Abstract
Plasma membrane Ca2+-ATPase (PMCA) plays a vital role in maintaining cytosolic calcium concentration ([Ca2+]i). Given that many diseases have modified PMCA expression and activity, PMCA is an important potential target for therapeutic treatment. This study demonstrates that the non-toxic, naturally-occurring polyphenol resveratrol (RES) induces increases in [Ca2+]i via PMCA inhibition in primary dermal fibroblasts and MDA-MB-231 breast cancer cells. Our results also illustrate that RES and the fluorescent intracellular calcium indicator Fura-2, are compatible for simultaneous use, in contrast to previous studies, which indicated that RES modulates the Fura-2 fluorescence independent of calcium concentration. Because RES has been identified as a PMCA inhibitor, further studies may be conducted to develop more specific PMCA inhibitors from RES derivatives for potential therapeutic use.
Abbreviations: RES, resveratrol; [Ca2+]i, cytosolic calcium concentration; PMCA, plasma membrane Ca2+-ATPase; EGCG, epigallocatechin gallate; SERCA, sarcoendoplasmic reticular Ca2+-ATPase; Fura-2, Fura-2-Acetoxymethyl ester; TG, thapsigargin; ROI, region of interest; HBSS, Ca2+- and Mg2+-free Hank's Balanced Salt Solution; FBS, fetal bovine serum; PBS, phosphate-buffered saline; BAPTA, BAPTA-Acetoxymethyl ester; DMSO, dimethyl sulfoxide; DMEM, Dulbecco's modified Eagle medium; ER, endoplasmic reticulum
Keywords: Plasma membrane Ca2+-ATPase, Resveratrol, Calcium signaling, Fura-2
Highlights
-
•
Resveratrol induces a rise in [Ca2+]i via plasma membrane Ca2+-ATPase inhibition.
-
•
FURA-2 is compatible with resveratrol in measuring [Ca2+]i.
-
•
PMCA inhibition is novel to resveratrol among naturally occurring polyphenols.
1. Introduction
Plasma membrane Ca2+-ATPase (PMCA) plays a critical role in maintaining the cytosolic calcium concentration ([Ca2+]i) in cells by extruding calcium from the cytosol into the extracellular space utilizing energy from ATP hydrolysis. PMCA consists of 10 transmembrane α-helices and 2 large cytosolic loops [1]. PMCA is part of a series of pumps and channels that maintain a calcium concentration gradient with a homeostatic [Ca2+]i of 100 nM. In contrast to the cytosol, the endoplasmic reticulum (ER) and extracellular milieu have calcium concentrations of approximately 1 mM. The elevated ER calcium concentration is sustained by the sarcoendoplasmic reticular Ca2+-ATPase (SERCA), which hydrolyzes ATP to transfer calcium from the cytosol to the ER. Channels on the ER membrane (inositol 1,4,5-trisphosphate (IP3) and ryanodine receptors) and plasma membrane (voltage-gated calcium channels, store-operated channels, and receptor-operated channels), when open, enable calcium to move down the concentration gradient from the ER or extracellular space into the cytosol. When [Ca2+]i rises above the resting concentration, PMCA expels calcium from the cytosol into the extracellular space. Together, these pumps and channels facilitate the use of Ca2+ as an important signaling mechanism inside the cell [2].
Changes in [Ca2+]i regulate critical cellular functions such as apoptosis, muscle contraction, neuronal synapse firing, and cellular motility [2]. The cellular response to [Ca2+]i modulation is dictated by the duration, magnitude, subcellular location, and the Ca2+-binding protein expression profile of each cell [3], [4]. PMCA activation controls the location, duration and magnitude of the changes in [Ca2+]i, and thereby helps determine the cellular response to the calcium flux.
Because PMCA is a critical component in defining [Ca2+]i, genetic mutations, overexpression, down-regulation, dysregulation, and inhibition of PMCA have an array of biological effects in mammalian cells. Alzheimer's disease, hypertension, male infertility, cardiovascular disease, deafness, diabetes, and cancer have all been correlated with altered PMCA activity [5], [6], [7], [8], [9]. More specifically, some of these diseases are characterized by modulated expression or activity of the four separate PMCA isoforms. For example, upregulation of PMCA is found in several gastric (KATO-III) and colon (HT-29, Caco-2, DLD-1, LS-174 T) cancer cell types during cell differentiation as well as in several breast cancer cell types (MDA-MB-231, T47D) [6], [10], [11]. Therapeutic options aiming to inhibit PMCA could offer new treatment options for cancers and other diseases with upregulated PMCA profiles as PMCA inhibition could generate the high [Ca2+]i typically associated with apoptosis [2]. Current inhibitors of PMCA have significant off-target effects due in part to a lack of isoform-specific inhibition [7]. The discovery of an additional PMCA inhibitor is an important step in the development of a more isoform-specific treatment for diseases exhibiting modulated PMCA profiles.
Resveratrol (trans-3, 4′, 5-trihydroxystilbene) (RES), a polyphenolic, cell-permeable phytoalexin found in grapes, peanuts and berries, exhibits chemotherapeutic, anti-aging, and cardioprotective properties [12], [13], [14], while exhibiting minimal toxicity in humans and animal models [15], [16]. One possible explanation for the pleiotropic biologic effects of RES is that RES modulates calcium signaling. RES has been shown to increase [Ca2+]i in breast cancer cells [17] and modulate the magnitude and duration of the calcium signal in excitable cells, such as neurons and muscle [18]. However, the mechanism by which the increase in [Ca2+]i is induced is unclear. In this study, we use a PMCA activity assay [19] to assess if RES directly inhibits PMCA and thereby induces increases in [Ca2+]i in both normal and cancerous cell lines. This study demonstrates that RES inhibits PMCA activity in both normal primary dermal fibroblasts (PDF) and MDA-MB-231 (MDA) breast cancer cells.
2. Materials and methods
The MDA-MB-231 human breast cancer (HTB-26) (MDA) and human primary dermal fibroblast (PCS-201–012) (PDF) cell lines were purchased from ATCC (Manassas, VA). The passage numbers of MDA cells used in experiments ranged from 10 to 35. Fura-2-Acetoxymethyl ester (14591) (Fura-2), thapsigargin (10522) (TG), (-)-epigallocatechin gallate (70935) (EGCG), quercetin (10005169), trans-resveratrol (70675) (RES), and BAPTA-Acetoxymethyl ester (15551) (BAPTA) were purchased from Cayman Chemical (Ann Arbor, MI). Lanthanum (III) chloride heptahydrate (LaCl3) (262072) was purchased from Sigma-Aldrich (St. Louis, MO). Fura-2, pentasodium salt (50032) was purchased from Biotium (Hayward, CA).
2.1. Cell culture
Both MDA and PDF cells were cultured in Dulbecco's Modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic-antimycotic. Cells were cultured at 37° C in 5% CO2.
2.2. Intracellular calcium imaging
Cells were prepared for imaging by seeding at a density of 20,000 (PDF) or 30,000–40,000 (MDA) cells per well in an 8-well chamber Lab-Tek #1.0 Borosilicate Coverglass (Thermo Scientific, Rochester, NY). Cells were grown for 48–72 h after initial seeding. No significant changes were recorded as a result of variation in initial cell density or time allowed for cells to grow. Prior to imaging, cells were washed twice with 1:1 FBS:PBS solution. Cells were then incubated in Ringer solution (NaCl 150 mM, glucose 10 mM, HEPES 5 mM, KCl 5 mM, MgCl2 1 mM, CaCl2 2 mM, pH 7.4) with 8 µM Fura-2 for 15 min at 37° C in 5% CO2. Where indicated, 20 µM BAPTA was co-loaded with Fura-2. The Fura-2/Ringer solution was then removed, and cells were incubated in fresh Ringer solution for 30 min at 37° C in 5% CO2. Ringer solution was then removed and replaced with a Ca2+-free buffer which was also used as vehicle for treatments. The Ca2+-free buffer used was either Ca2+- and Mg2+-free Hank's Balanced Salt Solution (HBSS) or, if treating with LaCl3, a Ca2+, Mg2+, and phosphate-free HEPES buffer (NaCl 150 mM, glucose 10 mM, KCl 5 mM, HEPES 5 mM, 10 mM EGTA, pH 7.4) to prevent La3+ ions from complexing with phosphate ions in HBSS. Cells were imaged following Fura-2 loading with an Olympus IX51 inverted microscope. All treatments were manually pipetted into the wells in 100 µL increments at the indicated time points. In all trials, cells were imaged for 1 min before the first treatment was added in order to establish a baseline. Subsequently, the cells were treated with 5 µM TG. In all trials, RES, LaCl3 and EGCG were dissolved in 1% DMSO in vehicle. Quercetin was dissolved in 2% DMSO in vehicle. Other details concerning specific treatments are included in the Results section. Data acquired from intracellular calcium imaging was analyzed with CellSens software by Olympus. Regions of interest (ROI) representing 1 cell each were selected from each individual experiment and analyzed for changes in [Ca2+]i. The resulting data from at least 10 ROIs per trial were then normalized (n≥3).
2.3. Emission spectra
Spectra measurements were taken by exciting at 340 nm and 380 nm while collecting the emission spectrum from 400 to 650 nm in Ca2+-free and 1 mM Ca2+ PBS buffers. RES and Fura-2 fluorescence were measured on the BMG LABTECH FLUOstar OPTIMA plate reader (BMG LABTECH Inc., Cary, NC). Triplicate 100 µL solutions were made and placed on a Greneir Bio-One Cellstar 96-well plate. Measurements of the Ca2+-containing solution (1 mM CaCl2 in PBS), and Ca2+-free solution (10 mM EGTA in PBS) with DMSO concentrations of 0.001%, 0.01%, 0.025%, 0.05%, 0.075%, or 0.1% were used as blanks. The Ca2+-containing solution and Ca2+-free solution were then treated to make up a 5 µM Fura-2-pentasodium salt solution with either 0, 1, 10, 25, 50, 75 or 100 µM RES. Spectra measurements were taken by exciting at 340 nm and 380 nm and collecting emissions at 510 nm.
2.4. Statistical analysis
All P values were calculated using a two-tailed t-test. P values from intracellular calcium imaging experiments were calculated by comparing the peak magnitude of [Ca2+]i of each treatment to the vehicle control. Statistical significance was determined by p<0.01.
3. Results
3.1. RES inhibits PMCA leading to an increased [Ca2+]i
We tested RES-induced PMCA inhibition because of the potential RES has as a therapeutic for PMCA-modulated diseases. Our interest in MDA-MB-231 cells stems from previous research that indicated PMCA upregulation in this cell type [6]. Previously, it was demonstrated that in MDA cells, RES had an indirect effect on PMCA by activating its degradation [17]. Additionally, RES has been shown to increase [Ca2+]i in many cell types, including MDA cells [17], [20], [21], [22]. Because of evidence supporting interactions between RES and PMCA, in this study, we use a PMCA activity assay to explore the RES-induced calcium signal [19]. We studied MDA cells while using PDF cells as a control to determine the direct effect of RES on PMCA inhibition in cells with and without a mutated PMCA profile.
As described in a previous study, we isolated endogenous PMCA activity [19]. We used Ca2+-free media to eliminate the possibility that changes in [Ca2+]i were due to an influx of Ca2+ from the extracellular space. The SERCA pump inhibitor thapsigargin (TG) was used to induce a rise in [Ca2+]i (Fig. 1). The TG-induced increase in [Ca2+]i is due to Ca2+ leaking from the ER, paired with the inhibited reuptake of Ca2+ by SERCA pumps. The subsequent decrease in [Ca2+]i is the result of PMCA activity. [Ca2+]i changes were measured using Fura-2, a Ca2+-binding, dual-excitation fluorescent compound [23]. Fura-2 is excited at 380 nm when Ca2+ is not bound and at 340 nm when Ca2+ is bound. We measured relative [Ca2+]i by loading Fura-2 into cells, alternately exciting Fura-2 at 340 nm and 380 nm, and measuring emission at 510 nm.
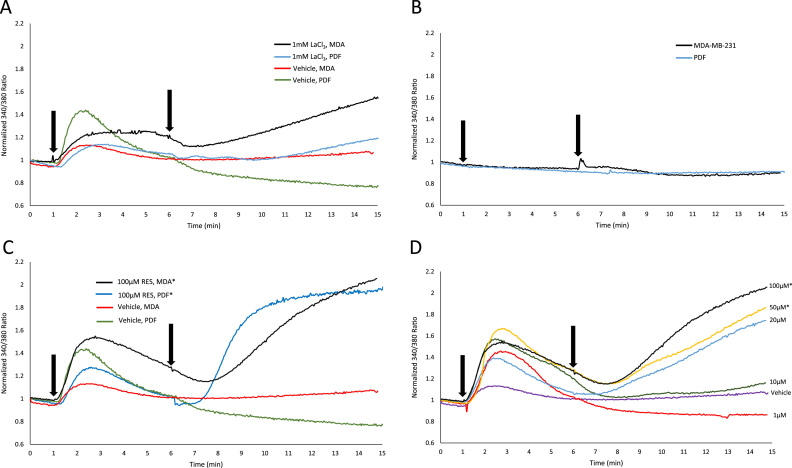
Fig. 1.
PMCA activity is inhibited by RES. Cells were monitored for changes in [Ca2+]i for 15 min using Fura-2. Graphs depict changes in [Ca2+]i as measured by 340 nm/380 nm ratio signal intensity. PMCA inhibition with LaCl3 (A) (arrows mark addition of 5 µM TG and 1 mM LaCl3 at 1 min and 6 min, respectively). LaCl3 induced an increase in [Ca2+]i in PDF (blue) and MDA (black) cell types as compared to vehicle only treatment in the respective cell types (PDF cells represented by green, MDA cells represented by red), indicative of PMCA inhibition. To ensure there were no calcium independent effects, BAPTA and Fura-2 were co-loaded into cells (B) (arrows mark addition of 5 µM TG and 50 µM RES at 1 min and 6 min, respectively). PMCA inhibition by RES (C), (arrows mark addition of 5 µM TG and 100 µM RES at 1 min and 6 min, respectively) in both PDF (blue) and MDA (black) cells. RES induced a statistically significant rise in [Ca2+]i as compared to a vehicle only treatment (* signifies a statistically significant difference from respective vehicle controls at p<0.01) in the respective cell types (PDF cell vehicle treatment represented by green, MDA cell vehicle treatment represented by red). RES dose-dependent PMCA inhibition (D). Arrows mark addition of 5 µM TG and followed by either 1 (red), 10 (green), 20 (blue), 50 (yellow), or 100 µM (black) RES at 1 min and 6 min, respectively. RES induced statistically significant increases in [Ca2+]i (* signifies a statistically significant increase from vehicle control (violet) at p<0.01) in 50 µM and 100 µM RES treatments.
As shown in Fig. 1, TG was added in all experiments after 1 min of imaging, followed by a treatment of RES or LaCl3, a known PMCA inhibitor [24], 5 min after TG addition. As a positive control for PMCA inhibition, we treated MDA and PDF cells with 1 mM LaCl3 (Fig. 1A) and measured changes in relative [Ca2+]i. Each treatment with LaCl3 induced an increase in [Ca2+]i in MDA and PDF cells, though this did not yield statistically significant results because of the variability of the peak magnitude of [Ca2+]i, which was used to quantify the results in Fig. 1.
To determine if there were any calcium-independent effects of TG or RES on Fura-2 fluorescence, we co-loaded both cell types with Fura-2 and 20 µM BAPTA (a cell-permeable calcium chelator). Co-loaded cells were treated with TG and 50 µM RES (Fig. 1B). Neither TG nor RES elicited a response in the [Ca2+]i of co-loaded cells in either cell type, indicating that neither TG nor RES modulated Fura-2 fluorescence independent of changes in [Ca2+]i. Upon treatment with 100 µM RES in Fura-2 loaded cells, both MDA and PDF cell lines responded with a significant increase in [Ca2+]i compared to vehicle treatments (p<0.01) (Fig. 1C), similar to the increase seen upon treatment with LaCl3. We also found that PMCA inhibition by RES is dose-dependent in MDA cells (Fig. 1D). Similar in design to the above experiments, we treated MDA cells 5 min after TG treatment with varying concentrations of RES (1, 10, 20, 50, or 100 µM). At the concentrations of 50 and 100 µM, RES induced significant increases in [Ca2+]i (p<0.01). Our findings indicate that at 50 and 100 μM, RES induces PMCA inhibition.
3.2. RES PMCA inhibition is unique in comparison to similar polyphenols
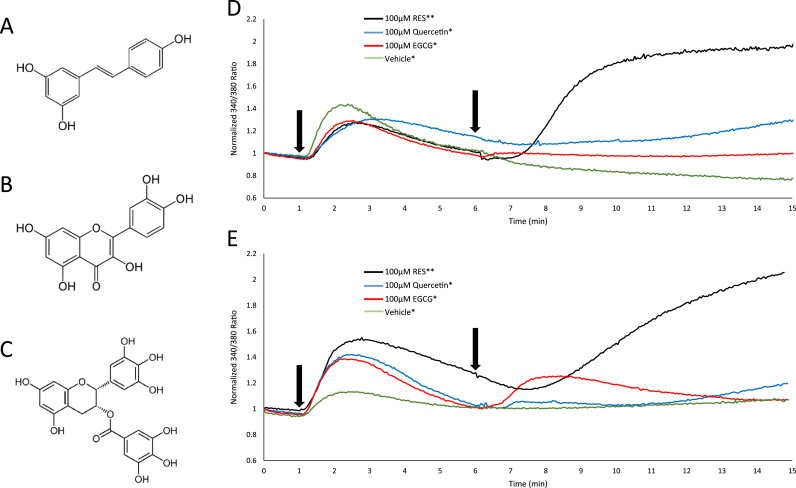
Quercetin and epigallocatechin gallate (EGCG) are systemically nontoxic, naturally-occurring polyphenols that share structural characteristics with RES (Fig. 2A–C) [25], [26]. Treatment with quercetin and EGCG was designed to assess if these compounds, which have chemical makeup similar to RES, also have a similar inhibitory effect upon PMCA. Treatment of PDF (Fig. 2D) and MDA cells (Fig. 2E) with either 100 µM quercetin or EGCG following TG treatment produced a change in [Ca2+]i that was statistically different from that induced by RES. The lack of apparent PMCA inhibition demonstrated by two similar compounds, EGCG and quercetin, suggests that PMCA inhibition is unique to RES.
Fig. 2.
PMCA inhibition by RES is unique among polyphenols. Structures of resveratrol (A), quercetin (B), and epigallocatechin gallate (EGCG) (C). PDF cells (D) were imaged for 15 min using Fura-2. Arrows represent treatment with 5 µM TG, and either 100 µM RES (black), 100 µM quercetin (blue), 100 µM EGCG (red) or vehicle (green) at 1 min and 6 min, respectively. Quercetin, EGCG and vehicle all showed statistically significant differences in [Ca2+]i changes from RES in PDF cells (* signifies a statistically significant difference from RES at p<0.01,** signifies that RES-treated cells differed significantly from vehicle-treated cells at p<0.01). MDA cells (E) were imaged for 15 min using Fura-2. Arrows represent treatment with 5 µM TG, and either 100 µM RES (black), 100 µM quercetin (blue), 100 µM EGCG (red) or vehicle (green) at 1 min and 6 min, respectively. Quercetin, EGCG and vehicle all showed statistically significant differences in [Ca2+]i changes from RES in MDA cells (* signifies a statistically significant difference from RES at p<0.01,** signifies that RES-treated cells differed significantly from vehicle-treated cells at p<0.01). Differences in calcium response to quercetin and EGCG indicate that neither molecule inhibits PMCA to the same degree as RES.
3.3. Calcium independent effects of RES on Fura-2
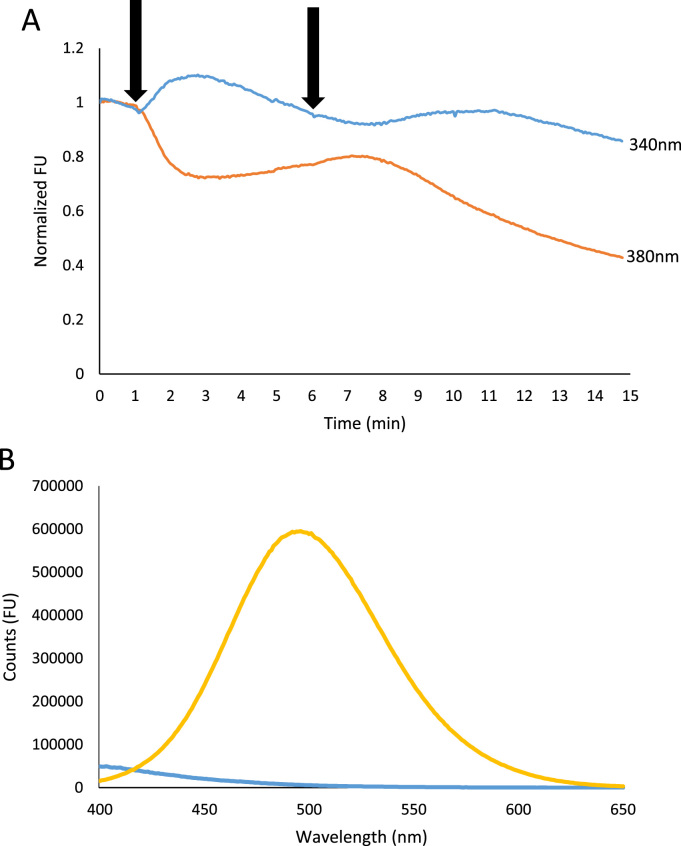
Previous studies have shown that RES elicits a calcium-independent increase of the calcium-bound (340 nm) Fura-2 signal through an additive fluorescent effect without exhibiting a change in the calcium-free (380 nm) signal [27], [28], [29]. These past studies question the validity of research conducted using RES and Fura-2 together. In contrast with these studies, our data illustrates that treatment of 100 µM RES in MDA cells increases the 340 nm signal and decreases the 380 nm signal (Fig. 3A).
Fig. 3.
Emission spectra of RES and Fura-2. Separate measurements (A) of 340 nm (Ca2+-bound, blue) and 380 nm (Ca2+-free, orange) signal intensity in MDA cells with 5 µM TG and 100 µM RES treatments at 1 min and 6 min, respectively, as indicated by the arrows. This data (A) is representative of all experiments conducted at these concentrations. Emission spectra (B) in 1 mM Ca2+ solution when excited at 340 nm of 100 µM RES (blue), and 5 µM Fura-2 (yellow).
To further verify that RES does not interfere with Fura-2, we performed in vitro measurements of the emission spectra of RES and Fura-2 when excited at 340 nm and 380 nm in Ca2+-free, and 1 mM Ca2+ solutions. We measured Fura-2 fluorescence at its estimated intracellular concentration of 5 µM [30]. To our knowledge, the intracellular concentration of RES has not been reported; therefore, we measured the spectra at 100 µM, which is close to the upper limit for RES solubility in aqueous solutions. Fura-2 fluorescence when excited at 340 nm and 380 nm in a 1 mM Ca2+ solution was approximately 100 and 15 times larger than RES at the same wavelengths, respectively. In the absence of calcium, 340 nm and 380 nm Fura-2 510 nm emission was 120 and 140 fold higher than that of 100 µM RES (data not shown). RES, in the same buffer and at a concentration 20 times higher than Fura-2, only emitted approximately 1% of the signal that Fura-2 did, therefore any additive effect from RES would be minor (Fig. 3B). However, synergistic effects between RES and Fura-2, if any, could explain why previous studies have seen a calcium-independent spike in the Fura-2 calcium-bound signal in RES-treated cells.
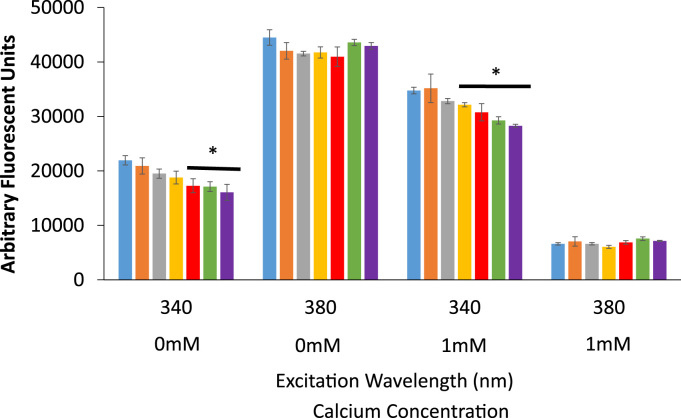
Although we estimated that the additive effect of RES on Fura-2 fluorescence would be minimal, we wanted to ensure that there was no synergistic effect by RES on Fura-2 fluorescence. Solutions were made containing 5 µM Fura-2 alone and Fura-2 with 1, 10, 25, 50, 75, and 100 µM RES. RES significantly decreased the Fura-2 510 nm emission when excited at 340 nm (Fig. 4) at concentrations above 50 µM in Ca2+-free solution and above 25 µM in 1 mM Ca2+ solution. RES showed no statistically significant effect on Fura-2 fluorescence when excited at 380 nm in either the Ca2+-free or 1 mM Ca2+ solution (Fig. 4). The diminished Fura-2 fluorescence by RES is likely due to spectral overlap as RES absorbance spectra shows significant absorbance at 340 nm, but no absorbance at 380 nm [31]. Taken together, this data indicates that RES does not increase the calcium-bound fluorescence of Fura-2, but rather has a quenching effect, which may mask the full magnitude of the RES-induced calcium signal.
Fig. 4.
The direct effects of RES on Fura-2 fluorescence. Solutions containing 5 µM Fura-2 pentasodium salt were titrated with RES at 0 µM (blue), 1 µM (orange), 10 µM (gray), 25 µM (yellow), 50 µM (red), 75 µM (green), and 100 µM (purple). The samples were excited at 340 nm and 380 nm and the emission of each sample was measured at 510 nm. Each measurement was performed in triplicate in PBS with 0 mM Ca2+ or 1 mM Ca2+ as outlined in the figure (*signifies a statistically significant difference from Fura-2 emission at 510 nm at p<0.01). Error bars represent the standard deviation.
Although this data is sufficient to conclude that RES does not interfere with Fura-2 fluorescence in vitro, it does not address what occurs in vivo. To provide evidence that RES is not directly interfering with Fura-2 in vivo, we co-loaded cells with the calcium chelator, BAPTA, and Fura-2 (Fig. 1B). BAPTA pretreatment eliminates any effects of [Ca2+]i changes in the cell by sequestering free, cytosolic Ca2+ ions. Upon treatment of the co-loaded cells with RES, no change in Fura-2 fluorescence was observed, indicating that the RES-induced change in Fura-2 fluorescence is Ca2+-dependent and not artifact. Because the Fura-2 340 nm/380 nm ratio is higher in the 1 mM Ca2+ solution than in the Ca2+-free solution, despite spectral overlap between Fura-2 and RES, we conclude that, in our hands, the RES-induced calcium signal appears to be reflective of the changes in [Ca2+]i due to PMCA inhibition.
4. Discussion
In this paper, we demonstrate that RES inhibits PMCA in a dose-dependent manner. PMCA is an important therapeutic target given that changes in PMCA activity and expression are prevalent in diseased cells. Current putative therapeutic options for PMCA inhibition are generally limited by their non-specificity to PMCA isoforms and/or impermeability to the cell [7], [32]. The novel discovery of RES as a PMCA inhibitor sets the foundation for further study on the effects of RES or RES derivatives on diseases with modulated PMCA activity or expression. RES has also been shown to be cell-permeable and, as a result, may act on PMCA via a different mechanism than current PMCA inhibitors [33]. We also demonstrate that RES and Fura-2 are compatible for simultaneous use, thereby confirming the validity of the measurement methods used in this study. We illustrate that inhibition of PCMA is unique to RES in comparison to two polyphenols of similar chemical structure, EGCG and quercetin. Our data suggest that other polyphenolic compounds do not demonstrate PMCA inhibition. The use of binding assays to explore the effects of such structural differences on the RES-PMCA interaction could yield the information necessary to synthesize RES derivatives capable of inhibiting specific PMCA isoforms without the off-target effects normally induced by RES.
One of the known effects of RES on tumorigenic cells is apoptosis. Previous studies have indicated that the apoptotic response to RES is tumor-cell specific [33], [34], [35], [36]. Our results indicate that tumor-specific apoptosis induced by RES is not solely the result of PMCA inhibition because the associated changes in [Ca2+]i are similar in both tumorigenic and non-tumorigenic cells. We draw this conclusion because PMCA inhibition profiles are similar in non-tumorigenic PDF and tumorigenic MDA cells. However, previous studies have shown that calcium signaling is an essential part of the RES-dependent apoptotic effect on tumor cells by using the intracellular Ca2+ chelator, BAPTA, to block RES-dependent apoptosis [37]. Indeed, further research in other cell types, animal and human models will be needed to understand the relation between PMCA inhibition and the RES-dependent apoptotic response. Given that PMCA is inhibited in tumorigenic and non-tumorigenic cells, but apoptosis is differentiated by cell type, we postulate that cancer cells have modified or additional Ca2+-binding proteins downstream from the calcium signaling event that trigger the apoptotic response.
Another section of this study worth further exploration involves the separate isoforms of PMCA. As previously mentioned, PMCA exists in four isoforms, with each isoform possessing multiple splice variants. PMCA1 and 4 are generally universally expressed and serve housekeeping functions whereas PMCA2 and 3 are differentiated by cell type [38]. Specific PMCA-related diseases are typically associated with the modification of one or more PMCA isoforms [6], [7]. In MDA cells, PMCA2 and 4 are upregulated [6], which may confer cancer the ability to avoid the high [Ca2+]i levels associated with apoptosis. We speculate that one of the many facets of RES-induced apoptosis may be the demonstrated inhibition of PMCA isoforms in cancer cells, thus inducing the sufficiently high [Ca2+]i necessary for apoptosis. In relation to our data, PMCA1 and 4 are present in PDF cells, while PMCA1, 2 and 4 are present in MDA cells [39], [40]. Because RES caused an increase in [Ca2+]i in a similar fashion in both cell types, there are several possibilities as to the isoform specificity of RES as a PMCA inhibitor. As PMCA1 and 4 are present in both cell types, RES may be acting to inhibit one or both of these isoforms. The possibility also exists that RES is a pan-PMCA inhibitor, meaning that it inhibits all isoforms of PMCA. It seems unlikely that RES is acting as a PMCA2-specific inhibitor as both cell types react similarly to RES treatment in our experiments while PMCA2 is found in MDA cells and not in PDF cells. Further study of RES and development of RES derivatives may yield isoform-specific PMCA inhibitors with the capability of acting as non-toxic treatments for cancers and other diseases with upregulated PMCA profiles.
5. Conclusion
Our data more fully elucidates the relationship between RES and PMCA by demonstrating RES-dependent PMCA inhibition in primary human fibroblasts and MDA-MB-231 breast cancer cells. Additionally, our data shows that PMCA inhibition is novel to RES in comparison to other naturally occurring polyphenols, EGCG and quercetin. Previous studies have shown that RES and Fura-2 have spectral overlap, which led to an artifactual rise in the Fura-2 calcium measurement. However, we demonstrate that Fura-2 can be used to measure intracellular calcium changes induced by RES. Further study of PMCA and RES may yield isoform-specific PMCA inhibitors capable of non-toxic treatment of specific diseases.
Conflict of interest
None declared.
Acknowledgments
We appreciate the assistance of Dr. Scott Weber and Claudia Tellez for their guidance in using their calcium microscope. We would also like to thank Jackson Kenealey (age 7) for drawing structures for us on ChemDraw. This work was funded by BYU Life Sciences Start-up Fellowship.
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.06.019.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Lopreiato R., Giacomello M., Carafoli E. The plasma membrane calcium pump: new ways to look at an old enzyme. J. Biol. Chem. 2014;289:10261–10268. doi: 10.1074/jbc.O114.555565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M.J. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 4.Bootman M.D., Collins T.J., Peppiatt C.M., Prothero L.S., MacKenzie L., De Smet P., Travers M., Tovey S.C., Seo J.T., Berridge M.J., Ciccolini F., Lipp P. Calcium signalling – an overview. Semin. Cell Dev. Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- 5.Lehotsky J., Kaplan P., Murin R., Raeymaekers L. The role of plasma membrane Ca2+ pumps (PMCAs) in pathologies of mammalian cells. Front. Biosci. 2002;7:d53–d84. doi: 10.2741/A769. [DOI] [PubMed] [Google Scholar]
- 6.Lee W.J., Roberts-Thomson S.J., Monteith G.R. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem. Biophys. Res. Commun. 2005;337:779–783. doi: 10.1016/j.bbrc.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 7.Strehler E.E. Plasma membrane calcium ATPases as novel candidates for therapeutic agent development. J. Pharm. Pharm. Sci. 2013;16:190–206. doi: 10.18433/j3z011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brini M., Cali T., Ottolini D., Carafoli E. The plasma membrane calcium pump in health and disease. FEBS J. 2013;280:5385–5397. doi: 10.1111/febs.12193. [DOI] [PubMed] [Google Scholar]
- 9.Curry M.C., Luk N.A., Kenny P.A., Roberts-Thomson S.J., Monteith G.R. Distinct regulation of cytoplasmic calcium signals and cell death pathways by different plasma membrane calcium ATPase isoforms in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2012;287:28598–28608. doi: 10.1074/jbc.M112.364737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribiczey P., Tordai A., Andrikovics H., Filoteo A.G., Penniston J.T., Enouf J., Enyedi A., Papp B., Kovacs T. Isoform-specific up-regulation of plasma membrane Ca2+ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium. 2007;42:590–605. doi: 10.1016/j.ceca.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanHouten J., Sullivan C., Bazinet C., Ryoo T., Camp R., Rimm D.L., Chung G., Wysolmerski J. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc. Natl. Acad. Sci. USA. 2010;107:11405–11410. doi: 10.1073/pnas.0911186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal B.B., Bhardwaj A., Aggarwal R.S., Seeram N.P., Shishodia S., Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 13.Kasiotis K.M., Pratsinis H., Kletsas D., Haroutounian S.A. Resveratrol and related stilbenes: their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013;61:112–120. doi: 10.1016/j.fct.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Wu J.M., Hsieh T.C. Resveratrol: a cardioprotective substance. Ann. N. Y. Acad. Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- 15.Williams L.D., Burdock G.A., Edwards J.A., Beck M., Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Cottart C.-H., Nivet-Antoine V., Laguillier-Morizot C., Beaudeux J.-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 17.Sareen D., Darjatmoko S.R., Albert D.M., Polans A.S. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol. Pharm. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- 18.McCalley A.E., Kaja S., Payne A.J., Koulen P. Resveratrol and calcium signaling: molecular mechanisms and clinical relevance. Molecules. 2014;19:7327–7340. doi: 10.3390/molecules19067327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samad A., James A., Wong J., Mankad P., Whitehouse J., Patel W., Alves-Simoes M., Siriwardena A.K., Bruce J.I. Insulin protects pancreatic acinar cells from palmitoleic acid-induced cellular injury. J. Biol. Chem. 2014;289:23582–23595. doi: 10.1074/jbc.M114.589440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos-Toimil M., Elies J., Orallo F. Trans- and cis-resveratrol increase cytoplasmic calcium levels in A7r5 vascular smooth muscle cells. Mol. Nutr. Food Res. 2005;49:396–404. doi: 10.1002/mnfr.200400108. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J.Q., Wu P.F., Long L.H., Chen Y., Hu Z.L., Ni L., Wang F., Chen J.G. Resveratrol promotes cellular glucose utilization in primary cultured cortical neurons via calcium-dependent signaling pathway. J. Nutr. Biochem. 2013;24:629–637. doi: 10.1016/j.jnutbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Elies J., Cuinas A., Garcia-Morales V., Orallo F., Campos-Toimil M. Trans-resveratrol simultaneously increases cytoplasmic Ca(2+) levels and nitric oxide release in human endothelial cells. Mol. Nutr. Food Res. 2011;55:1237–1248. doi: 10.1002/mnfr.201100240. [DOI] [PubMed] [Google Scholar]
- 23.Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Chen Y.-F., Cao J., Zhong J.-N., Chen X., Cheng M., Yang J., Gao Y.-D. Plasma membrane Ca 2+-ATPase regulates Ca2+ signaling and the proliferation of airway smooth muscle cells. Eur. J. Pharmacol. 2014;740:733–741. doi: 10.1016/j.ejphar.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 25.Lu N.T., Crespi C.M., Liu N.M., Vu J.Q., Ahmadieh Y., Wu S., Lin S., McClune A., Durazo F., Saab S., Han S., Neiman D.C., Beaven S., French S.W., Phase A., Dose Escalation I. Study demonstrates quercetin safety and explores potential for bioflavonoid antivirals in patients with chronic hepatitis C. Phytother. Res. 2015 doi: 10.1002/ptr.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh B.N., Shankar S., Srivastava R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharm. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp R.F., Leech C.A., Roe M.W. Resveratrol interferes with Fura-2 intracellular calcium measurements. J. Fluoresc. 2013 doi: 10.1007/s10895-013-1312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paudel R.C., Kiviluoto S., Parys J.B., Bultynck G. Resveratrol is not compatible with a Fura-2-based assay for measuring intracellular Ca2+ signaling. Biochem. Biophys. Res. Commun. 2014;450:1626–1630. doi: 10.1016/j.bbrc.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 29.Santofimia-Castaño P., Salido G.M., Gonzalez A. Interferences of resveratrol with Fura-2-derived fluorescence in intracellular free-Ca2+ concentration determinations. Cytotechnology. 2015:1–12. doi: 10.1007/s10616-015-9898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran N.N., Leroy P., Bellucci L., Robert A., Nicolas A., Atkinson J., Capdeville-Atkinson C. Intracellular concentrations of Fura-2 and Fura-2/am in vascular smooth muscle cells following perfusion loading of Fura-2/am in arterial segments. Cell Calcium. 1995;18:420–428. doi: 10.1016/0143-4160(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 31.Camont L., Cottart C.H., Rhayem Y., Nivet-Antoine V., Djelidi R., Collin F., Beaudeux J.L., Bonnefont-Rousselot D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal. Chim. Acta. 2009;634:121–128. doi: 10.1016/j.aca.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Pande J., Szewczyk M.M., Grover A.K. Allosteric inhibitors of plasma membrane Ca pumps: invention and applications of caloxins. World J. Biol. Chem. 2011;2:39–47. doi: 10.4331/wjbc.v2.i3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyagi A., Gu M., Takahata T., Frederick B., Agarwal C., Siriwardana S., Agarwal R., Sclafani R.A. Resveratrol selectively induces DNA damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin. Cancer Res. 2011;17:5402–5411. doi: 10.1158/1078-0432.CCR-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Ginkel P.R., Darjatmoko S.R., Sareen D., Subramanian L., Bhattacharya S., Lindstrom M.J., Albert D.M., Polans A.S. Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Invest. Ophthalmol. Vis. Sci. 2008;49:1299–1306. doi: 10.1167/iovs.07-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baarine M., Thandapilly S.J., Louis X.L., Mazue F., Yu L., Delmas D., Netticadan T., Lizard G., Latruffe N. Pro-apoptotic versus anti-apoptotic properties of dietary resveratrol on tumoral and normal cardiac cells. Genes Nutr. 2011;6:161–169. doi: 10.1007/s12263-011-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jing X., Cheng W., Wang S., Li P., He L. Resveratrol induces cell cycle arrest in human gastric cancer MGC803 cells via the PTENregulated PI3K/Akt signaling pathway. Oncol. Rep. 2016;35:472–478. doi: 10.3892/or.2015.4384. [DOI] [PubMed] [Google Scholar]
- 37.Ma X., Tian X., Huang X., Yan F., Qiao D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol. Cell Biochem. 2007;302:99–109. doi: 10.1007/s11010-007-9431-8. [DOI] [PubMed] [Google Scholar]
- 38.Krebs J. The plethora of PMCA isoforms: alternative splicing and differential expression. Biochim. Biophys. Acta. 1853;2015:2018–2024. doi: 10.1016/j.bbamcr.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Reisner P.D., Brandt P.C., Vanaman T.C. Analysis of plasma membrane Ca(2+)-ATPase expression in control and SV40-transformed human fibroblasts. Cell Calcium. 1997;21:53–62. doi: 10.1016/s0143-4160(97)90096-8. [DOI] [PubMed] [Google Scholar]
- 40.Lee W.J., Roberts-Thomson S.J., Holman N.A., May F.J., Lehrbach G.M., Monteith G.R. Expression of plasma membrane calcium pump isoform mRNAs in breast cancer cell lines. Cell Signal. 2002;14:1015–1022. doi: 10.1016/s0898-6568(02)00049-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material