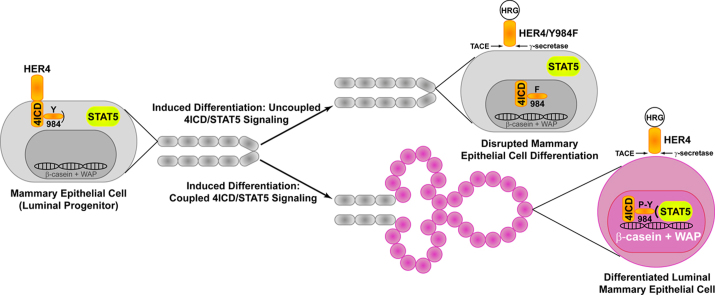
Abstract
The HER4 receptor tyrosine kinase and STAT5A cooperate to promote mammary luminal progenitor cell maturation and mammary epithelial cell differentiation. Coupled HER4 and STAT5A signaling is mediated, in part, through association of the HER4 intracellular domain (4ICD) with STAT5A at STAT5A target gene promoters where 4ICD functions as a STAT5A transcriptional coactivator. Despite an essential role for coupled 4ICD and STAT5A signaling in mammary gland development, the mechanistic basis of 4ICD and STAT5A cooperative signaling remains unexplored. Here we show for the first time that 4ICD and STAT5A directly interact through STAT5A recruitment and binding to HER4/4ICD residue Y984. Accordingly, altering the 4ICD Y984 to phenylalanine results in a dramatic reduction of STAT5A and 4ICD-Y984F interacting complexes coimmunoprecipitated with HER4 or STAT5A specific antibodies. We further show that disrupting the 4ICD and STAT5A interaction has an important physiological impact on mammary epithelial cell differentiation. HC11 mammary epithelial cells with stable expression of 4ICD undergo differentiation with significantly increased expression of the STAT5A target genes and differentiation markers β-casein and WAP. In contrast, HC11 cells stably expressing 4ICD-Y984F failed to undergo differentiation with basal expression levels of β-casein and WAP. Differentiation in this cell system was induced in the absence of exogenous prolactin indicating that 4ICD activity is sufficient to induce mammary epithelial cell differentiation. Finally, we show that suppression of STAT5A expression abolishes the ability of 4ICD to induce HC11 differentiation and activate β-casein or WAP expression. Taken together our results demonstrate for the first time that direct coupling of 4ICD and STAT5A is both necessary and sufficient to drive mammary epithelial differentiation. In conclusion, our findings that 4ICD and STAT5A directly interact to form a physiologically important transcriptional activation complex, provide a mechanistic basis for the in vivo observations that HER4/4ICD and STAT5A cooperate to promote mammary gland progenitor cell maturation and initiate lactation at parturition.
Abbreviations: 4ICD, HER4 intracellular domain; ATCC, American type culture collection; EGF, epidermal growth factor; EGFP, enhanced green fluorescent protein; EGFR, epidermal growth factor family; ERα, estrogen receptor alpha; FBS, fetal bovine serum; HEK, human embryonic kidney; HRGα, heregulin alpha; HRGα1, heregulin beta 1; NLS, nuclear localization signal; PCR, polymerase chain reaction; PI3K, phosphoinositide 3-kinase; RIP, regulated intramembrane cleavage; RT, reverse transcription; RTK, receptor tyrosine kinase; SH2, src homology 2; STAT5A, signal transducer and activator of transcription 5A; TACE, tumor necrosis factor-α-converting enzyme; WAP, whey acidic protein; YAP, yes-associated protein
Keywords: Mammary epithelial differentiation, EGFR-family, STAT5A, HER4/ERBB4, Gene expression, Transactivation
Graphical abstract
Highlights
-
•
HER4/4ICD tyrosine 984 mediates a direct interaction with STAT5A.
-
•
4ICD expression with an intact Y984 is sufficient to induce mammary differentiation.
-
•
Mammary differentiation is abolished by disrupting the 4ICD and STAT5A interaction.
-
•
STAT5 expression is required for 4ICD-induced mammary differentiation.
1. Introduction
Mammary epithelial differentiation requires the complex interplay between receptor tyrosine kinases (RTK), cytokine receptors, and downstream transcription factors. It is well established that signal transducer and activator of transcription 5A (STAT5A) represents the obligate transcription factor regulating mammary epithelial differentiation. For example, mammary specific deletion of STAT5A results in multiple defects including disengaged secretory epithelial differentiation, loss of milk-gene expression, and catastrophic lactational failure resulting in the death of offspring [1], [2], [3], [4]. Significantly, recent experimental evidence indicates that STAT5A is required to establish the luminal progenitor cell population [4], [5], providing a mechanistic basis for the loss of differentiated secretory epithelium and subsequent failure to lactate observed in STAT5A-null mice. Ultimately, activation of STAT5A in the differentiating mammary gland is regulated through cooperation between cytokine and the RTK receptor families. Although cytokine signaling through the prolactin receptor contributes to STAT5A activity during early pregnancy [2], [6], the RTK HER4 (also referred to as ERBB4) appears to be the obligate regulator of STAT5A mediated luminal progenitor function and differentiation at late pregnancy and parturition [5], [6].
HER4 is a unique member of the epidermal growth factor receptor (EGFR) family. Although, all four members of this RTK family localize to the cell nucleus, under specific experimental conditions [7], HER4 is the only EGFR-family member that undergoes regulated intramembrane cleavage (RIP) to release an independently signaling intracellular domain. Accordingly, ligand activation of HER4 results in tumor necrosis factor-α-converting enzyme (TACE)-mediated cleavage of the extracellular domain followed by γ-secretase catalyzed RIP to release the HER4 intracellular domain (4ICD) [8]. Interestingly, nuclear 4ICD functions as a potent transcriptional coactivator and is recruited to target gene promoters through association with the transcriptional coactivator yes-associated protein (YAP) [9] or the DNA-binding transcription factors estrogen receptor alpha (ERα) [10], [11], [12] or STAT5A [13].
Compelling developmental and experimental evidence indicates that HER4 employs multiple novel activities to regulate STAT5A induced luminal progenitor cell maturation and mammary epithelial cell differentiation. The HER4 ligand heregulin alpha (HRGα), HER4, and STAT5A all have overlapping mammary gland phenotypes [1], [2], [4], [5], [6], [8], [14], [15]. Accordingly, HRGα stimulation of HER4 regulates STAT5A activation and subsequent luminal progenitor cell maturation during late pregnancy [5]. Moreover, HRGα promotes proteolytic processing of HER4 to generate 4ICD, which functions as a STAT5A nuclear chaperone and transcriptional coactivator by binding with STAT5A at target gene promoters [13]. STAT5A association with 4ICD and subsequent nuclear cotranslocation is essential for STAT5A mediated gene expression and mammary epithelial differentiation [6], [13], [16]. Mechanistically the association between STAT5A and 4ICD requires an intact STAT5A Src homology 2 (SH2) domain [13] suggesting that STAT5A activity requires direct interaction with a specific phosphorylated tyrosine within 4ICD. However, the 4ICD residue targeted by this developmentally and functionally important phosphorylation event has remained elusive. Here, we demonstrate that phosphorylation of 4ICD Y984 is required for 4ICD interaction with STAT5A and activation of STAT5A target genes during mammary epithelial differentiation. Our results provide the first evidence for a direct interaction between 4ICD and STAT5A, further establishing 4ICD as a unique and obligate regulator of STAT5A activity during mammary progenitor cell maturation and mammary epithelial cell differentiation.
2. Materials and methods
2.1. Expression plasmids
To generate the p4ICD expression vector, the HER4 intracellular domain (4ICD) (residues 673-1308), was subcloned into pEGFP-N3 (Clontech) with the N-terminal EGFP removed. The Y984F mutation was produced by PCR-assisted site-directed mutagenesis of p4ICD using the primer 5′-GGCTCGAGACCCTCAAAGATTCCTAGTTATTCAGGG-3′ (altered base underlined) and the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent). The full length mouse STAT5A expression vector pEFneo-STAT5A has been described previously [14].
2.2. Cell lines
All cell lines were obtained from American Type Culture Collection (ATCC). The human embryonic kidney cell line HEK 293T was cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS) and 2 mM L-glutamine. HC11 mouse mammary epithelial cells were cultured in RPMI 1640 (Gibco) supplemented with 10% FBS, 10 ng/ml epidermal growth factor (EGF) (Invitrogen), 5 µg/ml insulin (Sigma-Aldrich), and 1 µg/ml hydrocortisone (Sigma-Aldrich). For stable expression of 4ICD or 4ICD-Y984F, HC11 cells were transfected with p4ICD, p4ICD-Y984F or the empty vector using Fugene6 (Roche) and selected with G418 to generate the pooled cell lines HC11/4ICD, HC11/4ICD-Y984F, and HC11/vector, respectively. Where indicated, differentiation of HC11 stable cell lines was induced by culturing confluent cells in FBS and EGF free culture media.
2.3. Immunoprecipitation and western blot analysis
Co-immunoprecipitation of STAT5A and 4ICD or 4ICD-Y984F was performed exactly as described previously [13], [17] using HEK 293T cells were transfected with pEFneo-STAT5A, p4ICD or p4ICD-Y984F using Lipofectamine with Plus Reagent (Invitrogen). At 2 days post-transfection, cell lysates were prepared in EBC buffer (50 mM Tris, pH 7.5, 50 mM Tris, pH 7.5, 0.5% NP-40, with 1 mM phenylmethylsulfonyl fluoride) and 500 μg of lysate was immunoprecipitated overnight at 4 °C with antibodies directed against HER4 (Santa Cruz; sc-283) or STAT5 (Santa Cruz; sc-835). Immune complexes were collected by incubating with Protein A sepharose (Roche) at 4 °C for 3 h. and eluted by boiling in 60 μl of NuPAGE LDS Sample Buffer (4X) (Life Technologies) containing NuPAGE Sample Reducing Agent (10X) (Life Technologies). Twenty μl of each immunoprecipitate was loaded in each lane of a NuPAGE 4–12% Bis–Tris Gel (Life Technologies). Total cell lysates were collected using RIPA Buffer (10 mM NaPO4, pH 7.2, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Na-deoxycholate, 1% Nonidet P40) containing Complete EDTA-free Protease Inhibitor Cocktail (Roche) and PhosSTOP phosphatase inhibitor (Roche) and western blot analysis was performed exactly as described previously [18] using the primary antibodies directed against HER4 (Abcam; E200), Stat5 (Santa Cruz; sc-835), and α-tubulin (Upstate Biotechnology; 05829).
2.4. Quantitative RT-PCR
Triplicate total RNA samples were isolated from stable HC11 cell lines induced to undergo differentiation and quantitative RT-PCR was performed exactly as described elsewhere [10] using the following PCR primer pairs to amplify β-actin 5′-CGACTGTGTCATGACATGTAC and 5′-TTGAAAGCATTATGTTCTCTCTGG, β-casein 5′-CGACTGTGTCATGACATGTAC and 5′-TTGAAAGCATTATGTTCTCTCTGG or whey acidic protein (WAP) 5′-CATCCTCGCCTGCCTTGTGGC and 5′-CCATGAGATTCACCTTCTGAAG. The Ct analysis for each reaction was performed using the supplied 7500 Software v2.0.5 (Applied Biosystems) and gene expression levels were normalized to β-actin using the ΔΔCt method.
2.5. STAT5A knockdown
To suppress expression of endogenous STAT5, the HC11 stable cell lines HC11/vector and HC11/4ICD were grown to confluency and transfected with siRNA targeting STAT5 (Sigma; WD03944063) or scramble siRNA (Sigma; WD03148542) in FBS- and EGF-free RPMI 1640 using Lipofectamine RNAiMAX Reagent (Invitrogen) according to the manufacturer's instructions.
3. Results and discussion
3.1. HER4 Y984 mediates the interaction between 4ICD and STAT5A
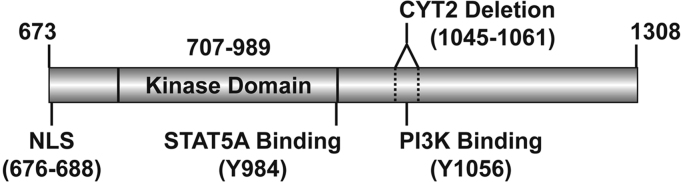
Our previously published data suggests that 4ICD directly interacts with STAT5A [8], [13], [17]. Furthermore, this interaction requires an intact STAT5A Src homology 2 (SH2) domain [13], which mediates STAT5A interaction with phosphorylated tyrosine residues. Although HER4 harbors a total of 27 intracellular tyrosine residues one report indicates that only 19 undergo phosphorylation in response to the HER4 ligand HRGβ1. However, STAT5A was not included in the subsequent unbiased phospho-peptide screen designed to identify SH2-mediated HER4 interacting partners [19]. This limitation precluded identification of potential HER4 phosphotyrosines required to recruit STAT5A. A similar unbiased interactome of all 27 HER4 cytosolic tyrosines revealed STAT5A interaction with a phosphotyrosine representing Y984 positioned within the final carboxyl residues of the intrinsic tyrosine kinase domain [20] (Fig. 1). However, the synthetic peptide-peptide interaction was not confirmed experimentally and the physiological relevance of a HER4/STAT5A interaction remains to be established.
Fig. 1.
Schematic of the HER4 intracellular domain (4ICD) residues 673-1308 showing the position of a putative STAT5A binding site, Y984, relative to the HER4 nuclear localization sequence (NLS) [13], and the phosphoinositide 3-kinase (PI3K) binding site, Y1056, located in the region deleted in the HER4 CYT2 isoform.
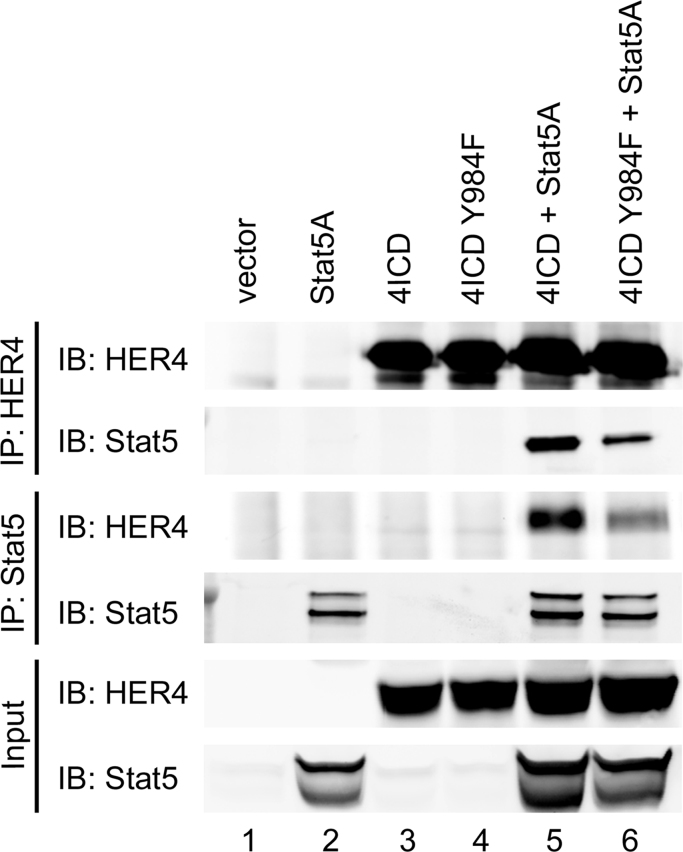
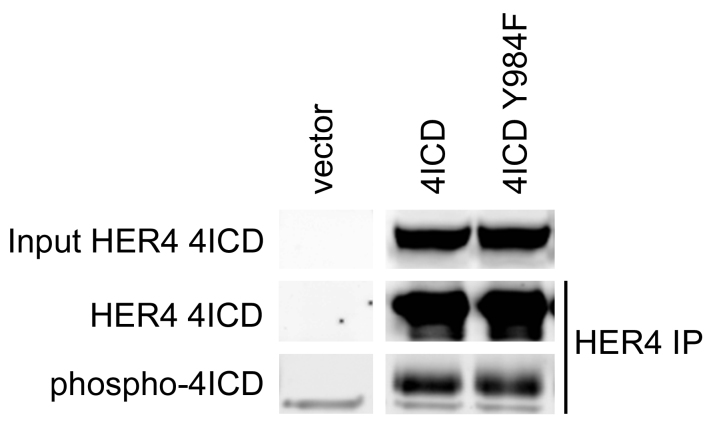
To confirm that HER4 Y984 mediates an interaction between 4ICD and STAT5A we used site-directed mutagenesis of 4ICD to replace Y984 with phenylalanine (Y984F). Both ectopically expressed 4ICD and 4ICD Y984F displayed relatively low but equivalent levels of autophosphorylation (Supplementary Fig. S1) indicating that the Y984F alteration failed to dramatically impact the 4ICD intrinsic tyrosine kinase activity. Using a coimmunoprecipitation assay of HEK 293T transiently transfected with STAT5A and 4ICD or 4ICD-Y984F, we show that, consistent with previously published results [13], [17], both the HER4 and STAT5A specific antibodies coimmunoprecipitate the HER4 and STAT5A interacting complex (Fig. 2; lane 5). Significantly, altering the 4ICD Y984 to phenylalanine results in a dramatic reduction of STAT5A and 4ICD-Y984F interacting complexes coimmunoprecipitated with HER4 or STAT5A specific antibodies (Fig. 2; lane 6). These results indicate that Y984 is the major HER4 phosphotyrosine required to mediate a direct interaction between 4ICD and STAT5A.
Fig. 2.
Direct interaction between 4ICD and STAT5A is mediated by HER4 Y984. HEK 293T cells were transfected with the indicated expression vectors and cell lysates were prepared at 48 h. post-transfection in EBC buffer. For immunoprecipitations, 500 μg of cleared cell lysates were incubated with HER4 or STAT5A specific antibodies at 4 °C overnight. Immune complexes were recovered by adding Protein A sepharose to each immunoprecipitation reaction and incubating for 3 h at 4 °C, and finally eluted into 60 μl of NuPAGE LDS Sample Buffer with Reducing Agent. Twenty μl of eluted immunoprecipitation reactions or 20 μg of EBC lysate (Input) was probed by western blot using the indicated immunoblot (IB) antibodies.
3.2. Mammalian epithelial cell differentiation requires an intact 4ICD Y984
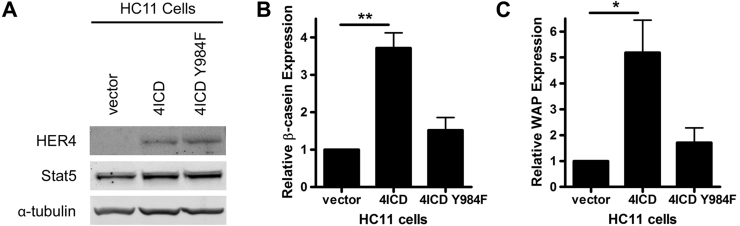
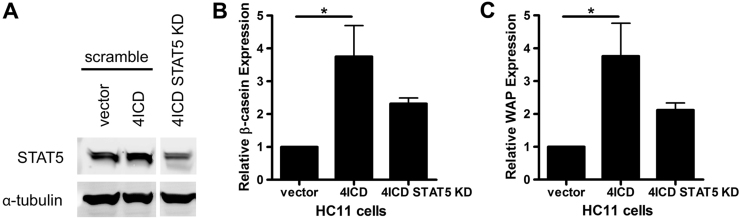
Previous reports have shown that expression of 4ICD is sufficient to induce mammary epithelial cell differentiation in both in vivo and in vitro experimental systems [21], [22]. The mouse mammary epithelial HC11 cell line is a commonly employed model of prolactin-induced mammary differentiation. These cells also undergo differentiation, in the absence of prolactin signaling, in response to 4ICD expression and serum starvation. To determine the physiological role for 4ICD Y984 during mammary epithelial cell differentiation, we induced differentiation of HC11 cells stably transfected with 4ICD or 4ICD-Y984F. The two pooled HC11/4ICD and HC11/4ICD-Y984F stable cell lines displayed equivalent levels of ectopic 4ICD expression (Fig. 3A). Consistent with a role for 4ICD in mammary epithelial cell differentiation, ectopic expression of 4ICD resulted in a significant increase in HC11 expression of the differentiation markers and STAT5A target genes β-casein (Fig. 3B) and WAP (Fig. 3C). In contrast, ectopic expression of 4ICD-Y984F failed to significantly increase HC11 expression of β-casein (Fig. 3B) or WAP (Fig. 3C). These results indicate that an intact 4ICD Y984 is required to induce mammary epithelial differentiation and suggests that phosphorylated Y984 recruits STAT5A to drive mammary differentiation.
Fig. 3.
4ICD induced mammary epithelial cell differentiation requires an intact Y984. (A) HER4/4ICD western blot analysis of HC11 mammary epithelial stable cell lines HC11/vector, HC11/4ICD, and HC11/4ICD-Y984F demonstrating equivalent levels of 4ICD and 4ICD-Y984F expression. Western blot of α-tubulin is included as a loading control. Each indicated HC11 stable cell line was induced to undergo differentiation in the absence of exogenous prolactin by serum starvation for two days and expression, normalized to β-actin, of the differentiation markers (B) β-casein and (C) WAP relative to HC11/vector control was determined by quantitative RT-PCR. Each quantitative RT-PCR sample was prepared in triplicate and the data represent the mean and standard error (SE) of at least three independent experiments and RNA extractions. Statistically significant differences between data sets were determined using one-way ANOVA with Bonferroni post-hoc test. Single or double asterisks indicates p<0.03 or p=0.003, respectively.
3.3. 4ICD-induced mammary epithelial cell differentiation requires STAT5A expression
Activation of STAT5A during 4ICD-induced HC11 cell differentiation suggests that STAT5A is the downstream effector of 4ICD activity in this experimental system [21]. Our findings that ablating 4ICD Y984, which is required for 4ICD to recruit STAT5A, abolishes the ability of 4ICD to induce HC11 differentiation, further substantiates an obligate role for STAT5A in 4ICD-induced mammary epithelial cell differentiation. To confirm a role for STAT5A in 4ICD-induced HC11 differentiation, we determined the impact of siRNA-mediated suppression of STAT5A on the differentiation of HC11/4ICD cells. Consistent with previous results (Fig. 3), differentiation of HC11/4ICD cells transfected with non-specific siRNA (Fig. 4A) resulted in a significant increase in expression the β-casein (Fig. 4B) and WAP (Fig. 4C) differentiation genes when compared to control HC11/vector cells. We successfully used siRNA to suppress STAT5A expression in the HC11/4ICD cells stimulated to undergo differentiation (Fig. 4A). However, in the absence of STAT5A the HC11/4ICD cells failed to induce significant levels of β-casein (Fig. 4B) or WAP (Fig. 4C) expression demonstrating for the first time that STAT5A is required for 4ICD induced differentiation of HC11 cells. Prolactin stimulation of β-casein and WAP was also suppressed, further confirming functional knockdown of STAT5 in the HC11/4ICD cells (Supplementary Fig. S2).
Fig. 4.
STAT5 expression is required for 4ICD induced mammary epithelial cell differentiation. (A) STAT5 western blot analysis of the HC11 mammary epithelial stable cell lines HC11/vector and HC11/4ICD transfected with scramble siRNA control or STAT5 siRNA after cells were induced to undergo differentiation in the absence of exogenous prolactin, by serum starvation for two days. Western blot of α-tubulin is included as a loading control. Superfluous gel lanes between 4ICD and 4ICD STAT5 KD were removed to condense the figure panel. Expression, normalized to β-actin, of the differentiation markers (B) β-casein and (C) WAP relative to HC11/vector control treated with scramble siRNA was determined by quantitative RT-PCR. Each quantitative RT-PCR sample was prepared in triplicate and the data represent the mean and standard error (SE) of at least three independent experiments and RNA extractions. Statistically significant differences between data sets were determined using one-way ANOVA with Bonferroni post-hoc test. Asterisks indicate p<0.05.
In conclusion, compelling in vivo and in vitro evidence suggests that 4ICD and STAT5A cooperate to drive mammary epithelial cell differentiation; however, experiments demonstrating direct coupling of these two signaling pathways have remained elusive. Here we show for the first time that STAT5A directly interacts with 4ICD and this interaction occurs through STAT5A binding to Y984 of 4ICD. We further demonstrate that the 4ICD and STAT5A interaction is physiologically relevant and required to drive mammary epithelial cell differentiation. Taken together our findings are significant because they indicate that 4ICD and STAT5A can induce mammary epithelial cell differentiation in the absence of prolactin signaling. Furthermore, our findings that 4ICD and STAT5A directly interact to form a physiologically important functional complex provide a mechanistic basis for the observations that HER4/4ICD and STAT5A cooperate to promote mammary gland progenitor cell maturation and initiate lactation at parturition.
Acknowledgments
We thank Elenore Semmes and Alicia Meyer for assistance with experiments and other members of the Jones lab for helpful discussions. This work was supported by NIH/NCI Grants RO1CA095783 (FEJ) and RO1CA096717 (FEJ), Tulane University Office of Research Bridge Funding (FEJ), and the Gerald and Flora Jo Mansfield Piltz Professorship in Cancer Research (FEJ).
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.07.015.
Supplementary data associated with this article can be found in the online version at 10.1016/j.bbrep.2016.07.015.
Appendix A. Transparency document
Supplementary material
.
Appendix B. Supplementary material
Fig. S1.
Western blot analysis of the indicated cell lines following HER4 immunoprecipitation indicating equivalent levels of 4ICD tyrosine phosphorylation in the HC11/4ICD and HC11/4ICD Y984F cell lines.
.
Fig. S2.
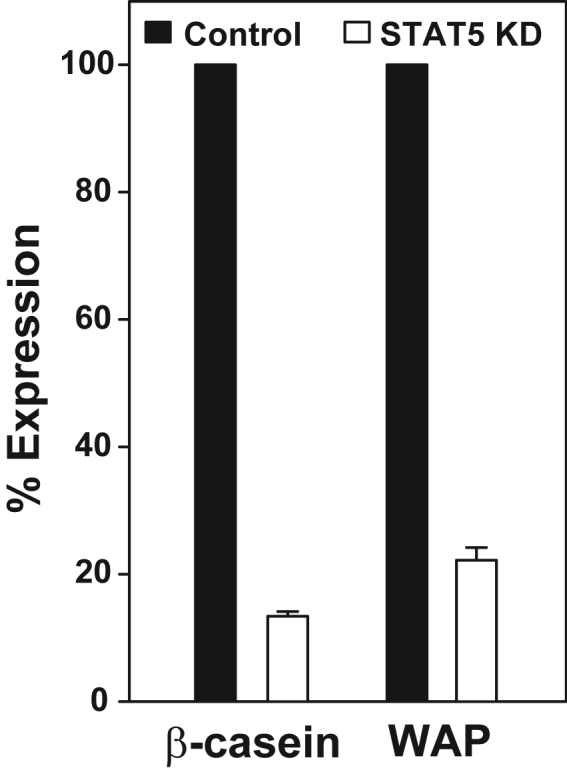
The HC11/4ICD cell line was treated with scrambled (Control) or STAT5 siRNA (STAT5 KD) for 24 h. as indicated in Fig. 4 and then treated with vehicle or 5 μg/ml of prolactin for 48 h. RNA was extracted and analyzed for β-casein and WAP expression by quantitative RT-PCR as described in Fig. 4. Results indicate that as predicted STAT5 knockdown resulted in suppressed prolactin stimulation of β-casein and WAP when compared to the scrambled control.
.
References
- 1.Liu X., Robinson G.W., Wagner K.-U., Garrett L., Wynshaw-Boris A., Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Miyoshi K., Shillingford J.M., Smith G.H., Grimm S.L., Wagner K.U., Oka T., Rosen J.M., Robinson G.W., Hennighausen L. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 2001;155:531–542. doi: 10.1083/jcb.200107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teglund S., McKay C., Schuetz E., vanDeursen J.M., Stravopodis D., Wang D., Brown M., Bodner S., Grosveld G., Ihle J.N. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 4.Yamaji D., Na R., Feuermann Y., Pechhold S., Chen W., Robinson G.W., Hennighausen L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009;23:2382–2387. doi: 10.1101/gad.1840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forster N., Saladi S.V., van Bragt M., Sfondouris M.E., Jones F.E., Li Z., Ellisen L.W. Basal cell signaling by p63 controls luminal progenitor function and lactation via NRG1. Dev. Cell. 2014;28:147–160. doi: 10.1016/j.devcel.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long W., Wagner K.-U., Lloyd K.C.K., Binart N., Shillingford J.M., Hennighausen L., Jones F.E. Impaired differentiation and lactational failure in ErbB4-deficient mammary glands identify ERBB4 as an obligate mediator of Stat5. Development. 2003;130:5257–5268. doi: 10.1242/dev.00715. [DOI] [PubMed] [Google Scholar]
- 7.Wang S.C., Hung M.C. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin. Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones F.E. HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer. J. Mammary Gland Biol. Neoplasia. 2008;13:247–258. doi: 10.1007/s10911-008-9076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskins J.W., Nguyen D.X., Stern D.F. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci. Signal. 2014;(7 ():ra116. doi: 10.1126/scisignal.2005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han W., Jones F.E. HER4 selectively coregulates estrogen stimulated genes associated with breast tumor cell proliferation. Biochem. Biophys. Res Commun. 2014;443:458–463. doi: 10.1016/j.bbrc.2013.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rokicki J., Das P.M., Giltnane J.M., Wansbury O., Rimm D.L., Howard B.A., Jones F.E. The ERa coactivator, HER4/4ICD, regulates progesterone receptor expression in normal and malignant breast epithelium. Mol. Cancer. 2010;9:150. doi: 10.1186/1476-4598-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y., Sullivan L.L., Nair S.S., Williams C.C., Pandey A., Marrero L., Vadlamudi R.K., Jones F.E. Coregulation of estrogen receptor by estrogen-inducible ERBB4/HER4 establishes a growth promoting autocrine signal in breast cancer. Cancer Res. 2006;66:7991–7998. doi: 10.1158/0008-5472.CAN-05-4397. [DOI] [PubMed] [Google Scholar]
- 13.Williams C.C., Allison J.G., Vidal G.A., Burow M.E., Beckman B.S., Marrero L., Jones F.E. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J. Cell Biol. 2004;167:469–478. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones F.E., Welte T., Fu X.-Y., Stern D.F. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J. Cell. Biol. 1999;147:77–87. doi: 10.1083/jcb.147.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L., Cleary S., Long W., Mandarano M.A., Birchmeier C., Jones F.E. The breast proto-oncogene, HRGa regulates epithelial proliferation and lobuloalveolar development in the mouse mammary gland. Oncogene. 2002;21:4900–4907. doi: 10.1038/sj.onc.1205634. [DOI] [PubMed] [Google Scholar]
- 16.Tidcombe H., Jackson-Fisher A., Mathers K., Stern D.F., Gassmann M., Golding J.P. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc. Natl. Acad. Sci. USA. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark D.E., Williams C.C., Duplessis T.T., Moring K.L., Notwick A.R., Lane W.S., Beuvink I., Hynes N.E., Jones F.E. ERBB4/HER4 potentiates STAT5A transcriptional activity by regulating novel STAT5A serine phosphorylation events. J. Biol. Chem. 2005;280:24175–24180. doi: 10.1074/jbc.M414044200. [DOI] [PubMed] [Google Scholar]
- 18.Huynh F.C., Jones F.E. MicroRNA-7 inhibits multiple oncogenic pathways to suppress HER2D16 mediated breast tumorigenesis and reverse trastuzumab resistance. PLoS One. 2014;9:e114419. doi: 10.1371/journal.pone.0114419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaushansky A., Gordus A., Budnik B.A., Lane W.S., Rush J., Macbeath G. System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem. Biol. 2008;15:808–817. doi: 10.1016/j.chembiol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulze W.X., Deng L., Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol. Syst. Biol. 2005;1:2005 0008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muraoka-Cook R.S., Sandahl M., Husted C., Hunter D., Miraglia L., Feng S.M., Elenius K., Earp H.S., 3rd The intracellular domain of ErbB4 induces differentiation of mammary epithelial cells. Mol. Biol. Cell. 2006;17:4118–4129. doi: 10.1091/mbc.E06-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muraoka-Cook R.S., Sandahl M.A., Strunk K.E., Miraglia L.C., Husted C., Hunter D.M., Elenius K., Chodosh L.A., Earp H.S., 3rd ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids and exert opposing effects on the mammary epithelium in vivo. Mol. Cell Biol. 2009;29:4935–4948. doi: 10.1128/MCB.01705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material