Abstract
Intravenous immunoglobulin (IVIG) and therapeutic plasma exchange (TPE) are well-known therapeutic modalities in Guillain–Barré syndrome (GBS). In developing countries like India, where plasma-derived products (IVIG) are not easily available, and affordable TPE is preferred. Here, we reported a case of severe GBS, who was treated with daily plasma exchange (PLEX) rather than recommended alternate day schedule. A 16-year-old male adolescent of severe GBS, i.e., on mechanical ventilator was treated with the plasmapheresis regimen consisted of removal of 1.3 plasma volumes in each cycle for total of five cycles, on daily basis. The patient's condition started improving after three cycles of TPE with power in the upper limbs 4/5 and lower limbs 3/5 and completely weaned off from ventilator after the 4th TPE, i.e. the 4th day of admission. This case emphasizes the need of daily PLEX regimen particularly in severe GBS patients because early weaning from ventilator reduces the ventilator-associated complications, hospital stay as wells as less morbidity, and mortality in severe GBS.
Keywords: Mechanical ventilation, plasma exchange, severe Guillain–Barré syndrome, weaning
Introduction
Guillain–Barré syndrome (GBS) is an autoimmune disease of the peripheral nervous system. About one-third of the patients with GBS will require mechanical ventilation, and most GBS-related deaths occur as a result of respiratory failure.[1] The annual incidence of GBS is 0.4–4 per 100,000 all over the world. Men are more frequently affected than women.[2] There are no incidence studies of GBS in the Indian population, but some case-based studies have been reported.[3] GBS includes subtypes, such as acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy, acute motor-sensory axonal neuropathy, and Miller Fisher syndrome (ophthalmoplegia, ataxia, and areflexia). GBS is a postinfectious, immune-mediated disease. Campylobacter jejuni, cytomegalovirus, Epstein–Barr virus, and Mycoplasma pneumoniae are the predominant causes associated with GBS.[4] Molecular mimicry between infectious agents and gangliosides plays an important role.[5] Winters et al. noted that the cost of intravenous immunoglobulin (IVIG) treatment in GBS is twice high as the cost of therapeutic plasma exchange (TPE) with equivalent clinical response.[6] In developing countries like India, where plasma-derived products (IVIG) are not easily available and affordable to all, TPE is a better option. Here, we reported a case of severe GBS, who was treated aggressively with daily plasma exchange (PLEX) and weaned off from Ventilator on 4th day, whereas average reported weaning period from ventilator is 21 days.
Case Report
A 16-year-old male adolescent was referred in our tertiary care hospital with chief complaints of inability to stand or walk for 8 days before which he was apparently asymptomatic. His symptoms began slowly following an upper respiratory tract infection that he had about 10 days prior. His upper respiratory symptoms improved, but 2 days later, he started having bilateral lower extremities weakness. The weakness progressed in his upper extremities. He was hospitalized in a private hospital 3 days later with complaints of difficulty in walking and weakness in all four limbs. He also developed respiratory muscle weakness and was not able to maintain oxygen saturation, so he was kept on mechanical ventilator. There was progressive deterioration in power of all four limbs. He was treated with antibiotics and steroids, but there was no improvement in his clinical condition, then he was shifted to our tertiary care hospital for further management after 10 days of the starting illness with GBS disability score 5, i.e., on ventilator.
On physical examination, he was calm, conscious, and oriented to time, place, and person with E4VTM6 on Glasgow Coma Scale. Pulse was 72/min. Blood pressure was 130/70 mm of Hg, and oxygen saturation was 100% with mechanical ventilator continuous positive airway pressure mode. Pupils were bilaterally reactive to light. Deep tendon reflexes were absent in all four limbs. Plantar was mute bilaterally. Power in the upper limbs was 3/5 and that in the lower limbs was 2/5. There was no sensory loss. Cardiovascular, respiratory, and abdominal examinations were unremarkable.
Routine studies of blood and urine gave normal results. His cerebrospinal fluid revealed elevated protein count of 200 mg/dl, with normal cell count, which supported the diagnosis of GBS. Nerve conduction studies of the patient showed predominantly motor demyelinating neuropathy with secondary axonal involvement which confirmed the diagnosis of AIDP. TPE was planned. The plasmapheresis regimen consisted of removal of 1.3 plasma volumes in each cycle for total of five cycles, on daily basis. Before starting the treatment, the patient's attendant was explained about the risks and benefits of treatment, and consent was taken. First TPE was performed emergently on the day of admission to our hospital in the Intensive Care Unit (ICU) on Terumo BCT COBE Spectra Apheresis System machine and continued daily for 5 days using plasma only as the replacement fluid instead of albumin due to nonaffordability of the patient. All procedures were completed uneventfully.
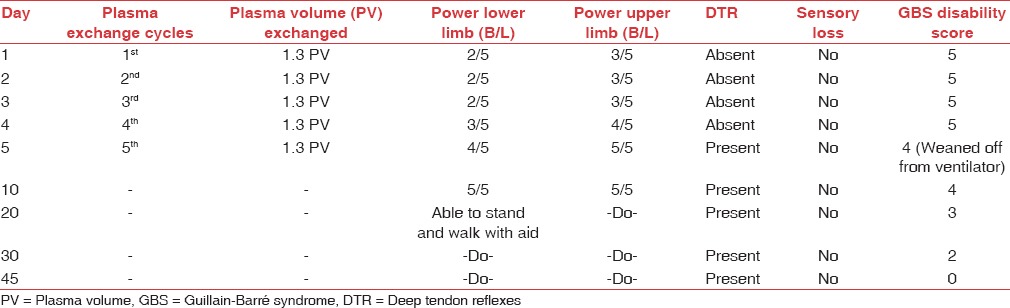
The patient's condition started improving after three cycles of TPE with power in the upper limbs 4/5 and lower limbs 3/5. The patient was also weaned off from ventilator after the 4th TPE. There was progressive improvement in the muscle power in all four limbs within 10 days of admission to our hospital. He started walking with aid in 20 days [Table 1].
Table 1.
Clinical outcome of the patient
Discussion
There are several scales to evaluate severity and prognosis of the disease. Here, we have used Hughes GBS disability score which is:
0: A healthy state
1: Minor symptoms and capable of running
2: Able to walk 10 m or more without assistance but unable to run
3: Able to walk 10 m across an open space with help
4: Bedridden or chair bound
5: Requiring assisted ventilation for at least part of the day
6: Dead.
Hughes score in our patient at the time of admission was 5. The American Society for Apheresis guidelines in 2013 recommend TPE as category I and Grade 1A indication in GBS and strategy is 5–6 one-volume TPE procedures every alternate day with 5% albumin replacement. This recommended schedule is irrespective of severity of disease. In AIDP patients with axonal involvement, TPE has been reported to be of greater potential benefit than IVIG,[7] and as our patient was also of AIDP subtype with secondary axonal involvement, as per nerve conduction study of the patient, we planned to do five TPE procedures daily with 1.3 plasma volume exchanges. Cochrane review in 2012 of TPE in AIDP found that it is most effective when initiated within 7 days of disease onset. It also analyzed three trials Farkkila in1987, McKhann in 1985, and Raphael in 1987 for the mean (standard deviation) duration on the ventilator in PLEX-treated group, and it was 11.7 (12.2) day, 27.5 (29.3) days, and 27 days, respectively.[8] However, in our case who was referred late to our hospital (after 10 days), started showing significant improvement in the muscle power after 3 TPE procedures with power 4/5 in the upper and 3/5 in the lower limbs. He was weaned off from ventilator after the 4th PLEX. On the 17th day of admission, the patient was able to stand and walk 2–4 steps with assistance. The mortality in the mechanically ventilated patients of GBS is higher; 20% mortality was reported by Lawn and Wijdicks.[9]
Chaudhari et al. found that the cost of plasmapheresis was significantly lower as compared to IVIG (P = 0.01.[8] However, in their study, they did not compare efficacy of daily versus alternate day plasmapheresis.[10] Sarkar et al. reported when IVIG is used as treatment modality, the mean duration of weaning from ventilator was 21.5 days.[11] Limitation of our study is that it is a case report without control. Hence, here is need to do control studies at centers with high load of GBS patients to compare the efficacy of daily versus alternate day PLEX particularly in severe GBS cases on ventilator support to help in early weaning from ventilator. As early weaning reduces ventilator-associated complications, the cost of ICU treatment, overall stay in hospital, and morbidity and mortality associated with severe GBS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zafar MS, Naqash MM, Bhat TA, Malik GM. Guillain-barré syndrome in pregnancy: An unusual case. J Family Med Prim Care. 2013;2:90–1. doi: 10.4103/2249-4863.109965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes RA, Rees JH. Clinical and epidemiologic features of Guillain-Barré syndrome. J Infect Dis. 1997;176(Suppl 2):S92–8. doi: 10.1086/513793. [DOI] [PubMed] [Google Scholar]
- 3.Ram S. India's contribution on “Guillain-Barre syndrome”: Mapping of 40 years research. Neurol India. 2013;61:375–82. doi: 10.4103/0028-3886.117612. [DOI] [PubMed] [Google Scholar]
- 4.Yuki N. Subclass IgG to motor gangliosides related to infection and clinical course in Guillain-Barré syndrome. J Neuroimmunol. 2008;194:181–90. doi: 10.1016/j.jneuroim.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Perera VN, Nachamkin I, Ung H, Patterson JH, McConville MJ, Coloe PJ, et al. Molecular mimicry in Campylobacter jejuni: Role of the lipo-oligosaccharide core oligosaccharide in inducing anti-ganglioside antibodies. FEMS Immunol Med Microbiol. 2007;50:27–36. doi: 10.1111/j.1574-695X.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 6.Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C., Jr Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barré syndrome. BMC Health Serv Res. 2011;11:101. doi: 10.1186/1472-6963-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: The sixth special issue. J Clin Apher. 2013;28:145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 8.Raphaël JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database of Systematic Reviews. 2012;7:CD001798. doi: 10.1002/14651858.CD001798.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Lawn ND, Wijdicks EF. Fatal Guillain-Barré syndrome. Neurology. 1999;52:635–8. doi: 10.1212/wnl.52.3.635. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri JR, Alladi S, Mridula KR, Demudu BB, Rao MV, Hemanth C, et al. Clinical outcome of Guillain-Barré syndrome with various treatment methods and cost effectiveness: A study from tertiary care center in South India: Yashoda GBS Registry. Neurol Asia. 2014;19:263–70. [Google Scholar]
- 11.Sarkar UK, Menon L, Sarbapalli D, Pal R, Zaman FA, Kar S, et al. Spectrum of Guillain-Barré syndrome in tertiary care hospital at Kolkata. J Nat Sci Biol Med. 2011;2:211–5. doi: 10.4103/0976-9668.92320. [DOI] [PMC free article] [PubMed] [Google Scholar]


