Abstract
Metal-on-metal (MoM) hip replacements, often manufactured from a cobalt-chrome alloy, are associated with adverse reactions including soft tissue necrosis and osteolysis. Histopathological analysis of MoM peri-implant tissues reveals an inflammatory cell infiltrate that includes macrophages, monocytes and neutrophils.
Toll-like receptor 4 (TLR4) is an innate immune receptor activated by bacterial lipopolysaccharide. Recent studies have demonstrated that cobalt ions from metal-on-metal joints also activate human TLR4, increasing cellular secretion of inflammatory chemokines including interleukin-8 (IL-8, CXCL8) and CCL2. Chemokines recruit immune cells to the site of inflammation, and their overall effect depends on the chemokine profile produced.
This study investigated the effect of cobalt on the secretion of inflammatory cytokines CCL20 and IL-6. The chemotactic potential of conditioned media from a cobalt-stimulated human monocyte cell line on primary monocytes and neutrophils was investigated using an in vitro transwell migration assay. The role of TLR4 in observed effects was studied using a small molecule TLR4-specific antagonist.
Cobalt ions significantly increased release of CCL2 and IL-6 by MonoMac 6 cells (P<0.001). Conditioned media from cobalt-stimulated cells significantly increased monocyte and neutrophil chemotaxis in vitro (P<0.001). These effects were abrogated by the TLR4 antagonist (P<0.001) suggesting that they occur through cobalt activation of TLR4.
This study demonstrates the role of TLR4 in cobalt-mediated immune cell chemotaxis and provides a potential mechanism by which cobalt ions may contribute to the immune cell infiltrate surrounding failed metal hip replacements. It also highlights the TLR4 signalling pathway as a potential therapeutic target in preventing cobalt-mediated inflammation.
Abbreviations: MoM, metal-on-metal; ARMD, adverse reactions to metal debris; CoCl2, Cobalt chloride hexahydrate
Keywords: Cobalt, TLR4, Metal-on-metal, Chemotaxis, Inflammation
Highlights
-
•
Cobalt ions from metal-on-metal hip implants promote migration of primary monocytes.
-
•
Cobalt ions also increase migration of primary neutrophils.
-
•
These effects are TLR4-dependent as they were inhibited by a small molecule TLR4 antagonist.
-
•
These results show the potential role of TLR4 in inflammatory responses to metal hips.
1. Introduction
Metal-on-metal (MoM) hip replacements are associated with elevated failure rates due to the development of adverse reactions to metal debris (ARMD) which include osteolysis, soft tissue necrosis, pain, and benign but inflammatory pseudotumours [1], [2]. Tissues surrounding failed MoM joints are infiltrated by immune cells including mononuclear phagocytes [3], lymphocytes [4] and neutrophils [3]. Once inside tissues these cells can carry out their effector functions that lead to an inflammatory response but the mechanisms by which they are recruited are currently unclear.
Cobalt ions released during wear of MoM implants activate human Toll-like receptor 4 (TLR4) [5], [6], [7], [8], an innate immune receptor expressed on the surface of numerous cell types, including macrophages, dendritic cells and endothelial cells. TLR4 is also activated by lipopolysaccharide (LPS) to protect against Gram negative bacterial infection and resulting sepsis. The recent discovery of cobalt as a TLR4 agonist has led to several studies highlighting the subsequent cellular events, specifically the release of pro-inflammatory cytokines and chemokines including interleukin-6 (IL-6), interleukin-8 (IL-8) [9], chemokine (C-X-C motif) ligand 10 (CXCL10) [9] and chemokine (C-C motif) ligand 2 (CCL2) [10].
Chemokines are small (10–12 kDa) secreted molecules that co-ordinate the immune response. In vivo they form a chemokine gradient to guide target cells towards the site of inflammation, and exert their effects through G protein-coupled receptors on these cells [11]. Each chemokine produces a different response within its target cell; for example IL-8 is chemotactic for neutrophils [12], while CCL2 acts on monocytes to direct them to the site of inflammation [13].
The individual effect of most chemokines is well-described. However the immunological outcome is dependent on the overall chemokine profile produced in response to a pathogen and the chemotactic effect of cobalt is currently unknown.
This study set out to determine the effect of cobalt ions on the migration of immune cells and the role of TLR4 in any observed responses to establish whether cobalt may contribute to the inflammatory infiltrate surrounding failed MoM hip replacements.
2. Materials and methods
2.1. Cell culture
MonoMac 6 cells are a human cell line derived from acute monocytic leukaemia. They are known to be LPS-responsive through their expression of TLR4 [14]. Cells were cultured in RPMI-1640 medium (Sigma Aldrich, Gillingham, UK) supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mM L-glutamine (all Sigma Aldrich).
2.2. Metal ion stimulation
Cobalt chloride hexahydrate (CoCl2) (Sigma Aldrich) was diluted in complete cell culture media prior to cell stimulation. MonoMac 6 cells were treated with 0.75 mM CoCl2, a clinically-relevant concentration optimised in a previous study [9]. 100 ng/ml TLR4-specific LPS (from E.coli serotype J5, Alexis Biochemicals, San Diego, USA) as a positive control for 24 h after which supernatant (termed 'conditioned media') was collected.
2.3. TLR4 antagonist
CLI-095 (Invivogen, Toulouse, France) is a small molecule antagonist that binds to the intracellular domain of TLR4 to prevent recruitment of adaptor proteins and subsequent downstream signalling. MonoMac 6 cells were pre-treated with 1 μg/ml CLI-095 for 6 h prior to stimulation with 0.75 mM CoCl2 or 100 ng/ml LPS. Enzyme-linked immunosorbent assay (ELISA).
Cytokine secretion was quantified by enzyme-linked immunosorbent assay (ELISA). An IL-6 ELISA kit (Peprotech, London, UK) was used according to the manufacturer’s protocol with the same minor modification as described previously for an IL-8 ELISA [9]. CCL20 was quantified using a DuoSet ELISA kit (R&D Systems, Abingdon, UK) according to the manufacturer’s protocol.
2.4. Monocyte and neutrophil isolation
Monocytes and neutrophils were isolated from whole blood of healthy volunteers. Monocytes were isolated using Lympholyte (Cedarlane, Burlington, USA) according to the manufacturer's protocol and neutrophils were isolated by dextran sedimentation (Dextran T500, Pharmacosmos, Holbaek, Denmark) and centrifugation on Percoll (GE Healthcare, Buckinghamshire, UK) density gradients as previously described [15].
2.5. Transwell migration assay
Following isolation cells were resuspended in RPMI-1640 medium (Sigma Aldrich) supplemented with 10% FBS and 2 mM L-glutamine. Conditioned media from MonoMac 6 cells was added to the lower chamber of a 24-well companion plate (BD Falcon, New Jersey, USA). LPS conditioned media was used as a positive control and complete RPMI-1640 media provided a negative control. Three technical replicates were included for each condition. A Falcon cell culture insert with 3 µm pores (VWR Systems, Pennsylvania, USA) was placed in each well. 500,000 monocytes or neutrophils were added to the upper chamber of each filter and incubated at 37 °C for 2 h to allow for chemotaxis. Migrated monocytes and neutrophils pass through the pores in the cell culture insert and adhere to the underside of the filter.
Filters were fixed in methanol before staining migrated cells with haematoxylin. Following fixation, filters were air-dried for 3 h, excised, and mounted using DPX mountant (Sigma Aldrich). Five different visual fields were selected per filter and attached cells were counted at ×40 magnification.
2.6. Statistical analysis
Data was analysed using GraphPad Prism 6 software (GraphPad, California, USA). A Student’s t test was used for statistical analysis unless otherwise stated, with p<0.05 considered significant. All error bars show the standard error of the mean (SEM).
3. Results
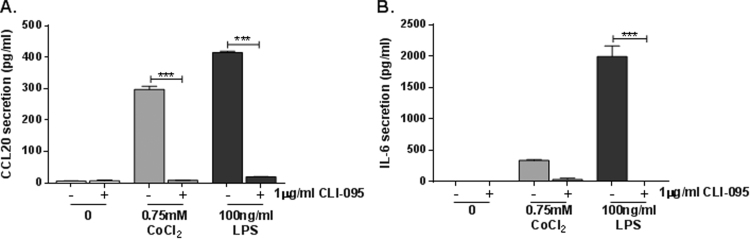
We have previously described TLR4-dependent IL-8 and CXCL10 secretion by cobalt-stimulated MonoMac 6 cells [9], as well as increased CCL20 and IL1A expression [16]. For the present study we have further investigated the effects of cobalt-mediated TLR4 activation on cytokine secretion by studying CCL20 and IL-6. MonoMac 6 cells were pre-treated with 1 μg/ml CLI-095 for 6 h prior to 24 h stimulation with 0.75 mM CoCl2 or 100 ng/ml TLR4-specific LPS as a positive control. In response to CoCl2 there was a significant increase in CCL20 secretion to approximately 300 pg/ml (P<0.001) (Fig. 1A). Pre-treatment with the TLR4 antagonist decreased CoCl2-mediated CCL20 release to <10 pg/ml (P<0.001). This was comparable to the CCL20 response to LPS, which was also significantly increased by the ligand and inhibited by CLI-095 (both P<0.001) (Fig. 1A). LPS increased IL-6 secretion by MonoMac 6 cells to approximately 2000 pg/ml (P<0.001), and this was significantly inhibited by CLI-095 (P<0.001) (Fig. 1B). In response to CoCl2 stimulation there was also a significant upregulation in IL-6 release but only to 350 pg/ml (P=0.045) (Fig. 1B). Although CLI-095 reduced CoCl2-mediated IL-6 release to approximately 50 pg/ml, this did not reach statistical significance (P=0.080) (Fig. 1B). However from the data obtained it is clear that cobalt ions can increase secretion of pro-inflammatory cytokines and chemokines through activation of TLR4.
Fig. 1.
Effect of cobalt-mediated TLR4 activation on CCL20 and IL-6 secretion. MonoMac 6 cells were pre-treated with 1 μg/ml CLI-095 for 6 h prior to 24 h stimulation with 0.75 mM CoCl2 or 100 ng/ml LPS. A. CCL20 and B. IL-6 secretion was assessed by ELISA. Data is representative of three independent experiments and statistical significance was calculated by one-way ANOVA with Tukey's test for multiple comparisons comparing al samples to each other.
The effect of cobalt ions on monocyte and neutrophil chemotaxis was investigated due to the presence of these innate immune cells around failed MoM implants, increased chemokine secretion in response to cobalt, and the role of TLR4 in innate immunity [9], [10], [17]. The TLR4 antagonist CLI-095 has previously been shown to inhibit cobalt-induced chemokine secretion by MonoMac 6 cells [9] and was used to investigate the role of TLR4 in cobalt-mediated monocyte and neutrophil chemotaxis.
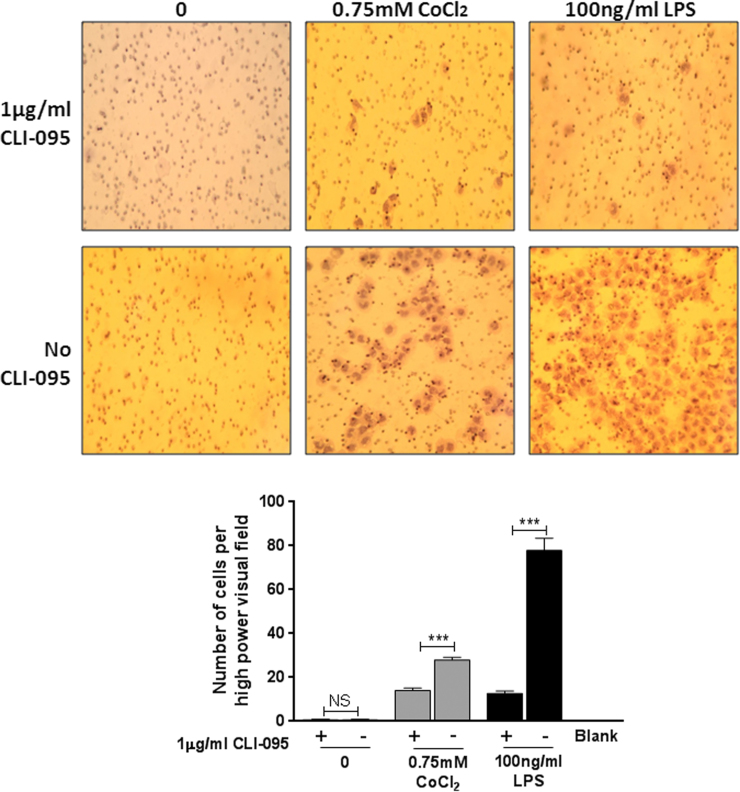
MonoMac 6 cells were left untreated or pre-treated with 1 μg/ml CLI-095 for 6 h, followed by 24 h treatment with 100 ng/ml LPS or 0.75 mM CoCl2. A transwell migration assay was then conducted using the conditioned media. Monocyte chemotaxis was significantly upregulated in response to CoCl2 conditioned media compared to the untreated control (P<0.001) (Fig. 2A and B). This increase was significantly inhibited by pre-treatment of MonoMac 6 cells with CLI-095 (P<0.001). Monocyte migration was also increased in response to conditioned media from TLR4-specific LPS and the effect was also abrogated by CLI-095 (P<0.001) demonstrating that CLI-095 specifically inhibits the TLR4 signalling pathway. Untreated conditioned media did not affect monocyte chemotaxis and there was no difference with the addition of CLI-095 (P=0.78). This shows that CLI-095 alone does not affect chemokine secretion, nor does it cause monocyte chemotaxis down a concentration gradient.
Fig. 2.
Effect of cobalt ions on monocyte chemotaxis. A. Filters stained with haematoxylin showing migrated monocytes at ×40 magnification. B. Migrated monocytes were counted at ×40 magnification. There was a significant increase in monocyte migration in response to 0.75 mM CoCl2 and 100 ng/ml LPS (24 h treatment) conditioned media compared to untreated controls. 1 µg/ml CLI-095 abrogated the CoCl2-induced effect (P<0.001). The effect of LPS was also inhibited demonstrating that the antagonist is specific for TLR4. CLI-095 had no effect on migration in untreated controls (P=0.78). Data is representative of three independent experiments each using a different monocyte donor. ***=P<0.001, Student's t-test.
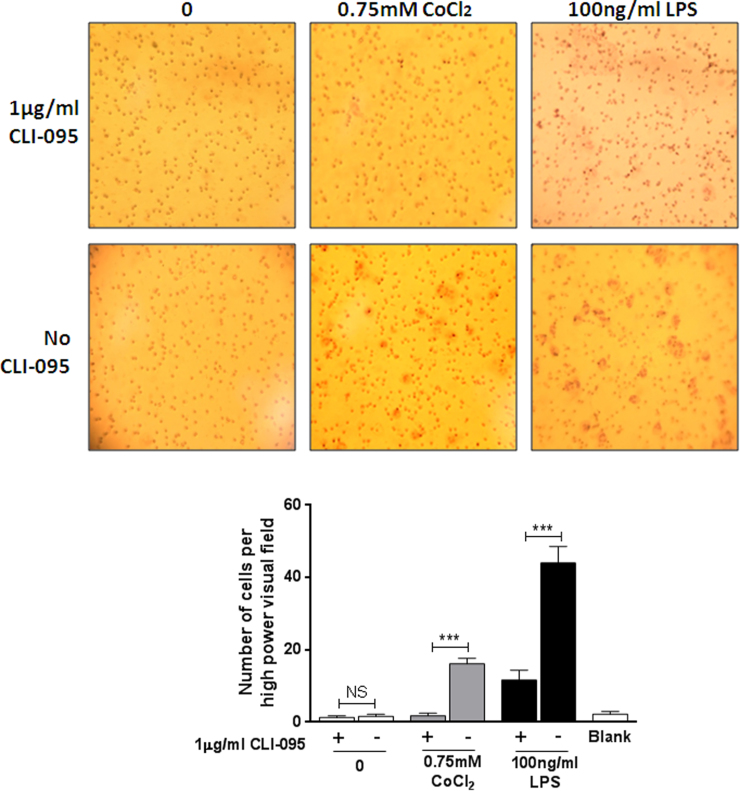
Neutrophil chemotaxis was also investigated and similar effects were observed to those in monocytes (Fig. 3A and B). Cobalt conditioned media induced neutrophil chemotaxis (P<0.001) and was significantly decreased in the presence of the TLR4 inhibitor (P<0.001), as was LPS-stimulated migration as a positive control (P<0.001). The untreated controls with and without CLI-095 had no effect on neutrophil migration and were not significantly different from each other (P=0.61).
Fig. 3.
Effect of cobalt ions on neutrophil chemotaxis. A. Filters stained with haematoxylin showing migrated neutrophils at ×40 magnification. B. Migrated neutrophils were counted at ×40 magnification. Neutrophil migration was significantly increased in response to 0.75 mM CoCl2 and 100 ng/ml LPS (24 h) conditioned media from MonoMac 6 cells compared to untreated controls. 1 µg/ml CLI-095 significantly abrogated the observed CoCl2-mediated chemotaxis (P<0.001). LPS-induced neutrophil chemotaxis was also inhibited by CLI-095 (P<0.001). Data is representative of three independent experiments each using a different neutrophil donor. ***=P<0.001, Student's t-test.
4. Discussion
This study set out to determine the chemotactic properties of cells stimulated with cobalt ions and the role of TLR4 in these responses. We have clearly shown that cobalt-mediated activation of TLR4 increases the release of inflammatory cytokines, specifically CCL20 and IL-6. This supports out previous work showing similar effects on release of IL-8 and CXCL10 [9]. Furthermore, we have demonstrated that conditioned media from cobalt-stimulated MonoMac 6 cells causes increased chemotaxis of primary monocytes and neutrophils. The observed monocyte and neutrophil chemotaxis can be attributed to TLR4 as the small molecule TLR4 antagonist CLI-095 abrogated the response. This demonstrates that chemokines capable of attracting innate immune cells are secreted via direct cobalt activation of the TLR4 signalling pathway.
Given the increased chemokine secretion observed in response to cobalt in previous studies it could be assumed that any conditioned media from cobalt-stimulated cells would be chemotactic. However it is important to note that many cytokines and chemokines, such as IL-10, can dampen an inflammatory response in order to modulate immunity and prevent systemic shock [18], [19]. Furthermore, while research has shown that cobalt induces certain individual pro-inflammatory factors including IL-8 [9] and CCL2 [10], it is the cumulative effect of chemokines that ultimately influences migration. Before the current study it was not fully understood whether cobalt ions would cause a chemotactic response or induce a chemokine profile that skewed the response against migration. Data presented here shows clearly that the overall cobalt-induced chemokine profile in monocytes is pro-migratory for both primary monocytes and neutrophils.
Based on our understanding of the effects of factors such as IL-8 and CCL2 on neutrophil and monocyte migration respectively, coupled with their known secretion in response to TLR4 activation by cobalt [9], [20], it can be surmised that they are at least in part responsible for the observed chemotaxis. Future work will involve neutralising different chemokines and their receptors to further investigate their individual role in the chemotaxis response to cobalt ions. Work will also be undertaken to determine whether or not the migration of monocytes and neutrophils affects subsequent recruitment of adaptive immune cells such as lymphocytes, which are also known to be present around failed MoM hip replacements [4], [21].
In conclusion, this study demonstrates the role of TLR4 in cobalt-mediated inflammatory cytokine secretion and innate immune cell chemotaxis, and highlights TLR4 as a potential therapeutic target in preventing inflammatory cell infiltration of MoM peri-implant tissues.
Acknowledgements
The authors acknowledge Mr Jonathan Scott for his help with neutrophil isolation.
This work was funded by a studentship from the Newcastle NIHR Biomedical Research Centre and Newcastle NIHR Biomedical Research Centre (grant number BH135138) and DePuy Sythes Ltd (grant number BH140255).
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.07.003.
Contributor Information
Helen Lawrence, Email: helen.lawrence@ncl.ac.uk.
David J. Deehan, Email: david.deehan@nuth.nhs.uk.
James P. Holland, Email: jim.holland@nuth.nhs.uk.
Sami A. Anjum, Email: s.a.anjum@ncl.ac.uk.
Amy E. Mawdesley, Email: amymawdesley@outlook.com.
John A. Kirby, Email: john.kirby@ncl.ac.uk.
Alison J. Tyson-Capper, Email: alison.tyson-capper@ncl.ac.uk.
Appendix A. Transparency document
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Wiley K.F., Ding K., Stoner J.A., Teague D.C., Yousuf K.M. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal-on-metal hip articulations: a meta-analysis. J. Arthroplast. 2013;28:1238–1245. doi: 10.1016/j.arth.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Daniel J., Holland J., Quigley L., Sprague S., Bhandari M. Pseudotumors associated with total hip arthroplasty. J. Bone Jt. Surg. – Ser. A. 2012;94:86–93. doi: 10.2106/JBJS.J.01612. [DOI] [PubMed] [Google Scholar]
- 3.Mahendra G., Pandit H., Kliskey K., Murray D., Gill H.S., Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties: relation to implant failure and pseudotumor formation. Acta Orthop. 2009;80:653–659. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natu S., Sidaginamale R.P., Gandhi J., Langton D.J., Nargol A.V.F. Adverse reactions to metal debris: Histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J. Clin. Pathol. 2012;65:409–418. doi: 10.1136/jclinpath-2011-200398. [DOI] [PubMed] [Google Scholar]
- 5.Tyson-Capper A.J., Lawrence H., Holland J.P., Deehan D.J., Kirby J.A. Metal-on-metal hips: cobalt can induce an endotoxin-like response. Ann. Rheum. Dis. 2013;72:460–461. doi: 10.1136/annrheumdis-2012-202468. [DOI] [PubMed] [Google Scholar]
- 6.Rachmawati D., Bontkes H.J., Verstege M.I., Muris J., Von Blomberg B.M.E., Scheper R.J., Van Hoogstraten I.M.W. Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermat. 2013;68:331–338. doi: 10.1111/cod.12042. [DOI] [PubMed] [Google Scholar]
- 7.Potnis P.A., Dutta D.K., Wood S.C. Toll-like receptor 4 signaling pathway mediates proinflammatory immune response to cobalt-alloy particles. Cell. Immunol. 2013;282:53–65. doi: 10.1016/j.cellimm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Raghavan B., Martin S.F., Esser P.R., Goebeler M., Schmidt M. Metal allergens nickel and cobalt facilitate TLR4 homodimerization independently of MD2. EMBO Rep. 2012;13:1109–1115. doi: 10.1038/embor.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence H., Deehan D., Holland J., Kirby J., Tyson-Capper A. The immunobiology of cobalt: demonstration of a potential aetiology for inflammatory pseudotumours after metal-on-metal replacement of the hip. Bone Jt. J. 2014;69B:1172–1177. doi: 10.1302/0301-620X.96B9.33476. [DOI] [PubMed] [Google Scholar]
- 10.Queally J.M., Devitt B.M., Butler J.S., Malizia A.P., Murray D., Doran P.P., O'Byrne J.M. Cobalt ions induce chemokine secretion in primary human osteoblasts. J. Orthop. Res. 2009;27:855–864. doi: 10.1002/jor.20837. [DOI] [PubMed] [Google Scholar]
- 11.Marchese A., George S.R., Kolakowski L.F., Jr, Lynch K.R., O’Dowd B.F. Novel GPCRs and their endogenous ligands: expanding the boundaries of physiology and pharmacology. Trends Pharmacol. Sci. 1999;20:370–375. doi: 10.1016/s0165-6147(99)01366-8. [DOI] [PubMed] [Google Scholar]
- 12.Baggiolini M., Walz A., Kunkel S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuentes M.E., Durham S.K., Swerdel M.R., Lewin A.C., Barton D.S., Megill J.R., Bravo R., Lira S.A. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J. Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- 14.Huang L.Y., DuMontelle J.L., Zolodz M., Deora A., Mozier N.M., Golding B. Use of toll-like receptor assays to detect and identify microbial contaminants in biological products. J. Clin. Microbiol. 2009;47:3427–3434. doi: 10.1128/JCM.00373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dransfield I., Buckle A.M., Savill J.S., McDowall A., Haslett C., Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (FcγRIII) expression. J. Immunol. 1994;153:1254–1263. [PubMed] [Google Scholar]
- 16.Lawrence H., Mawdesley A., Holland J., Kirby J., Deehan D., Tyson-Capper A. Targeting toll-like receptor 4 prevents cobalt-mediated inflammation. Oncotarget. 2016;7:7578. doi: 10.18632/oncotarget.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zughaier S.M., Ryley H.C., Jackson S.K. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect. Immun. 1999;67:1505–1507. doi: 10.1128/iai.67.3.1505-1507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vries J.E. De. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann. Med. 1995;27:537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- 19.Latifi S.Q., O'Riordan M.A., Levine A.D. Interleukin-10 controls the onset of irreversible septic shock. Infect. Immun. 2002;70:4441–4446. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K.S., Rajagopal V., Gonsalves C., Johnson C., Kalra V.K. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J. Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- 21.Watters T.S., Cardona D.M., Menon K.S., Vinson E.N., Bolognesi M.P., Dodd L.G. Aseptic lymphocyte-dominated vasculitis-associated lesion: a clinicopathologic review of an underrecognized cause of prosthetic failure. Am. J. Clin. Pathol. 2010;134:886–893. doi: 10.1309/AJCPLTNEUAH8XI4W. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material