Abstract
Background and Aims:
Sepsis is a major worldwide cause of morbidity and mortality. Most sepsis epidemiologic data are from the Western literature. Sparse data from India describe the epidemiology of infection rather than sepsis which is a host response to infection. This study describes the epidemiology of sepsis in the Intensive Care Unit (ICU) of an Indian tertiary care hospital.
Subjects and Methods:
A prospective study conducted between June 2006 and May 2011. All consecutively admitted patients during the 5 year study >=18 years of age were included and data obtained from hospital in-patient records. Variables measured were the incidence of severe sepsis, ICU, hospital, and 28-day mortality, the median length of ICU stay, median Acute Physiology and Chronic Health Evaluation II (APACHE II) score, infection site, and microbial profile.
Results:
There were 4711 admissions during the study with 282 (6.2%, 95% confidence interval 2.3, 13.1) admissions with severe sepsis. ICU mortality, hospital mortality, and 28-day mortality were 56%, 63.6%, and 62.8%, respectively. Predominant infection site was respiratory tract. The most common organisms were Gram-negative microbes. The most common microbe was Acinetobacter baumanni. Median APACHE II score on admission was 22 (interquartile range 16–28) and median length of ICU stay was 8 days. Severe sepsis attributable mortality was 85%.
Conclusion:
Severe sepsis is common in Indian ICUs and is mainly due to Gram-negative organisms. ICU mortality is high in this group and care is resource intensive due to increased length of stay.
Keywords: Epidemiology, intensive care patients, mortality, prevalence, severe sepsis
INTRODUCTION
Sepsis is a major cause of morbidity and mortality and the second leading cause of death worldwide.[1] Epidemiologic data on sepsis varies depending on the origin of database– community-based or hospital-based, nature of data collection-retrospective chart review, discharge diagnoses, diagnosis in death certificates, or prospective observational studies. A robust epidemiological study methodology should be prospective in nature conducted over a prolonged period and should include heterogeneous case mix representative of the disease, thus allowing generalizability of observed data. Epidemiological data on sepsis come mostly from western literature.[1,2,3,4,5,6] Data from India are sparse and in the form of epidemiology of infection (both community and hospital acquired) rather than sepsis which is a host response to infection.[7,8,9,10] Moreover, literature and surveys conducted in India concentrate on the microbiological profile, resistance pattern, antibiotic usage, and outcome rather than sepsis epidemiology. To address this deficiency, we conducted a prospective observational study on severe sepsis for 5 years in a tertiary care hospital in India.
SUBJECTS AND METHODS
Study setting
This study was conducted as a prospective observational cohort study at a 43-bedded Intensive Care Unit (ICU) (medical and surgical, neurology, and trauma care) of a tertiary care hospital in India. The study protocol was reviewed by the hospital ethical committee and informed consent was waived because the nature of this study was observational.
Study population
All consecutive adult (age ≥18 years) admissions in the ICUs between June 1, 2006 and May 31, 2011 were included in the study. Patient data were extracted from in-patient record charts and investigation reports daily. Physicians in charge of patients were also interviewed to ensure consistency of data. Patients who stayed in the ICU for <24 h for routine postoperative surveillance or those who were discharged alive from ICU within 24 h without developing sepsis or complications were excluded, and no further data were collected from them. Patients admitted to ICU on more than one occasion during the study were counted as a new case on every admission.
Identification of severe sepsis patients
Patients who stayed in ICU for ≥24 h were screened daily for features of systemic inflammatory response syndrome (SIRS), sepsis and severe sepsis as per the ACCP/SCCM criteria.[11] SIRS was considered to be present when patients had two or more of the following clinical findings; (i) body temperature higher than 38°C or lower than 36°C; (ii) heart rate higher than 90/min; (iii) hyperventilation evidenced by respiratory rate higher than 20/min or PaCO2 lower than 32 mmHg; (iv) white blood cell count higher than 12,000 cells/μl or lower than 4000/μl.[11] Sepsis was defined by the presence of both infection and SIRS and severe sepsis referred to the presence of sepsis with at least one criterion for organ dysfunction.[11] The criteria for organ dysfunction were adapted from those used in the PROWESS study.[12] Patients were screened daily for new sepsis episodes if they remained in ICU after recovery from the initial septic episode. The new episode was counted separately from the previous one. The source of infection was defined by adopting criteria from ANZICS study.[3] Variables that were recorded on admission included age, sex, admission category (medical, surgical, trauma), primary diagnosis, chronic comorbidities, clinical and laboratory data to calculate Acute Physiology and Chronic Health Evaluation II (APACHE II) score (using worst variable reading during the 1st 24 h of ICU admission and worst Glasgow Coma Score before sedation or anesthesia.[13] The predicted mortality rate (PMR) and standardized mortality ratio (SMR) were calculated using APACHE II scores.
Additional data collected included infection site (defined by treating physician applying ANZICS study criteria)[3] and infection source (outside or in ICU). Body fluid samples as indicated (blood, urine, sputum, broncho-alveolar lavage fluid, tracheal secretions, pus from any site, pleural, and peritoneal fluids) were sent for microbial assessment on the 1st day of presumed sepsis and at anytime during ICU stay if clinically indicated (new onset fever, new chest infiltrates, and hypotension) for identifying causative pathogens. All severe sepsis patients were screened for their vital status (alive or dead) on day 28 using hospital discharge records. Outcome variables calculated were the prevalence of adult ICU admissions with severe sepsis, ICU mortality rate, hospital mortality rate, and 28-day mortality rate.
Data were collected and fed into a computerized database. Any data discrepancy was cross-checked with patient records for errors during entry. All patient personal identifiers were removed from data files to maintain patient confidentiality. Quality of data was assessed by screening 50 randomly selected records every 3 months. Any difference was resolved by consensus. Patients with incomplete data were included only for relevant data analysis.
Statistical analysis
Univariate distribution of variables was computed and noted for their deviations from normalcy, skewness, and kurtosis. The normally distributed data are presented as mean and standard deviation, whereas median and interquartile range (IQR) were used to describe nonnormally distributed data. The occurrence rate of severe sepsis was calculated as percentage, dividing number of severe sepsis episodes by number of adult ICU admissions, who stayed for more than 24 h in ICU. The prevalence of sepsis and ICU mortality between men and women were compared using Chi-square tests. We used t-tests to compare mean ICU LOS between men and women. The prevalent site of infection was calculated as the observed proportion of infection in each site with their 95% confidence intervals. Prevalence of microbial organisms was the observed proportion of microbes among the total spectrum of microbial organisms observed in ICU. Two-tailed tests were used with a significance level set at α =0.05. All statistical analysis was performed using the PC-SAS program (V9.2, SAS Institute, Cary, NC, USA). The crude mortality rate, PMR, and the SMR were calculated with the help of the Medcalc statistical software (MedCalc Software, Mariakerke, Belgium).
RESULTS
The study included patients from three ICU's (medical-surgical ICU, neurology ICU, and trauma ICU) in the same center. There were a total of 4711 patients during the study which included 4657 first admissions and 54 (1%) readmissions. Most admissions were due to medical reasons (86.4%). A total of 790 (16.7%) patients did not show features of SIRS during ICU stay; 73.4% had SIRS only, whereas SIRS with organ dysfunction was found in 463 (9.8%) patients. SIRS with organ dysfunction due to an infection (severe sepsis) was noted in 264 patients (6%) who had 282 sepsis episodes [Figure 1].
Figure 1.
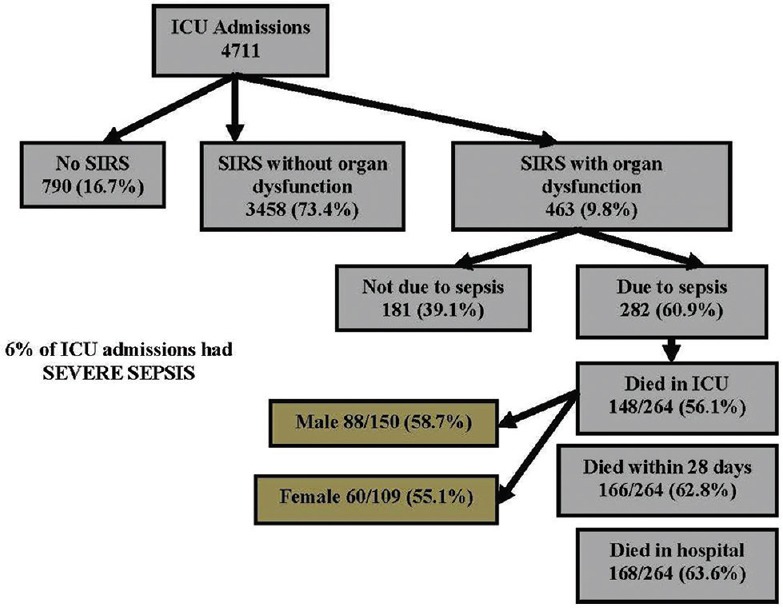
Summarizes the total number of severe sepsis patients and their outcomes during the study
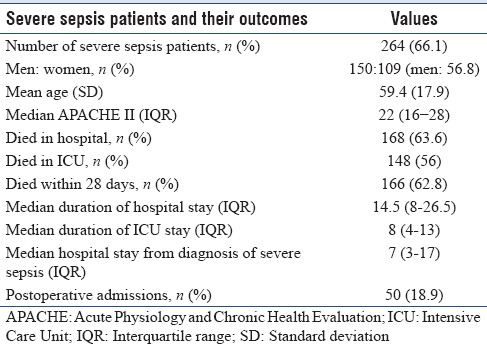
Characteristics of patients with severe sepsis are described in Table 1. Nonsignificant increase of severe sepsis episodes occurred in men (56.8%; P = 0.4). There was no significant difference of age between severe septic and nonsevere septic patients (P = 0.7) and this was also irrespective of gender (P = 0.6). The median length of stay in ICU of severe sepsis patients was 8 (IQR: 4–13) days and was similar between men and women (P = 0.6). The median duration of hospital stay for the severe sepsis patients was 14.5 (IQR 8–26.5) days.
Table 1.
Characteristics of severe sepsis patients and outcome
The most frequent site of infection among severe sepsis patients was respiratory tract (53.3%), followed by abdomen (14.9%), blood stream (14.3%) and urinary tract infection (12.9%) [Table 2]. Severe sepsis was the reason for admission in 84% of severe sepsis cohort, whereas 16% of patients had ICU acquired infections. Microbiological documentation was available in 172 (61%) episodes. Majority of infections were caused by Gram-negative organisms (73%); 6.2% of severe sepsis patients had fungal infections (candidemia, aspergillosis), whereas tropical diseases (malaria, dengue, leptospirosis) were documented in 7.2% of severe sepsis patients. The commonly isolated microbes were Acinetobacter baumannii (21.2%), Pseudomonas Aeruginosa (17%) and equal prevalence of Klebsiella and Escherichia coli (15.4%) [Table 3].
Table 2.
Sites of infection according to their predominance
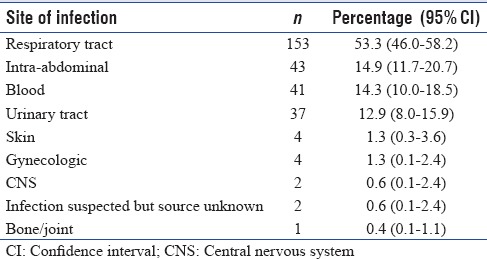
Table 3.
Microbial profile of severe sepsis patients

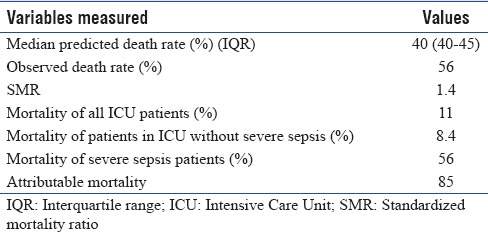
Of the total 264 severe sepsis patients admitted to the ICU during the study, 148 (56%) died in the ICU, and 166 (62.8%) patients died within 28 days. The in-hospital mortality was 63.6%. There was no significant mortality difference between men and women (58.7% and 55.1%; P = 0.9). The majority (77%) of deaths occurred in patients with multi-organ failure which did not respond to treatment. The median APACHE II score of the severe sepsis cohort was 22 (IQR 16–28). Observed death rate (57.9%) was higher than predicted death rate (40%). The SMR was 1.4. Mortality of the study cohort without severe sepsis was 8.4% giving rise to an attributable mortality rate of 85% in severe sepsis patients [Table 4].
Table 4.
Standardized mortality ratio and attributable mortality
DISCUSSION
This study documents the prevalence, patient demographics, microbial profile, and outcome of sepsis with organ dysfunction in a heterogeneous case mix of adult ICU population over 5 years in India. In a similar study conducted in 23 multi-disciplinary ICUs in Australia New Zealand (ANZICS),[3] 5878 consecutive ICU admission were prospectively followed during their ICU stay. SIRS criteria in the ANZICS study were not fulfilled in 8.6% as opposed to 16.7% in our patient population. SIRS without organ dysfunction was noted in 26.6% and with organ dysfunction in 28.2% of patients in ANZICS study. The comparable numbers in our study were 73.4% and 9.8%, respectively. Nearly 54.8% of patients with SIRS and organ dysfunction in ANZICS[3] study did not have any evidence of infection, and 41.5% had evidence of infection compared to 39.1% and 60.9% in our study. SIRS without organ dysfunction was comparatively more in our study probably due to a different case mix.
In this study, 6% of all ICU admission was due to severe sepsis out of which 16% were ICU acquired. This is a much lower prevalence than described in a point prevalence study (INDICAP)[14] conducted in 120 ICUs across India. The INDICAP study analyzed 4038 patient data and reported a prevalence of severe sepsis of 28.3% out of which 20.5% were ICU acquired. This discrepancy could be due to differences in study design as our study was prospective with data collected over 5 years in contrast to a 1 day (staggered) observation in INDICAP study. Somewhat similar rate of sepsis incidence of 10% like ours has been described in the ANZICS[3] study and studies conducted in Italy[15] and United States.[16] However, a French study[17] noted an incidence of 27%, similar to INDICAP study.
The median APACHE II score of all ICU admission in our study was 13 with an observed mortality of 11.1%. A similar observation was made in INDICAP study[14] with average APACHE II score of 15.7 with ICU mortality of 11.7% thereby reflecting a similar case mix with other Indian ICUs. In our cohort of septic patients, ICU mortality, 28-day mortality and hospital mortality were 56.1%, 62.8%, and 63.6%, respectively. The median APACHE II score for severe sepsis patients in our ICU was 22 with a predicted mortality of 40% and SMR of 1.4 [Table 4]. INDICAP study, on the other hand, observed a median APACHE II score of 21.7 and ICU mortality of 34% in severe sepsis patients. This observation may be due to single center versus multicenter nature of these two studies. In the ANZICS[3] study, ICU mortality, 28-day mortality and hospital mortality rates of 26.5%, 32.4%, and 37.5%, respectively, were much lower with similar APACHE II scores which could be due to different case-mix of predominant surgical patients. In a recent study from Australia–New Zealand, sepsis mortality has been found to have an annual rate of decrease of 1.3% and reduced from 35% in the year 2000-18.4% in 2012.[18] This is in sharp contrast with mortality of severe sepsis in the study. The reason for these discordant results could be related more to the difference of health-care delivery pattern. In a retrospective analysis of an international database predominantly from high-income countries from 1995 to 2015 the incidence of sepsis was 437/100,000 person-years and for severe sepsis 270/100,000 person-years. Hospital mortality was 17% for sepsis and 26% for severe sepsis during this period.[19] All these studies are, however, limited by their retrospective nature. Furthermore, population-based sepsis epidemiology data are lacking from India at present.
The median (IQR) length of ICU stay in our study for the entire population during the study was 3 (2–5) days and of severe sepsis patient of 8 (4–13) days. Comparable figures from the INDICAP study was 6 (3–13) and 10 (5–15) days. The duration of ICU stay in our study was comparable to ANZICS study of 6 (3–12) days. Male patients constituted 57% of severe sepsis in ANZICS and 63.4% in INDICAPS, which is similar to our observation of 56.8%. The ANZICS study population was of comparatively higher mean age on admission 60.7 years as opposed to a comparative younger patient cohort in INDICAPS (Mean age 53.8) and 59.7 in our study.
The predominant source of infection was pulmonary (53.3%) similar to ANZICS (50.3%) and INDICAPS (35%) followed by abdominal, blood stream and urosepsis. In ANZICS study Gram positive infection constituted 48.3% and Gram-negative 38.5% of all infections as opposed to 12.6% and 73.4%, respectively, in our study cohort. In ANZICS study, E. coli was the most common (9.3%) Gram-negative organism and methicillin sensitive Staphylococcus aureus, the most common Gram-positive organism as opposed to Acinetobacter (21.2%) and methicillin-resistant S. aureus (8.7%), respectively, in our study.
The strength of our study is its prospective nature studying all consecutive ICU admissions over a long period-a desirable requisite for epidemiologic studies. Point prevalence studies fail to capture the variations inherent in a highly heterogeneous syndrome like sepsis. Most of the studies in India have concentrated on the infectious syndromes and microbial profile rather than sepsis which is host response to infection. As the study was conducted in ICU with mandatory critical care consult, all patients were adequately screened and followed during ICU stay and a dedicated team of data collectors ensured adequate and complete data collection. Severity scoring by APACHE II to predict hospital mortality also helped to calculate SMR and attributable mortality in the study cohort.
The limitation of our study is its single center nature which may preclude generalizability. Ours is a tertiary care private teaching hospital located in a metro city. The epidemiology of sepsis patients, especially the severity of illness and outcomes might be different in public hospitals, primary and secondary care hospitals, nonmetro hospitals and nonteaching hospitals. The mandatory critical care consult model might also affect the outcome which may differ from that seen in ICUs with no formal critical care team. The study was conducted in adult mixed medical surgical, trauma, neurology ICU and the epidemiology may be different in pediatric, neonatal, transplant, and oncology ICUs. Applicability of data in the current period after a latency of 5 years is not unusual in epidemiological studies where data are published with similar latency.[3,20,21] During the time of data collection, the current criteria for sepsis definition (SEPSIS 3) were not available and were not used.
In future, similar multicenter studies should be conducted with a broader representation of sufficient duration which may reflect the current trend of sepsis outcome and its relation to the variability within health-care delivery systems in our country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: Sampling, selection, and society. Crit Care. 2004;8:222–6. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand Intensive Care Units. Intensive Care Med. 2004;30:589–96. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 4.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–21. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: Secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;10:R42. doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European Intensive Care Units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 7.Ghanshani RV, Gupta R, Sood S, Bansal A, Joad SH, Khedar RS. Epidemiology of infections in a medical ICU in India. Intensive Care Med. 2014;40:456–7. doi: 10.1007/s00134-013-3208-1. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Gupta D, Ray P, Aggarwal AN, Jindal SK. Epidemiology, risk factors and outcome of nosocomial infections in a Respiratory Intensive Care Unit in North India. J Infect. 2006;53:98–105. doi: 10.1016/j.jinf.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Ghanshani R, Gupta R, Gupta BS, Kalra S, Khedar RS, Sood S. Epidemiological study of prevalence, determinants, and outcomes of infections in medical ICU at a tertiary care hospital in India. Lung India. 2015;32:441–8. doi: 10.4103/0970-2113.164155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradhan NP, Bhat SM, Ghadage DP. Nosocomial infections in the medical ICU: A retrospective study highlighting their prevalence, microbiological profile and impact on ICU stay and mortality. J Assoc Physicians India. 2014;62:18–21. [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 12.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 14.Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, et al. Intensive care in India: The Indian intensive care case mix and practice patterns study. Indian J Crit Care Med. 2016;20:216–25. doi: 10.4103/0972-5229.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, et al. The Italian SEPSIS study: Preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med. 1995;21(Suppl 2):S244–9. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- 16.Pittet D, Rangel-Frausto S, Li N, Tarara D, Costigan M, Rempe L, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: Incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995;21:302–9. doi: 10.1007/BF01705408. [DOI] [PubMed] [Google Scholar]
- 17.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in Intensive Care Units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–74. [PubMed] [Google Scholar]
- 18.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–16. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 19.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–72. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 20.Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian sepsis epidemiological study (BASES study) Crit Care. 2004;8:R251–60. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–40. [PubMed] [Google Scholar]


