Abstract
Introduction:
Pain is highly prevalent in critically ill trauma patients, especially those with a traumatic brain injury (TBI). Behavioral pain tools such as the behavioral pain scale (BPS) and critical-care pain observation tool are recommended for sedated noncommunicative patients. Analysis of heart rate variability (HRV) is a noninvasive method to evaluate autonomic nervous system activity. The analgesia nociception index (ANI) device (Physiodoloris®, MDoloris Medical Systems, Loos, France) allows noninvasive HRV analysis. The ANI assesses the relative parasympathetic tone as a surrogate for antinociception/nociception balance in sedated patients. The primary aim of our study was to evaluate the effectiveness of ANI in detecting pain in TBI patients. The secondary aim was to evaluate the impact of norepinephrine use on ANI effectiveness and to determine the correlation between ANI and BPS.
Methods:
We performed a prospective observational study in 21 deeply sedated TBI patients. Exclusion criteria were nonsinus cardiac rhythm; presence of pacemaker; atropine or isoprenaline treatment; neuromuscular blocking agents; and major cognitive impairment. Heart rate, blood pressure, and ANI were continuously recorded using the Physiodoloris® device at rest (T1), during (T2), and after the end (T3) of the painful stimulus (tracheal suctioning).
Results:
In total, 100 observations were scored. ANI was significantly lower at T2 (Median [min – max] 54.5 [22–100]) compared with T1 (90.5 [50–100], P < 0.0001) and T3 (82 [36–100], P < 0.0001). Similar results were found in the subgroups of patients with (65 measurements) or without (35) norepinephrine. During procedure, a negative linear relationship was observed between ANI and BPS (r2 = −0.469, P < 0.001). At the threshold of 50, the sensitivity and specificity of ANI to detect patients with BPS ≥ 5 were 73% and 62%, respectively, with a negative predictive value of 86%.
Discussion:
Our results suggest that ANI is effective in detecting pain in ventilated sedated TBI patients, including those patients treated with norepinephrine.
Keywords: Behavioral pain scale, Intensive Care Unit, pain assessment, traumatic brain injury
INTRODUCTION
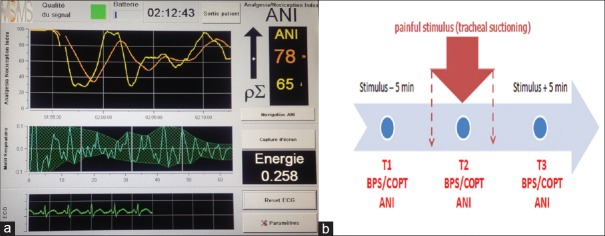
Pain is highly prevalent in critically ill trauma patients, especially those with a traumatic brain injury (TBI).[1] Behavioral pain tools such as the behavioral pain scale (BPS) and critical-care pain observation tool (CPOT) are recommended for sedated noncommunicative patients.[2,3] Analysis of heart rate variability (HRV) is a noninvasive method to evaluate autonomic nervous system activity. The analgesia nociception index (ANI) device (Physiodoloris®, MDoloris Medical Systems, Loos, France) [Figure 1a] allows noninvasive HRV analysis. The ANI assesses the relative parasympathetic tone as a surrogate for antinociception/nociception balance in sedated patients.[4] The primary aim of our study was to evaluate the effectiveness of ANI in detecting pain in TBI patients. The secondary aim was to evaluate the impact of norepinephrine use on ANI effectiveness and to determine the correlation between ANI and BPS.
Figure 1.
(a) PhysioDoloris™ analgesia monitor (In this screenshot: instantaneous analgesia nociception index value = 65; mean analgesia nociception index value = 78). (b) Time points for analgesia nociception index measurements
METHODS
We performed a prospective observational study in 21 deeply sedated TBI patients. Exclusion criteria were nonsinus cardiac rhythm; presence of pacemaker; atropine or isoprenaline treatment; neuromuscular blocking agents; and major cognitive impairment. Heart rate, blood pressure, and ANI were continuously recorded using the Physiodoloris® device at rest (T1), during (T2), and after the end (T3) of the painful stimulus (tracheal suctioning) [Figure 1b].
RESULTS
In total, 100 observations were scored. Patients' characteristics were resumed in Table 1.
Table 1.
Baseline demographic and clinical characteristics

The mean values of the changes in BPS, CPOT, ANI, MAP, and HR at the 3 times (baseline, during painful stimulation, and at recovery time) are shown in Table 1. The mean BPS, CPOT, MAP, and HR values were significantly changed overtime, increasing during suctioning, and decreasing at recovery time (5 min after the procedure) (P < 0.05) [Table 2].
Table 2.
Variables at different time points
ANI was significantly lower at T2 (Median [min – max] 54.5 [22–100]) compared with T1 (90.5 [50–100], P < 0.0001) and T3 (82 [36–100], P < 0.0001) [Table 2]. Similar results were found in the subgroups of patients with (65 measurements) or without (35) norepinephrine [Table 3]. During procedure, a negative linear relationship was observed between ANI and BPS (r2 = −0.469, P < 0.001). At the threshold of 50, the sensitivity and specificity of ANI to detect patients with BPS ≥5 were 73% and 62%, respectively, with a negative predictive value of 86%.
Table 3.
Variables at different time points: Impact of norepinephrine
DISCUSSION
Our results suggest that ANI is effective in detecting pain in ventilated sedated TBI patients, including those patients treated with norepinephrine.
To the best of our knowledge, our study is the first to evaluate the ANI device for pain assessment in deeply sedated mechanically ventilated patients with TBI.
Pain assessment is an immense challenge for clinicians, especially in the context of the Intensive Care Unit (ICU), where the patient is often unable to communicate verbally; current guidelines recommend that intensivists should use some valid observable behavioral scales and physiological indicators, such as the BPS and COPT in patients with intact motor function.[5]
However, these scores have some limitations, including the inter-rater variability and the lack of determination of the level of anxiety and discomfort.[6]
There have been no formal validation studies of the reliability of theses scores to evaluate the behavior of pain in the TBI population. The literature review found that TBI patients were underrepresented (<17%) in studies validating the use of behavioral pain tools in critically ill adults.[1,7]
Another criterion used to assess pain in ICU patients is vital signs. Although hemodynamic changes are easily accessible in the ICU, their validity for pain assessment is not strongly confirmed.[8]
The performance of ANI was evaluated in the prediction of immediate postoperative pain after adult general anesthesia.[9,10]
Few studies have shown the value of this device in the monitoring of pain in sedated critically ill patients.[11]
Scientific evidence is not yet sufficient to conclude on the validity of the ANI in assessing pain in the context of the ICU, especially in TBI patients.
In the current study, the ANI scores, when compared to baseline values, decreased approximately 40% during endotracheal suctioning and increased >50% by the recovery time.
In the study by Broucqsault-Dédrie et al.,[11] ANI decreased approximately 19% during painful situations compared to baseline. The chosen painful stimulus was patient turning for washstand. It should be noted that in that study, the majority of patients included were admitted for respiratory or hemodynamic failure and they were under deep sedation.
Our findings are in line with those of Broucqsault-Dédrie et al.[11] who found that ANI is effective in detecting pain in deeply sedated critically ill patients, including those patients treated with norepinephrine.
In this preliminary study, no sample size calculation was performed, which may limit the precision of our estimates. Further large interventional studies are required to confirm our results and to determine the value of this device in titrating the analgesic requirements of this vulnerable group of critically ill patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Berben SA, Meijs TH, van Dongen RT, van Vugt AB, Vloet LC, Mintjes-de Groot JJ, et al. Pain prevalence and pain relief in trauma patients in the accident and emergency department. Injury. 2008;39:578–85. doi: 10.1016/j.injury.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29:2258–63. doi: 10.1097/00003246-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15:420–7. [PubMed] [Google Scholar]
- 4.Logier R, Jeanne M, De Jonckheere J, Dassonneville A, Delecroix M, Tavernier B, et al. PhysioDoloris: A monitoring device for analgesia/nociception balance evaluation using heart rate variability analysis. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:1194–7. doi: 10.1109/IEMBS.2010.5625971. [DOI] [PubMed] [Google Scholar]
- 5.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the Intensive Care Unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 6.Skrobik Y, Chanques G. The pain, agitation, and delirium practice guidelines for adult critically ill patients: A post-publication perspective. Ann Intensive Care. 2013;3:9. doi: 10.1186/2110-5820-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aïssaoui Y, Zeggwagh AA, Zekraoui A, Abidi K, Abouqal R. Validation of a behavioral pain scale in critically ill, sedated, and mechanically ventilated patients. Anesth Analg. 2005;101:1470–6. doi: 10.1213/01.ANE.0000182331.68722.FF. [DOI] [PubMed] [Google Scholar]
- 8.Herr K, Coyne PJ, Key T, Manworren R, McCaffery M, Merkel S, et al. Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. Pain Manag Nurs. 2006;7:44–52. doi: 10.1016/j.pmn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ledowski T, Tiong WS, Lee C, Wong B, Fiori T, Parker N, et al. Analgesia nociception index: Evaluation as a new parameter for acute postoperative pain. Br J Anaesth. 2013;111:627–9. doi: 10.1093/bja/aet111. [DOI] [PubMed] [Google Scholar]
- 10.Boselli E, Bouvet L, Bégou G, Dabouz R, Davidson J, Deloste JY, et al. Prediction of immediate postoperative pain using the analgesia/nociception index: A prospective observational study. Br J Anaesth. 2014;112:715–21. doi: 10.1093/bja/aet407. [DOI] [PubMed] [Google Scholar]
- 11.Broucqsault-Dédrie C, De Jonckheere J, Jeanne M, Nseir S. Measurement of heart rate variability to assess pain in sedated critically ill patients: A Prospective observational study. PLoS One. 2016;11:e0147720. doi: 10.1371/journal.pone.0147720. [DOI] [PMC free article] [PubMed] [Google Scholar]