with TRs (82.8%) in the present study as it was the most common blood component used for transfusion. This also contributed to the mean volume in patients developing TRs (223 ± 71.4 mL and 236 ± 98.8 mL in pediatrics and surgical patients respectively). Studies conducted in other parts of India report a lesser volume in this regard since PCV was suspected in substantially less number of cases.[19,25] Other blood components, used in relatively less number of patients, were less frequently associated with TRs in the present study.
FNHTR was the most common TR in pediatric (44.9%) and surgical patients (50.8%) in the present study. It is reported as the most common TR in studies conducted in various parts of the world.[16,24,27,28] Reaction was common with packed cells, most commonly used blood component in the present study since it is commonly observed with cellular blood components.[25] It is caused by white cell Ag-Ab interaction and release of cytokines during storage of blood components. Use of leukodepleted packed cells and platelets,[29] initiation of transfusion within 30 min of issue of blood component and cold chain maintenance are recommended to reduce the occurrence.[9,14]
Allergic reactions were the second most common acute TRs in children (11.6%) and surgical patients (19%) in the present study. Varying incidence of allergic TRs, i.e., 0.2%–5.1% is reported in literature[16,19,30] since these primarily depend on individual susceptibility and cannot be predicted. Transfusion of IgA deficient plasma is recommended to reduce such reactions;[23] however, it may be an unlikely option in a resource-constrained setting. A detailed history and premedication of susceptible individuals with antihistamines can reduce the occurrence of these reactions.[31] Delayed allergic reactions observed could not be definitely related to transfusion because of a weak temporal relation.
Abdominal pain (7.2% children and 15.8% surgical patients) and vomiting (2.9% children) were reported in few patients in the present study, which could not be classified. These type of reactions are reported from other parts of the world also i.e., Japan, Pakistan and Italy.[28,29,32] and may be attributed to comorbid illness or mild unrecognized hemolysis.[9] Since urine analysis for hemoglobinuria was not performed in the present study, hemolysis could not be confirmed.
Most common delayed TR (29%) in children in the present study was iron overload, which resulted from repeated transfusions in thalassemic patients. These patients were prescribed oral deferasirox to prevent the ill effect of excess iron. Iron overload was not observed in surgical patients. Most studies do not classify iron overload as a TR and hence, the finding was not compared.
In the current study, FNHTR manifested as chills with rigors (14.4%), fever with chills (13%) or headache (13%) or malaise (1.4%) and mild dyspnea (2.9%) in children and fever with chills (31.7%), chills (14.3%) and fever with headache (4.8%) in surgical patients. Different manifestations have been reported in various studies, i.e., chills and rigors, fever, vomiting, myalgia, anxiety and hypotension.[4,19,26] Of these, chills with rigors and fever are reported as most frequent manifestations, similar to our observations.[4,16] Variability with regard to other manifestations requires further evaluation in a larger scale study. Allergic reactions commonly manifested as urticarial rash, pruritus and flushing with anxiety in the present study. Rash and pruritus are reported as the most common manifestations by other studies also.[4,16,19] Wheals (8%) and periorbital edema (10.8%)[16] reported elsewhere were not observed in the present study. Allergic reactions are likely to have diverse manifestations which can explain variability in manifestations. Iron overload in children was primarily diagnosed by elevated serum ferritin levels in the present study.
Nearly half of TRs (50.7%) in children were observed in thalassemic patients with a history of transfusion. Bhattacharya et al. also reported that 52.3% patients developing TRs had a history of transfusion.[16] Thus, the risk of TRs increases with each transfusion,[33] which was evident in the present study. Interestingly, it was detected that risk of TRs increased when transfusion was initiated after 30 min of issue of blood components in children. This coheres with the WHO recommendation of initiation of transfusion within 30 min.[9] The finding, however, was not evident in surgical patients.
Acute hemolytic reactions were not observed in the present study. This indicated an efficient blood grouping and cross matching practices by blood bank and lack of administration errors. Delayed hemolytic reactions were also not observed in the present study. These reactions, often being asymptomatic, are difficult to diagnose.[16] Absence of transfusion-associated circulatory overload and transfusion transmitted infections suggested good transfusion practices and efficient screening by blood bank respectively. Transfusion-related acute lung injury, more common when the donors are multiparous women,[30] was not observed in the present study. It is rare in Indian subcontinent where most donors are males.[16]
In the present study, 54.5% patients developing TRs received concomitant medications such as antimicrobials, antiemetics, and intravenous fluids. Some of these were implicated in causation of adverse reactions and influenced causality. Majority of reactions (87.1%) were treated symptomatically with drugs such as antihistamines (69.6%), paracetamol (47.7%), ondansetron (1.5%), glucocorticoids (0.7%) and deferasirox (15.1%). A temporary stoppage of transfusion was required in 5.3% patients. Treatment of TRs is largely supportive[34] and all reactions, except iron overload in children, recovered completely.
In the current study, in majority of cases, blood component was probably associated with TR[10,11] since no other likely cause for reaction was detected. A possible causal relation was attributed to concomitant drugs or comorbid illnesses, leading to similar symptoms. Delayed allergic reactions (7.5%) had an unlikely relation with suspect blood component because of a weak temporal relationship. Majority of TRs (99.2%) in the current study were nonserious since only one case of prolongation of hospitalization was observed. Similar findings were reported by Kato et al. in Japan.[35] Majority of reactions in children (75.4%) and surgical patients (50.8%) were probably preventable since adequate preventive measures, i.e., leukofiltered blood components were not administered due to unavailability in the present setup. Allergic or nonspecific reactions, occurrence of which could not be predicted, were considered not preventable. A single case of FNHTR was definitely preventable since patient was not administered any prophylactic treatment despite a history of similar reaction. Since majority of reactions in children (89.8%) and surgical patients (84.1%) required symptomatic treatment, these were considered moderately severe.[13] Temporary stoppage of transfusion or no requirement of treatment implied mild nature of reactions. Severe reactions were not detected in the present study. Similar observations were reported in Iran and Namibia.[23,32]
Limitations of the study
Since the study focused on TRs in two populations, findings of the study cannot be generalized to other patients. Duration of transfusion could not be estimated due to a lack of availability of data regarding completion of transfusion. Furthermore, it was not possible for authors to monitor the entire transfusion chain. Causality and preventability assessment, primarily carried out by investigators, can be considered as subject to variation since clinicians' opinions were not sought. However, the extent of data collected leads to few important conclusions.
Conclusion
Transfusion reactions are common in pediatric and surgical patients in the present setup and observed with whole blood, packed cells, FFP and cryoprecipitate. Majority of reactions are acute and nonserious. Febrile nonhemolytic and allergic reactions are common TRs. Risk of TRs increases with multiple transfusions. Good blood grouping, cross-matching and transfusion practices are observed in the present setup.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sahu S, Hemlata, Verma A. Adverse events related to blood transfusion. Indian J Anaesth. 2014;58:543–51. doi: 10.4103/0019-5049.144650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuriyan M, Carson JL. Blood transfusion risks in the Intensive Care Unit. Crit Care Clin. 2004;20:237–53. doi: 10.1016/j.ccc.2003.12.001. ix. [DOI] [PubMed] [Google Scholar]
- 3.Callum JL, Pinkerton PH, editors. Bloody Easy: Blood Transfusions, Blood Alternatives and Transfusion Reactions, a Guide to Transfusion Medicine. Toronto (Ontario): Sunnybrook and Women's College Health Sciences Centre; 2005. Transfusion reactions; pp. 34–65. [Google Scholar]
- 4.Sidhu M, Meenia R, Yasmeen I, Akhtar N. A study of transfusion related adverse events at a tertiary care centre in North India: an initiative towards hemovigilance. Intern J Adv Med. 2015;2:206–10. [Google Scholar]
- 5.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–69. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 6.de Vries RR, Faber JC, Strengers PF Board of the International Haemovigilance Network. Haemovigilance: An effective tool for improving transfusion practice. Vox Sang. 2011;100:60–7. doi: 10.1111/j.1423-0410.2010.01442.x. [DOI] [PubMed] [Google Scholar]
- 7.Maiti R, Mukherjee S. Post Graduate Topics for Pharmacology. 2nd ed. Hyderabad: PARAS Medical Publisher; 2015. pp. 304–17. [Google Scholar]
- 8.Andreu G, Morel P, Forestier F, Debeir J, Rebibo D, Janvier G, et al. Hemovigilance network in France: Organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion. 2002;42:1356–64. doi: 10.1046/j.1537-2995.2002.00202.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organisation, Manual of Clinical Use of Blood in Surgery and Anaesthesia. 2002. [Last viewed on 2016 Jan 16]. Available from: http://www.who.int/bloodsafety/clinical_use/en/Manual_EN.pdf .
- 10.The Use of the WHO–UMC System for Standardised Case Causality Assessment. [Last accessed on 2016 Apr 27]. Available from: http://www.who-umc.org/Graphics/24734.pdf .
- 11.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. Amethod for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 12.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 13.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 14.Central Drugs Standard Control Organization. Adverse Drug Reaction Reporting Form, India. [Last accessed on 2016 Apr 27]. Available from: http://www.cdsco.nic.in/writereaddata/ADR%20form%20PvPI.pdf .
- 15.Venkatachalapathy TS. A prospective audit of blood transfusion reactions in tertiary care hospital for the use of blood and blood components. J Blood Disord Transfus. 2013;3:118. [Google Scholar]
- 16.Bhattacharya P, Marwaha N, Dhawan HK, Roy P, Sharma RR. Transfusion-related adverse events at the tertiary care center in North India: An institutional hemovigilance effort. Asian J Transfus Sci. 2011;5:164–70. doi: 10.4103/0973-6247.83245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widmann FK. Untoward effects of blood transfusion: Common problems and simple safeguards. Postgrad Med. 1981;69:40–4. doi: 10.1080/00325481.1981.11715669. 47-50, 52-3. [DOI] [PubMed] [Google Scholar]
- 18.Awasthi S, Das R, Verma T, Vir S. Anemia and undernutrition among preschool children in Uttar Pradesh, India. Indian Pediatr. 2003;40:985–90. [PubMed] [Google Scholar]
- 19.Kumar P, Thapliyal R, Coshic P, Chatterjee K. Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: A preliminary step towards hemovigilance. Asian J Transfus Sci. 2013;7:109–15. doi: 10.4103/0973-6247.115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UNICEF/United Nations University/World Health Organization Iron Deficiency Anemia. Assessment, prevention and control: A guide for programme managers. Document WHO/NHD/01.3. Geneva: World Health Organization; 2001. [Google Scholar]
- 21.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 22.Heddle NM, Klama L, Singer J, Richards C, Fedak P, Walker I, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625–8. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 23.Meza BP, Lohrke B, Wilkinson R, Pitman JP, Shiraishi RW, Bock N, et al. Estimation of the prevalence and rate of acute transfusion reactions occurring in Windhoek, Namibia. Blood Transfus. 2014;12:352–61. doi: 10.2450/2013.0143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mafirakureva N, Khoza S, Mvere DA, Chitiyo ME, Postma MJ, Van Hulst M. Incidence and pattern of 12 years of reported transfusion adverse events in Zimbabwe: A retrospective analysis. Blood Transfus. 2014;12:362–7. doi: 10.2450/2014.0156-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma KD, Datta S, Gupta A. Study of acute transfusion reactions in a teaching hospital of Sikkim: A hemovigilance initiative. Indian J Pharmacol. 2015;47:370–4. doi: 10.4103/0253-7613.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narvios AB, Lichtiger B, Neumann JL. Underreporting of minor transfusion reactions in cancer patients. MedGenMed. 2004;6:17. [PMC free article] [PubMed] [Google Scholar]
- 27.Khalid S, Usman M, Khurshid M. Acute transfusion reactions encountered in patients at a tertiary care center. J Pak Med Assoc. 2010;60:832–6. [PubMed] [Google Scholar]
- 28.Giampaolo A, Piccinini V, Catalano L, Abbonizio F, Hassan HJ. The first data from the haemovigilance system in Italy. Blood Transfus. 2007;5:66–74. doi: 10.2450/2007.0001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions. Management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128:991–5. doi: 10.5858/2004-128-991-FNTR. [DOI] [PubMed] [Google Scholar]
- 30.Moore SB. Anaphylactic transfusion reactions – A concise review. Ir Med J. 1985;78:54–6. [PubMed] [Google Scholar]
- 31.Dzieczkowski JS, Anderson KC. Transfusion biology and therapy. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrisson's Priniciple of Internal Medicine. 19th ed. New Delhi: McGraw Hill; 2014. [Google Scholar]
- 32.Payandeh M, Zare ME, Kansestani AN, Pakdel SF, Jahanpour F, Yousefi H, et al. Descriptions of acute transfusion reactions in the teaching hospitals of Kermanshah university of medical sciences, Iran. Int J Hematol Oncol Stem Cell Res. 2013;7:11–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Menitove JE, McElligott MC, Aster RH. Febrile transfusion reaction: what blood component should be given next? Vox Sang. 1982;42:318–21. doi: 10.1111/j.1423-0410.1982.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 34.Sokolovic M, Pastores SM. Transfusion therapy and acute lung injury. Expert Rev Respir Med. 2010;4:387–93. doi: 10.1586/ers.10.22. [DOI] [PubMed] [Google Scholar]
- 35.Kato H, Uruma M, Okuyama Y, Fujita H, Handa M, Tomiyama Y, et al. Incidence of transfusion-related adverse reactions per patient reflects the potential risk of transfusion therapy in Japan. Am J Clin Pathol. 2013;140:219–24. doi: 10.1309/AJCP6SBPOX0UWHEK. [DOI] [PubMed] [Google Scholar]


