Abstract
The incidence of ischaemic heart disease (IHD) is increasing. The patients with IHD with or without interventions coming for non-cardiac surgical procedures are also increasing. These patients have increased risk of myocardial ischaemia, myocardial infarction (MI), conduction disturbances, morbidity and mortality during the peri-operative period. The risks of these events are even higher in patients with recent MI. An anaesthesiologist should be aware of the pathophysiology and the need to thoroughly evaluate the patient for peri-operative management. We searched Pubmed using combinations of terms like “ischemic heart disease” and “anaesthesia”, “perioperative”, and “anaesthetic implications”. We reviewed the current practices and guidelines regarding evaluation, risk stratification and management.
Key words: Evaluation, myocardial infarction, revascularisation, risk stratification
INTRODUCTION
The life expectancy of general Indian population has increased, and there is also an increase in the incidence of coronary artery disease among the population. Ischaemic heart disease (IHD) is the leading cause of morbidity and mortality all over the world. Among the estimated 25 million patients in the United States who undergo surgery each year, approximately 7 million are considered to be at high risk of IHD. Goldman et al. reported that 500,000–900,000 myocardial infarctions (MIs) occur annually worldwide with subsequent mortality of 10%–25%.[1,2] The number of people with coronary artery disease with or without intervention coming for non-cardiac procedures has also increased.
Patients with coronary artery diseases undergoing non-cardiac surgery are at an increased risk for peri-operative complications such as myocardial ischaemia, MI, cardiac failure, arrhythmias, cardiac arrest and increased morbidity and mortality. These complications are much higher in patients with recent MI or unstable angina who require urgent or emergency cardiac surgery.[3,4] For this review, we searched Pubmed using combinations of terms like “ischemic heart disease” and “anaesthesia”, “perioperative”, and “anaesthetic implications”. We reviewed the current practices and guidelines regarding evaluation, risk stratification and management.
PRE-OPERATIVE EVALUATION
The purpose of pre-operative evaluation is to evaluate a patient's current medical status, to provide clinical risk profiling, to decide on further testing, to treat the modifiable risk factors and to plan the management of cardiac illness during the peri-operative period.
History and physical examination
A thorough history and physical examination are important. A history suggestive of angina pectoris, recent MI, unstable angina and cerebrovascular disease should be sought.
Prior myocardial infarction
The American College of Cardiology (ACC)/American Heart Association (AHA) practice guidelines considered the period within 6 weeks of acute MI as a period of high risk for a peri-operative cardiac event, as it is the mean healing time of the infarcted myocardium. The period from 6 weeks to 3 months is of intermediate risk. This period is extended beyond 3 months in cases with complications such as arrhythmias, ventricular dysfunction or continued medical therapy. Patients who have undergone coronary revascularisation procedure within 5 years and are asymptomatic have low perioperative risk surgery and can undergo surgery without any further evaluation.[5] Pre-operative questionnaires, preferably computer-based version filled by anaesthesiologists may be helpful.[5]
Cardiovascular disease
When active cardiac disease is suspected in patients posted for surgery, cardiologists should be involved. Patients may be taking several drugs such as antihypertensives, diuretics, anticoagulants, etc., for a variety of reasons. Whether or not to continue the drugs during the peri-operative period, and if discontinued, alternative drugs and restarting time should be planned.
Respiratory disease, smoking and obstructive sleep apnoea syndromes
Routine pre-operative diagnostic spirometry and chest X-ray are not recommended. Chest X-ray has limited value in patients with established risk factors older than 70 years, as it does not alter the peri-operative management. Patients with obstructive sleep apnoea syndrome should be evaluated carefully for a potential difficult airway. Continuous positive airway pressure use in the pre-operative period in such patients may be helpful in reducing hypoxic events. Pre-operative incentive spirometry can be of benefit in upper abdominal surgery to prevent post-operative pulmonary complications. Malnutrition should be corrected. Smoking should be stopped at least 4 weeks prior to surgery, and preferably for 6-8 weeks prior.
Renal disease
The risk index of Kheterpal et al. is useful for the identification of patients for postoperative renal impairment.[4] Calculated glomerular filtration rate is superior to serum creatinine for the identification of patients with pre-existing renal impairment. Urine output should be monitored throughout the peri-operative period, and adequate fluid management should be done.[4]
Diabetes mellitus
Pre-operative assessment includes a formal assessment of the risk of a patient having disordered glucose homoeostasis. Routine pre-operative blood sugar testing is not necessary in established well-monitored diabetic patients when they properly maintain their glycaemic status/glycated haemoglobin (HbA1c).[6] Patients at high risk of disordered glucose homeostasis should be identified as needing specific attention to peri-operative glucose control.[6] Known diabetics should be managed according to the guidelines.
Obesity
Pre-operative assessment of obese patients includes clinical evaluation, Berlin or STOP questionnaire,[6] electrocardiogram (ECG), polysomnography, oximetry, glucose/HbA1c concentrations and haemoglobin measurement.
Coagulation disorders
If a coagulation disorder is suspected based on patient's history and/or clinical examination, haematological evaluation should be done prior to the procedure. Correction of haemostasis decreases peri-operative bleeding.
Anaemia and pre-operative blood conservation strategies
Pre-operative supplementation of iron corrects anaemia.
The elderly
Risk, not the age, should be used to trigger increased assessment and preparation.
Concurrent medication
One should inquire about herbal medicines, particularly those (ginseng, garlic and gingko) which may cause increased bleeding in the peri-operative period or have other unwanted interaction/side effects. Herbal medicines should be discontinued 2 weeks before surgery.
Psychotropic medication
Patients on antidepressants, selective serotonin reuptake inhibitors and antipsychotic medications should not discontinue their treatment. Patients treated with tricyclic antidepressants should have cardiac evaluation. Monoamine oxidative inhibitors should be discontinued at least 2 weeks before anaesthesia. Lithium administration should be discontinued 72 h before surgery.
Peri-operative bridging of anticoagulation therapy
In high-risk patients under oral anticoagulation, bridging management for the peri-operative period is highly recommended. In minor surgical procedures such as cataract or minor soft tissue surgery, continuation of warfarin therapy can be considered.[7]
Pre-operative tests
12-lead electrocardiogram
It is useful to detect myocardial ischaemia, MI, cardiac rhythm and/or conduction disturbances, ventricular hypertrophy and electrolyte abnormalities.
Non-invasive cardiac investigations
The exercise ECG simulates stimulation of sympathetic nervous system and effects that may accompany peri-operative events such as laryngoscopy and surgical stimulation.
The pharmacological stress testing reproduces the cardiovascular effects of exercise and is particularly useful for patients who are unable to exercise. It includes dipyridamole, thallium myocardium imaging and dobutamine stress echocardiography.
Recommendations for peri-operative coronary angiography
The Class 1 recommendations for pre-operative coronary angiography apply only to the patients with[5]
Evidence for high risk of adverse outcome based on non-invasive test results
Angina pectoris unresponsive to medical therapy
Unstable angina, particularly when facing intermediate- or high-risk non-cardiac surgery
Equivocal non-invasive tests results in patients with high clinical risk undergoing high-risk surgery.
RISK STRATIFICATION
Overall assessment of peri-operative cardiac risk requires consideration of the type of surgery planned, presence and type of specific clinical indicators of coronary artery disease and patient functional status.
Earlier, the Goldman et al. multifactorial risk index[8] was used to stratify patients according to cardiac risk. Later, Detsky et al.[9] provided improved risk assessment in patients undergoing vascular surgery. In 1999, Lee et al. proposed much superior revised cardiac risk index.
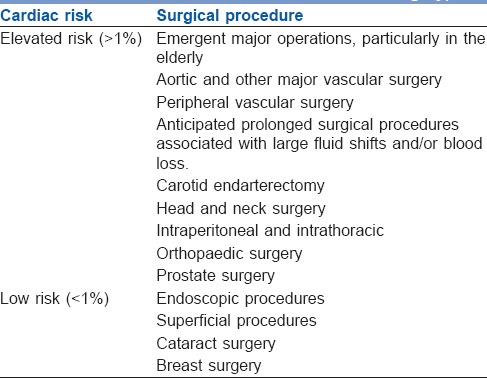
A low-risk procedure is one in which the combined surgical and patient characteristics predict a risk of a major adverse cardiac event (MACE) of death or MI of <1%. Procedures with a risk of MACE of ≥1% are considered elevated risk.[3] Risk factors are explained in Tables 1–4.[10,11,12,13]
Table 1.
Clinical predictors of increased peri-operative cardiovascular risk
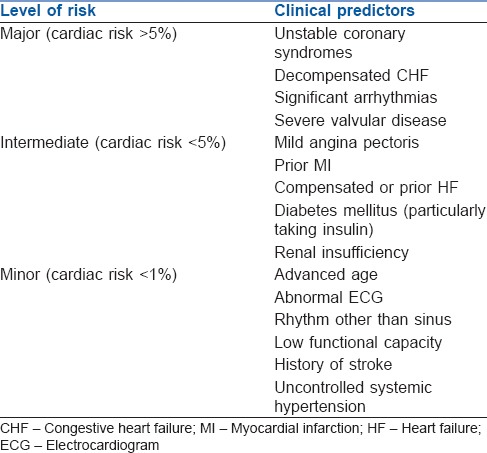
Table 4.
Risk factors identified by the American College of Physicians

Table 2.
Assessment of functional capacity: The duke activity index (approximate metabolic equivalents) 1 metabolic equivalent-represents an oxygen consumption of 3.5 ml/kg/min
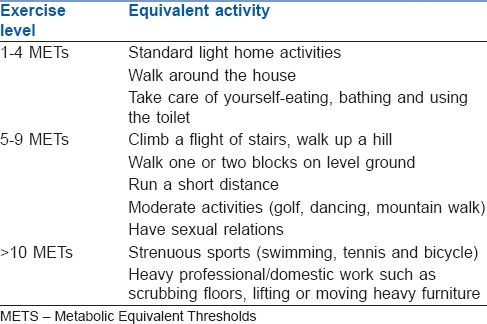
Table 3.
Cardiac risk classification of non-cardiac surgical procedures (American College of Cardiologists/American Heart Association guidelines for peri-operative cardiovascular evaluation for non-cardiac surgery)
Peri-operative myocardial infarction
Peri-operative MI may be due to myocardial oxygen supply/demand mismatch or due to acute plaque disruption because of increased sympathetic activities such as increased heart rate and blood pressure.
Prevention of peri-operative MI includes pre-operative coronary revascularisation and pharmacological intervention.
Coronary revascularisation
Studies showed that when the complications and mortality rates of CABG are taken into account, combined mortality of cardiac and non-cardiac procedures is not different from the mortality of non-cardiac surgery alone when IHD has been managed by medical treatment only.[5]
Indications for pre-operative coronary artery revascularisation are as follows:
Acceptable coronary revascularisation risk and viable myocardium with left main coronary artery stenosis
Three vessel coronary artery disease with left ventricular dysfunction
Left main equivalent (high-grade block in the left anterior descending artery and circumflex artery)
Intractable coronary ischaemia despite maximal medical therapy.
Major noncardiac procedures should wait at least 4–6 weeks (possibly 6 months).
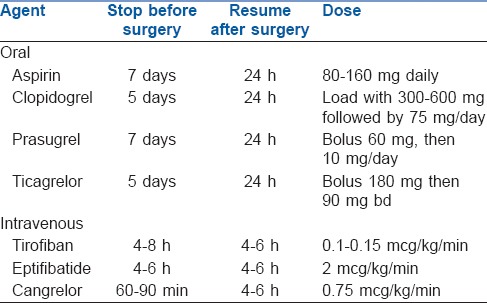
In a patient with recent coronary angioplasty and stenting, the risk of stent thrombosis and MI is increased if dual antiplatelet treatment is stopped, while the risk of surgical bleeding is increased on continuation of the drugs in the peri-operative period. The use of low dose aspirin (75 mg/day) in patients undergoing non-cardiac surgery should be based on an individual decision, which depends on the peri-operative bleeding risk, weighed against the risk of thrombotic complications[4] [Table 5].
Table 5.
Pre-operative interruption and resumption of antiplatelet therapy
The ACC/AHA guidelines recommend a delay of at least 6 weeks between insertion of bare metal stent and noncardiac surgery and 6 months (preferably 1 year) delay for drug eluting stents. This allows time for stent reendothelisation. In case stent insertion is required prior to surgery, either insertion of a bare metal stent or just percutaneous angioplasty is preferable.[14,15]
BRIDGING THERAPY
This therapy is for select patients who have a high thrombotic profile undergoing a surgical procedure, who are at risk for haemorrhage, in whom dual anti-platelet therapy (DAPT) is to be stopped as surgery cannot be delayed.
Currently available drugs for bridging therapy are tirofiban, eptifibatide and cangrelor. Oral antiplatelet drugs are stopped 5–7 days before planned surgical procedures and started on continuous intravenous infusion of tirofiban or eptifibatide until 4–6 h of procedures. These drugs are restarted postoperatively till DAPT can be reinstituted.
MEDICAL MANAGEMENT
Peri-operative beta-blockers
Beta-blockers are known to cause decrease in sympathetic tone and myocardial contractility. Hence, myocardial oxygen supply/demand balance is maintained. Metoprolol, atenolol and bisoprolol are the most common beta-blockers used. Target heart rate is 50–70 beats per minute. The ACC/AHA guidelines propose that patients already receiving beta blockers should continue taking them preoperatively and throughout the perioperative period. Beta-blockers should not be started acutely, but started at least 24 hours before elective surgery and dose titrated to achieve the target heart rate 50–60 beats/min without significant hypotension.[5,16]
Alpha adrenergic agonists
Clonidine is a centrally acting alpha adrenergic agonist. It is used as a sedative, anxiolytic and analgesic and reduces hypertension, tachycardia and norepinephrine release associated with surgical stress. Clonidine can cause bradycardia and hypotension.[17]
ANAESTHESIA MANAGEMENT
Anaesthetic goals
The primary goal of the anaesthetic management of a patient with coronary artery disease for non-cardiac surgery is the avoidance of myocardial ischaemia and MI. This is by avoiding the factors which impair myocardial oxygen supply-demand balance.
Anything which increases cardiac work such as physical work, emotional stress, surgical and anaesthesia stress increases myocardial oxygen demand which is compensated in normal individuals by increasing coronary blood flow. This is not so in patients with IHD where coronary flow already is compromised.
The factors which decrease myocardial oxygen supply are decreased coronary blood flow, tachycardia, hypotension, increased preload, hypoxia, coronary artery spasm, decreased oxygen content and its availability, anaemia, hypoxemia, etc. Factors which increase oxygen demand are tachycardia, increased wall tension, increased afterload (hypertension) and increased myocardial contractility.
All anaesthetic techniques must aim to keep myocardial oxygen supply greater than demand and thus avoid ischaemia. The essential requirements of general anaesthesia for IHD are avoiding tachycardia and extremes of blood pressure, both of which adversely affect balance between oxygen supply and demand.
Premedication
Anxiolytics such as short-acting benzodiazepines can be prescribed to these patients as anxiety can cause tachycardia and hypertension.
ANAESTHESIA TECHNIQUE
General or regional anaesthesia can be chosen alone or in combination as parts of balanced technique depending on the surgery and patient requirements.
General anaesthesia
Maintenance of haemodynamic stability with attenuation of the haemodynamic responses to intubation and surgical stimulation are the main goals.
Induction
Most of the induction agents are myocardial depressants and cause decrease in systemic vascular resistance with increased venous pooling. Etomidate is preferred because of minimal cardiovascular effects. Concern about etomidate is that it can inhibit cortisol synthesis. Propofol is an alternative. Ketamine is avoided because it can cause sympathetic stimulation.
Intubation
Stress response to laryngoscopy should be avoided by using drugs such as opioids, lidocaine or induction agents which can attenuate haemodynamic responses.
Maintenance
Maintenance of anaesthesia is either by volatile agents such as isoflurane, sevoflurane, desflurane or by total intravenous anaesthesia with propofol, analgesics (opioids) and using muscle relaxants. Volatile anaesthetics have cardioprotective effects but may not be significant in non-cardiac surgical patients.
Extubation
It should be smooth by avoiding sympathetic stimulation. This can be achieved using opioids and beta-blockers.
Regional anaesthesia
Either spinal or epidural anaesthesia can be good choices in intermediate- and low-risk surgeries involving extremities, perineum and lower abdomen. Guidelines have to be followed for those who are on anticoagulant drugs. Central neuraxial blockade can cause hypotension which should be treated with adequate preload and vasopressors such as phenylephrine.
A 2014 review of nine systematic reviews of randomised controlled clinical trials summarises the outcomes of neuraxial analgesia with or without general anaesthesia versus general anaesthesia alone in patients undergoing any type of surgery On comparison with general anaesthesia, use of neuraxial blockade alone reduces 0–30-day mortality and decreases the risk of pneumonia.[18]
MONITORING
Monitoring is very important to detect ischaemia, arrhythmias and haemodynamic instabilities. Essential monitors are as follows:
Pulse oxymetry, capnography, urine output, temperature and non-invasive blood pressure
Electrocardiography: Patients should have continuous ECG monitoring to detect myocardial ischaemia and arrhythmias. Computerised ST-segment analysis is superior and multiple lead monitoring is more sensitive. Lead 2 and V4 can detect 80% of ischaemic episodes; adding V5 may increase it up to 97%[19]
Invasive arterial pressure: Direct measurement of blood pressure is more reliable than indirect methods, especially in major surgeries and when large fluid shifts are expected
Central venous catheters: are indicated when significant blood loss, large fluid shifts and/or vasopressor infusions are expected
Pulmonary artery catheters: Usually not indicated unless patients requiring cardiac output monitoring e.g., patients with cardiomyopathies
Transoesophageal echocardiography: Though not proven, high-risk patients may be benefitted as TOE may help detect ischaemia, ventricular dysfunction and fluid status.[20]
TREATMENT OF ISCHAEMIA
The factors which alter myocardial oxygen supply/demand balances should be treated. Tachycardia and hypertension caused by pain and inadequate anaesthesia should be corrected by deepening the plane of anaesthesia using inhalational or intravenous anaesthetic, opioid or local anaesthetics through central neuraxial or peripheral nerve block catheters. Beta-blockers such as esmolol, metoprolol and labetalol are useful. If ST-segment changes still exist, vasodilators such as nitroglycerine can be used. Hypotension can be treated with fluid and vasopressors such as phenylephrine. Persistent hypotension can be treated with vasopressor infusion. Haemoglobin should be maintained >8 g/dl. Hypothermia has to be treated.
TREATMENT OF ARRHYTHMIAS
Arrhythmias are common in patients with IHD. Ventricular fibrillation, atrial fibrillation and bradycardia are some of the life-threatening arrhythmias and should be treated with drugs or DC shock accordingly.
POST-OPERATIVE MANAGEMENT
The patient should be monitored for ischaemia by continuous ECG in the Intensive Care Unit. Serial 12-lead ECG and troponin measurements are useful in patients suspected to have ischaemia.[21]
Effective pain management is important to reduce stress, adverse haemodynamics and hypercoagulable states. Epidural anaesthesia, use of opiates in subarachnoid block, other regional blocks and patient-controlled analgesia are other useful techniques. Non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors should be avoided in these patients.[4]
CONCLUSION
As the incidence if IHD increases in the population, the number of patients coming for non-cardiac surgery will also increase. As these patients are prone for myocardial ischaemia, infarction and arrhythmias during peri-operative period, a thorough evaluation has to be done regarding history and tests. Any modifiable risk factors have to be corrected. Whenever required, further testing has to be ordered. Since this is a team effort, we have to involve cardiologists, surgeons, treating physicians and patients. Anti-failure medications, beta-blockers and statins have to be continued throughout peri-operative period. For regional anaesthesia, guidelines have to be followed regarding anticoagulant medications. Factors which alter myocardial oxygen supply-demand ratio are to be taken care of. Monitoring is important to detect early ischaemia and rhythm disturbances. Post-operative pain management is other important aspect. Hence, proper evaluation and management peri-operatively are the key to success.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to acknowledge Sparsh Super Specality Hospital, Bengaluru, and Sri Jayadeva Institute Cardiovascular Sciences and Research, Bengaluru, Karnataka, India.
REFERENCES
- 1.Hall MJ, Owings MF, Hyattsville MD. Department of Health and Human Services 2002. Advance Data From Vital and Health Statistics, No. 329. National Hospital Discharge Survey (PHS) 2002 Jun 19; [Google Scholar]
- 2.Kaul TK, Tayal G. Anaesthetic considerations in cardiac patients undergoing non cardiac surgery. Indian J Anaesth. 2007;51:280–6. [Google Scholar]
- 3.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: Executive summary: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130:2215–45. doi: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen SD, Knuuti J. New ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management. Eur Heart J. 2014;35:2344. doi: 10.1093/eurheartj/ehu285. [DOI] [PubMed] [Google Scholar]
- 5.Eagle KA, Berger PB, Calkins H. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Non Cardiac Surgery) Anesth Analg. 2002;94:1052. doi: 10.1097/00000539-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 6.De Hert S, Imberger G, Carlisle J, Diemunsch P, Fritsch G, Moppett I, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684–722. doi: 10.1097/EJA.0b013e3283499e3b. [DOI] [PubMed] [Google Scholar]
- 7.Lobato EB, Bavry AA. The patient with coronary stents undergoing non cardiac surgery. In: Kaplan JA, Augoustides JG, editors. Kaplan's Cardiac Anaesthesia. 7th ed. Elsevier: 2017. p. 1493. [Google Scholar]
- 8.Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–50. doi: 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- 9.Detsky AS, Abrams HB, Forbath N, Scott JG, Hilliard JR. Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index. Arch Intern Med. 1986;146:2131–4. [PubMed] [Google Scholar]
- 10.Glance LG, Lustik SJ, Hannan EL, Osler TM, Mukamel DB, Qian F, et al. The surgical mortality probability model: Derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255:696–702. doi: 10.1097/SLA.0b013e31824b45af. [DOI] [PubMed] [Google Scholar]
- 11.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the duke activity status index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, et al. Exercise standards for testing and training: A statement for healthcare professionals from the American heart association. Circulation. 2001;104:1694–740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 13.L'Italies GJ, Paul SD, Hendel RC. Development and Validation of a Bayesian model for perioperative risk assessment in a cohort of 1,081 vascular surgical candidates. J Am Coll Cardiol. 1996;27:779. doi: 10.1016/0735-1097(95)00566-8. [DOI] [PubMed] [Google Scholar]
- 14.Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–72. doi: 10.1001/jama.2013.278787. [DOI] [PubMed] [Google Scholar]
- 15.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31:2501–55. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 16.Andersson C, Mérie C, Jørgensen M, Gislason GH, Torp-Pedersen C, Overgaard C, et al. Association of β-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: A Danish nationwide cohort study. JAMA Intern Med. 2014;174:336–44. doi: 10.1001/jamainternmed.2013.11349. [DOI] [PubMed] [Google Scholar]
- 17.Devereaux PJ, Sessler DI, Leslie K, Kurz A, Mrkobrada M, Alonso-Coello P, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–13. doi: 10.1056/NEJMoa1401106. [DOI] [PubMed] [Google Scholar]
- 18.Guay J, Choi P, Suresh S. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: An overview of Cochrane systemic reviews. Ccochrane Database Syst Rev. 2014:CD010108. doi: 10.1002/14651858.CD010108.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landesberg G, Luria MH, Cotev S, Eidelman LA, Anner H, Mosseri M, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet. 1993;341:715–9. doi: 10.1016/0140-6736(93)90486-z. [DOI] [PubMed] [Google Scholar]
- 20.London MJ, Tubau JF, Wong MG, Layug E, Hollenberg M, Krupski WC, et al. The “natural history” of segmental wall motion abnormalities in patients undergoing noncardiac surgery. S.P.I. Research Group. Anesthesiology. 1990;73:644–55. doi: 10.1097/00000542-199010000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Liu SS, Wu CL. The effect of analgesic technique on postoperative patient-reported outcomes including analgesia: A systematic review. Anesth Analg. 2007;105:789–808. doi: 10.1213/01.ane.0000278089.16848.1e. [DOI] [PubMed] [Google Scholar]


