Abstract
Objective
This aim is to evaluate the effect of Sijunzi decoction (SJZD) treating chronic atrophic gastritis (CAG).
Methods
We performed searches in seven databases. The randomized controlled trials (RCTs) comparing SJZD with standard medical care or inactive intervention for CAG were enrolled. Combined therapy of SJZD plus conventional therapies compared with conventional therapies alone was also retrieved. The primary outcome included the incidence of gastric cancer and the improvement of atrophy, intestinal metaplasia, and dysplasia based on the gastroscopy and pathology. The secondary outcomes were Helicobacter pylori clearance rate, quality of life, and adverse event/adverse drug reaction.
Results
Six RCTs met the inclusion criteria. The research quality was low in the trials. For the overall effect rate, pooled analysis from 4 trials showed that modified SJZD plus conventional medications exhibited a significant improvement (OR = 4.86; 95% CI: 2.80 to 8.44; P < 0.00001) and without significant heterogeneity compared with the conventional medications alone. None reported the adverse effect.
Conclusions
Modified SJZD combined with conventional western medicines appears to have benefits for CAG. Due to the limited number and methodological flaw, the beneficial and harmful effects of SJZD for CAG could not be identified. More high-quality clinical trials are needed to confirm the results.
1. Introduction
Chronic atrophic gastritis (CAG) is a common inflammatory condition typically characterized by the loss of gastric glandular structures or by glandular structures metaplastic atrophy [1]. The clinical symptoms include epigastric pain, fullness, belching, anorexia, and other nonspecific symptoms [2, 3]. Furthermore, it is worth noting that helicobacter pylori (HP) infection has a remarkable influence on the incidence of CAG. A systematic review published in 2010 reported that the rate ratio between HP infection and CAG incidence ranged from 2.4 to 7.6 [4]. In certain instances, a small subset of CAG cases eventually progress to gastric neoplasia [5, 6]. And the severity of CAG has been demonstrated to be a key risk factor for the development of gastric cancer from a 10-year prospective cohort study in Japan [7]. Therefore, the proper management of CAG will contribute to the prevention of gastric cancer.
In the viable therapies, pharmacotherapy is still dominant and has been widely applied in the treatment of CAG. These medications are regularly used to alleviate the clinical symptoms and improve quality of life, including acid-inhibitory drugs, HP eradication therapy, mucosal-protective agents, gastrointestinal prokinetic drugs, and digestants [8–11]. Additionally, antidepressant or antianxiety agent may be necessary for the CAG patients with obvious tendency of nervousness and emotional instability [12]. Mucosal-protective agents and proton pump inhibitor were most commonly used medications for chronic gastritis in China [13]. However, the medications still cannot meet clinical needs with respect to efficacy [14, 15]. And patients with long-term western medicine use such as proton pump inhibitor may have a higher possibility of experiencing either diffuse or linear/micronodular enterochromaffin-like cell hyperplasia [16]. In this situation, an increasing number of clinicians and patients are starting to choose herbal treatment for gastric inflammatory condition [17, 18]. As one of the most popular forms of alternative medicine, Chinese classical formula and materia medica have been gradually adopted in different cultures and regions [19, 20].
Sijunzi decoction (SJZD), a traditional Chinese herbal formula, has been frequently used for the treatment of various gastrointestinal disorders [21]. SJZD is composed of four commonly used herbs, including Radix Ginseng (Renshen), Poria cocos (Fuling), Rhizoma Atractylodis Macrocephalae (Baizhu), and Radix Glycyrrhizae (Gancao). According to the theory for Chinese prescription efficiency, SJZD is the representative formula for strengthening the spleen and replenishing Qi [22]. The existing researches on action mechanism have demonstrated that SJZD can ameliorate inflammation, reduce the histopathological injuries, enhance humoral and cellular immune responses, and improve immunological function of the rat through adjusting the genetic expression of JAK-STAT signal pathway [22–24].
In the last two decades, more and more clinical studies have reported the application of SJZD or modified SJZD for better effectiveness in patients with chronic gastritis or CAG, especially in China [25, 26]. Nevertheless, the evidence from the systematic review on SJZD for CAG is insufficient. To address these issues, this systematic review aims to synthesize available data and evaluate clinical evidence of SJZD treating CAG from randomized controlled trials (RCTs).
2. Methods
This study was designed and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement recommendations [27].
2.1. Information Sources and Search Strategies
We performed searches in PubMed, EMBASE, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), Wanfang database, and Chinese Biomedicine Literature Database (SinoMed) from their inception through December, 2016. No restrictions were placed on age, gender, or duration of symptoms. But the search language was limited to English and Chinese. Searching strategies were made through the way of title/abstract, key words, and MeSH terms. The search terms “chronic gastritis”, “chronic atrophic gastritis”, “precancerous lesions of gastric cancer”,“atrophic”, “Sijunzi decoction”, “Sijunzi formula”, “Sijunzi tang”, “Sijunzi pill”, “Sijunzi powder”, “Sijunzi capsule”, “Sijunzi granule” and “random” were applied in various combinations to identify relevant literature. The titles and abstracts of the previous studies were retrieved using the reference management software NoteExpress version 2.0.
2.2. Inclusion Criteria and Exclusion Criteria
To be included in the systematic review, the studies had to meet the following criteria: (1) the type of design was RCT; (2) the articles were published in English or Chinese peer-reviewed journals; (3) the trials compared SJZD with standard medical care or inactive intervention(s) for CAG, such as triple therapy or placebo, and combined therapy of SJZD plus conventional therapies compared with conventional therapies alone was also retrieved; (4) outcome measurement used a validated tool. The primary outcome measures included the incidence of gastric cancer and the improvement of atrophy, intestinal metaplasia, and dysplasia based on the gastroscopy and pathology [15]. Subsequently, we could calculate the overall effect rate according to the improvement of gastroscopy and pathology. Histologic grading score mainly referred to the updated Sydney system [28]. The secondary outcomes were Hp clearance rate, quality of life, and adverse event/adverse drug reaction. In addition, the doctors might use SJZD directly or use modified SJZD (modify some Chinese herbs in SJZD) through judging the patients' clinical symptoms or signs in clinical practice. So the modified SJZD was also included in the review.
The exclusion criteria were listed as follows: (1) non-RCTs or quasi-RCTs and animal study; (2) journal or conference proceedings with no associated full-text article; (3) inappropriate intervention or control, such as SJZD combined with other alternative therapies (herbal formula, acupuncture, moxibustion, cupping, Taichi, Baduanjin, Wuqinxi exercise, etc.) which lacked evidence; (4) nonrecognized outcomes, for instance, self-compiled assessment scale which was not validated. Two authors (D. N. Gan and A. L. Xu) independently searched and selected the eligible trials according to the inclusion and exclusion criteria. Disagreement was resolved by discussion.
2.3. Data Extraction and Quality Assessment
Two authors (A. L. Xu and H. B. Du) extracted the data using a predetermined form. After extraction, data were compared by A. L. Xu, with disagreements being solved by consensus. We contacted the authors of the original articles when we needed to clarify the study data.
All the included studies were evaluated by using the criteria from the Cochrane Handbook for Systematic Review of Interventions [29]. The items reported random generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias. The evaluated domains were judged as low, high, or uncertain risk of bias. Where the two reviewers were uncertain or cannot agree on the quality of individual studies, a third reviewer (Y. A. Ye) would act as an arbiter.
2.4. Data Synthesis
All analyses were performed with the Review Manager 5.2.0 software (Cochrane Collaboration). We chose odds ratio (OR) to present dichotomous outcomes and mean difference (MD) to calculate continuous outcomes with 95% confidence interval (CI). The χ2 test and I2 scores were used to measure statistical heterogeneity. If the result was P < 0.1 and I2 ≥ 50%, the heterogeneity was considered to be high. Random or fixed effect model for meta-analysis of included trials was used based on the heterogeneity between their results. To decrease heterogeneity and increase reliability, subgroup analysis was performed for the comparable group.
3. Results
3.1. Description of the Included Trials
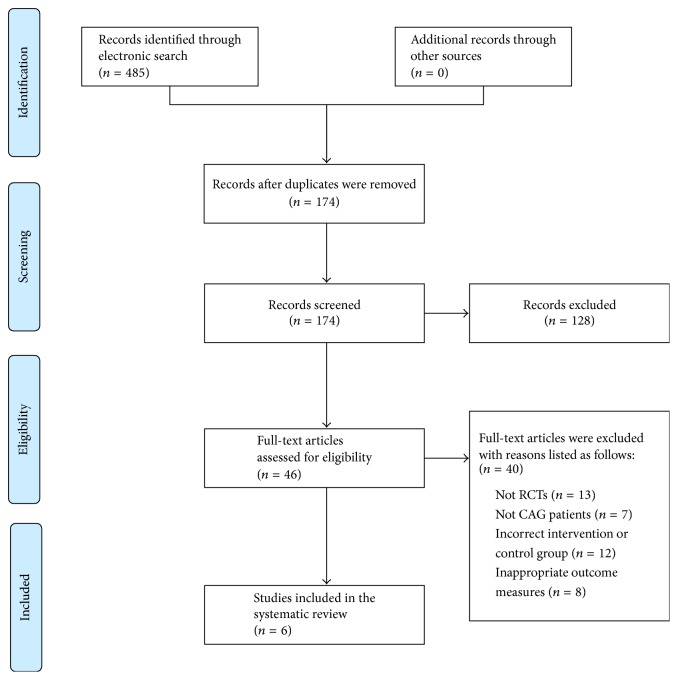
The details about the multistep literature screening process were outlined in Figure 1. We identified 485 new articles. Through removing the duplicated articles, 174 reports were reserved. After screening of titles and abstracts, we excluded 128 reports. Then, the remaining 46 were studied in detail and a further 40 were subsequently excluded. The reasons for exclusion were as follows: not RCTs (n = 13), not CAG patients (n = 7), incorrect intervention or control group (n = 12), and inappropriate outcome measures (n = 8). Eventually, 6 randomized trials that had been conducted in China and published in Chinese met our inclusion criteria [30–35]. They were published between 2009 and 2016. Of these 6 new trials, no trials were placebo-controlled.
Figure 1.
PRISMA 2009 flow diagram.
3.2. Essential Characteristics of the Included Trials
Characteristics of the RCTs in this review were described in Table 1. The sample size ranged from 64 to 126 with a total size of 502. Three trials applied the diagnosis criterion from clinical research guideline on new drugs of traditional Chinese medicine [30–32], one trial used diagnosis criterion of Chinese digestive endoscopy association (gastroscopy diagnosis) and second national consensus meeting on chronic gastritis in China (pathology diagnosis) [34], one trial only mentioned pathological examination with the help of gastroscopy [35], and the other one did not report any criterion [33]. Five trials compared modified SJZD plus conventional medicines with conventional medicines alone [30, 32–35], and one trial compared modified SJZD with conventional medicines [31]. We summarized the composition of the formula in Table 2. In the control group, the medications were recommended by international or Chinese clinical practice guidelines.
Table 1.
Characteristics of the included studies.
| Study ID | Sample size (T/C) | Diagnosis criteria | Intervention | Control | Course of treatment | Outcome assessment |
|---|---|---|---|---|---|---|
| Song et al., 2009 [30] | 68 (34/34) | CRGNDTCM (including gastroscopy and pathology diagnosis) |
Modified SJZD + control | Metronidazole (200 mg, twice a day), 1 week, and folate (10 mg, three times a day), 24 weeks | 24 weeks | Overall effect rate |
|
| ||||||
| Hu, 2011 [31] | 96 (48/48) | CRGNDTCM (including gastroscopy and pathology diagnosis) |
Modified SJZD | Domperidone (10 mg, three times a day), colloidal bismuth pectin (200 mg, four times a day), and omeprazole (20 mg, twice a day) | 4 weeks | Overall effect rate Hp clearance rate |
|
| ||||||
| Sun et al., 2012 [32] | 70 (36/34) | CRGNDTCM (including gastroscopy and pathology diagnosis) | Modified SJZD + control | Omeprazole (30 mg, once a day) and colloidal bismuth pectin (120 mg, three times a day) Abdominal distension or regurgitation onset: domperidone (10 mg, three times a day) HP infection: amoxicillin (500 mg, three times a day) and tinidazole (1 g, three times a day) |
3 weeks | Overall effect rate |
|
| ||||||
| Li, 2013 [33] | 126 (63/63) | Not reported | Modified SJZD + control | Bismuth potassium citrate (300 mg, three or four times a day) | Not reported | Overall effect rate |
|
| ||||||
| Zhang, 2016 [34] | 64 (32/32) | Gastroscopy diagnosis: DCBDACMA Pathology diagnosis: SNCMCG |
Modified SJZD + control | Omeprazole (20 mg, twice a day), clarithromycin (0.5 g, once a day), amoxicillin (1 g, twice a day), 14 days; and folate (10 mg, three times a day), 12 weeks | 12 weeks | Histologic grading score |
|
| ||||||
| Shen, 2016 [35] | 78 (39/39) | Not reported, but mentioned pathological examination with the help of gastroscopy | Modified SJZD + control | Metronidazole (400 mg, twice a day), Lansoprazole (30 mg, twice a day), and Levofloxacin (200 mg, twice a day) | 12 weeks | Overall effect rate |
T: treatment group; C: control group; CRGNDTCM: clinical research guideline on new drugs of traditional Chinese medicine; DCBDACMA: diagnosis criterion of Chinese digestive endoscopy association; SNCMCG: second national consensus meeting on chronic gastritis in China; SJZD: Sijunzi decoction.
Table 2.
Composition of formula.
| Study ID | Formula | Composition of formula |
|---|---|---|
| Song et al., 2009 [30] | Modified SJZD | Radix Codonopsis (Dangshen) 9 g, Poria Cocos (Fuling) 9 g, Rhizoma Atractylodis Macrocephalae (Baizhu) 9 g, Radix Glycyrrhizae (Gancao) 6 g, Trogopterus xanthipes Milne-Edwards (Wulingzhi) 8 g, Rhizoma Chuanxiong (Chuanxiong) 8 g, Herba Hedyotidis Diffusae (Baihuasheshecao) 6 g |
|
| ||
| Hu, 2011 [31] | Modified SJZD | Radix Ginseng (Renshen) 15 g, Poria Cocos (Fuling) 15 g, Rhizoma Atractylodis Macrocephalae (Baizhu) 15 g, Radix Glycyrrhizae (Gancao) 6 g, Radix Astragali (Huangqi) 30 g, Pericarpium Citri Reticulatae (Chenpi) 12 g, Radix Glehniae (Beishashen) 12 g, Radix Ophiopogonis (Maidong) 12 g, Herba Dendrobii (Shihu) 12 g, Fructus Amomi (Sharen) 6 g, Fructus Hordei Germinatus (Maiya) 30 g, Fructus Setariae Germinatus (Guya) 30 g |
|
| ||
| Sun et al., 2012 [32] | Modified SJZD | Radix Codonopsis (Dangshen) 20 g, Poria Cocos (Fuling) 20 g, Rhizoma Atractylodis Macrocephalae (Baizhu) 15 g, Radix Glycyrrhizae (Gancao) 5 g, Fructus Aurantii Immaturus (Zhishi) 15 g, Radix Salviae Miltiorrhizae (Danshen) 10 g, Radix Paeoniae Rubra (Chishao) 15 g, Radix Bupleuri (Chaihu) 5 g |
|
| ||
| Li, 2013 [33] | Modified SJZD | Radix Ginseng (Renshen) 10 g, Poria Cocos (Fuling) 10 g, Rhizoma Atractylodis Macrocephalae (Baizhu) 10 g, Radix Glycyrrhizae (Gancao) 5 g, Radix Astragali (Huangqi) 10 g, Radix Paeoniae Alba (Baishao) 10 g, Semen Lablab Album (Baibiandou) 10 g |
|
| ||
| Zhang, 2016 [34] | Modified SJZD | Radix Codonopsis (Dangshen) 10 g, Poria Cocos (Fuling) 10 g, Rhizoma Atractylodis Macrocephalae (Baizhu) 10 g, Radix Glycyrrhizae (Gancao) 6 g, Radix Astragali (Huangqi) 10 g, Rhizoma Curcuma (Eshu) 10 g, Rhizoma Chuanxiong (Chuanxiong) 10 g, Herba Hedyotidis Diffusae (Baihuasheshecao) 10 g |
|
| ||
| Shen, 2016 [35] | Modified SJZD | Radix Codonopsis (Dangshen) 15 g, Poria Cocos (Fuling) 15 g, Rhizoma Atractylodis Macrocephalae (Baizhu) 15 g, Radix Glycyrrhizae (Gancao) 10 g, Rhizoma Cyperi (Xiangfu) 15 g, Cortex Magnoliae Officinalis (Houpu) 15 g, Radix Angelicae Sinensis (Danggui) 15 g, Radix Paeoniae Alba (Baishao) 15 g, Rhizoma Zingiberis (Ganjiang) 10 g, Rhizoma Pinelliae (Banxia) 10 g, Fructus Aurantii (Zhike) 10 g, Rhizoma Coptidis (Huanglian) 7 g, Fructus Amomi (Sharen) 6 g |
The course of treatment varied from 3 to 24 weeks, but one trial did not report the treatment duration [33]. Five trials evaluated the overall effect rate including manifestations of gastroscopy and pathology [30–33, 35], and one trial assessed the histologic grading score [34], while the secondary outcome only observed Hp clearance rate in one trial [31].
3.3. Risk of Bias in the Included Trials
The methodological quality for the six included studies was presented in Table 3. The reporting quality was classified as high risk of bias in all the trials. The major reason for low quality was a lack of randomization and blinding. Only one trial reported the method generating random sequence [35], while the others simply mentioned that patients were randomly allocated without specific random method. The six trials were not explicit about the reporting of an appropriate method of allocation concealment, blinding of outcome assessor, and selective reporting. We considered the three items to be unclear risk of bias because of insufficient information. In the enrolled trials, no RCTs registered the research protocols. The item “blinding of participants and personnel” was judged as high risk of bias, because no placebo-controlled trials were designed and found. Only one trial described the dropout or withdrawal data in the article [30]. None of the trials had a pretrial sample size calculation. For the item “other bias,” only one trial did not report that the two groups had similarity at the baseline [33].
Table 3.
Risk of bias assessment based on the Cochrane handbook.
| Included studies | Random sequence generation | Concealment of allocation | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data |
Selective reporting | Other bias | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Song et al., 2009 [30] | ? | ? | — | ? | + | ? | + | High |
| Hu, 2011 [31] | ? | ? | — | ? | ? | ? | + | High |
| Sun et al., 2012 [32] | ? | ? | — | ? | ? | ? | + | High |
| Li, 2013 [33] | ? | ? | — | ? | ? | ? | ? | High |
| Zhang, 2016 [34] | ? | ? | — | ? | ? | ? | + | High |
| Shen, 2016 [35] | + | ? | — | ? | ? | ? | + | High |
+: low risk of bias; —: high risk of bias; ?: unclear risk of bias.
3.4. Efficacy of the Interventions
As for the existing different interventions, this study formed two separate comparisons: modified SJZD compared to conventional medicines and modified SJZD plus conventional medicines compared to conventional medicines alone.
3.4.1. Modified SJZD Compared to Conventional Medicines
The meta-analysis was not designed between the two groups. Overall effect rate and Hp clearance rate were evaluated, respectively, in a trial [31]. After treatment for 4 weeks, modified SJZD monotherapy showed better effect on improving overall effect rate and Hp clearance rate compared to combination of the conventional drugs (domperidone, colloidal bismuth pectin, and omeprazole).
3.4.2. Modified SJZD Plus Conventional Medicines Compared to Conventional Medicines Alone
The other 5 trials compared the effect of modified SJZD plus conventional medicines with conventional medicines alone [30, 32–35]. Histologic grading score was assessed in a trial [34]. The results indicated that modified SJZD plus conventional medications could improve significantly the histologic scores of atrophy, intestinal metaplasia, and dysplasia compared with the conventional medications (omeprazole, clarithromycin, and amoxicillin treatment for 14 days and folate treatment for 12 weeks) alone (P < 0.05).
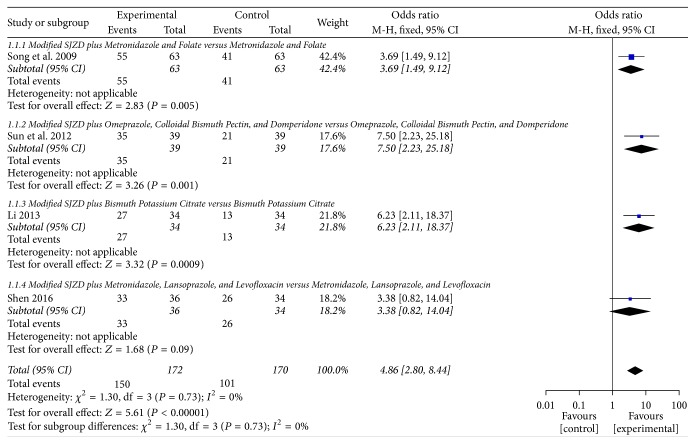
Overall effect rate was observed in the remaining 4 trials [30, 32, 33, 35]. Pooled analysis from 4 trials showed that modified SJZD plus conventional medications exhibited a significant improvement (OR = 4.86; 95% CI: 2.80 to 8.44; Z = 5.61, P < 0.00001) and without significant heterogeneity (χ2 = 1.30, P = 0.73; I2 = 0%) compared with the conventional medications. Fixed effect model was used to estimate the pooled effect. See Figure 2. A statistically significant difference between the intervention and control groups was also found in 3 trials [30, 32, 33].
Figure 2.
Forest plot of modified SJZD plus conventional medicines compared to conventional medicines alone; outcome: overall effect rate.
(1) Modified SJZD plus Metronidazole and Folate versus Metronidazole and Folate. After treatment for 24 weeks, there was statistically significant difference between the combination-therapy group and western medicines alone (OR = 3.69; 95% CI: 1.49 to 9.12; Z = 2.83, P = 0.005) [30].
(2) Modified SJZD plus Omeprazole, Colloidal Bismuth Pectin, and Domperidone versus Omeprazole, Colloidal Bismuth Pectin, and Domperidone. After treatment for 3 weeks, there was statistically significant difference between the combination-therapy group and conventional medicines alone on overall effect rate (OR = 7.50; 95% CI: 2.23 to 25.18; Z = 3.26, P = 0.001) [32].
(3) Modified SJZD plus Bismuth Potassium Citrate versus Bismuth Potassium Citrate. Modified SJZD plus bismuth potassium citrate was better than bismuth potassium citrate in improving the clinical overall effect rate (OR = 6.23; 95% CI: 2.11 to 18.37; Z = 3.32, P = 0.0009) [33].
(4) Modified SJZD plus Metronidazole, Lansoprazole, and Levofloxacin versus Metronidazole, Lansoprazole, and Levofloxacin. After treatment for 12 weeks, there was no statistical significance between the two groups (OR = 3.38; 95% CI: 0.82 to 14.04; Z = 1.68, P = 0.09) [35].
Additionally, we did not find any assessment on the incidence of gastric cancer and quality of life in these identified studies.
3.5. Adverse Effect of the Interventions
None of the trials reported the adverse event or adverse drug reaction in the previous studies.
4. Discussion
4.1. Overview of Findings
In the systematic review, we included 6 RCTs following the inclusion criteria. All the trials used the modified SJZD as the main intervention. Only one trial compared modified SJZD with conventional medicines, including domperidone, colloidal bismuth pectin, and omeprazole [31]. The results showed that modified SJZD was more effective than conventional medicines in improving overall effect rate and Hp clearance rate. Nevertheless, the analytical data was extracted from one trial with a small sample size, and the trial did not perform blinding. Five trials compared the clinical efficacy of modified SJZD plus conventional medicines with conventional medicines alone [30, 32–35]. One trial found that modified SJZD plus conventional medications could improve significantly the scores of histopathology compared with the conventional medications, including HP eradication therapy and folate treatment [34]. But the methodological flaw such as randomization and blinding was also found in the trial. The meta-analysis indicated that modified SJZD plus conventional medications had a significant improvement compared with the conventional medications in improving overall effect rate [30, 32, 33, 35]. Although the pooled analysis created a positive result, it was still difficult to draw a definite conclusion because of the limited sample size of outcome events (150 versus 101) and low-quality studies.
Meanwhile, no extra information on the incidence of gastric cancer, quality of life, and adverse events/adverse drug reaction could be available to assess the efficacy or adverse effect of SJZD for CAG. The course of treatment was also inconsistent in the included trials and might affect the effectiveness of Chinese herbal formula.
4.2. Comparison with the Previous Systematic Review
So far there was a systematic review reporting the modified SJZD treating CAG that preceded our study [36]. Both the two systematic reviews and meta-analysis demonstrated that modified SJZD plus conventional western medicine can significantly improve the overall effect in treating patients with CAG compared with conventional western medicine.
However, the differences could be distinguished between the two reviews. Firstly, the previous review included 7 trials, while 3 trials in that review were enrolled in our study [31–33]. Four trials were excluded, because 2 trials chose unconventional treatment as control group, while the others did not design recognized outcome measures. Secondly, we searched the literatures from their inception until December, 2016. One article published in 2009 [30] and two articles published in 2016 [34, 35] were screened. Thirdly, the outcomes including Hp clearance rate and histologic grading score were reported in our study.
4.3. Limitations and Implications
The quality of each of the included trials was evaluated by using the Cochrane Collaboration's tool. The methodology evaluation showed a high risk of bias in domain of blinding for participants and personnel, which directly weakened the strength of the positive results. In spite of difficulties, the double-blinding clinical trial should be strongly recommended to confirm the absolute effect of Chinese herbal formula [37].
SJZD came from the Chinese pharmacopoeia named “Tai Ping Hui Min He Ji Ju Fang” in Song dynasty. For SJZD, two limitations or questions should be noted to this review. Radix Ginseng (Renshen) was substituted by Radix Codonopsis (Dangshen) in the four studies [30, 32, 34, 35]. According to the traditional Chinese medicine theory, the role of strengthening the spleen and replenishing Qi might be weakened. On the other hand, the physicians always modified some herbs based on the original prescription of SJZD including just four herbs. In our studies, the number of modified herbs ranged from 3 to 10 kinds. Therefore, the modified SJZD was difficult to be standardized and the clinical effect of the interventions should be different from each other.
Additionally, we only searched electronic databases but did not conduct a manual retrieval, which might leave out the relevant clinical trials. As the sample size of the included studies was relatively small, we were unable to determine the effect estimates of the intervention.
Based on the existing problems of the current studies, more and more rigorous RCTs including multicenter, placebo-controlled clinical trials are needed to be launched to produce higher quality evidence. The study protocol of traditional Chinese medicine clinical trials should be registered or published in the future [38]. The appropriate randomization method and sample size calculation would be applied. In respect of trial reporting, the researchers should follow the checklist of the Consolidated Standards for Reporting Trials (CONSORT) [39].
4.4. Conclusion
Modified SJZD combined with conventional western medicines appears to have benefits for the patients with CAG compared with conventional western medicines. Due to the limited number and methodological flaw of the previous studies, the beneficial and harmful effects of SJZD or modified SJZD for CAG could not be identified. More rigorous RCTs and further clinical evidence are needed to confirm the results.
Acknowledgments
The authors are thankful for the financial support from the National Twelfth Five-Year Key Science and Technology Project (no. 2012ZX10005010-002-005).
Disclosure
D. N. Gan and A. L. Xu are co-first authors of this paper.
Conflicts of Interest
All the authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
D. N. Gan and A. L. Xu contributed equally to this paper.
References
- 1.Vannella L., Lahner E., Annibale B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: A critical reappraisal. World Journal of Gastroenterology. 2012;18(12):1279–1285. doi: 10.3748/wjg.v18.i12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman M., Lee E. L. Gastritis. In: Feldman M., editor. Sleisinger and Fordtran’s Gastrointestinal and Liver Disease. 10th. chapter 52. Philadelphia, Pa, USA: Elsevier Saunders; 2016. pp. 870–872. [Google Scholar]
- 3.Zhang Y., Zhou A., Liu Y., et al. Exploratory factor analysis for validating traditional chinese syndrome patterns of chronic atrophic gastritis. Evidence-Based Complementary & Alternative Medicine. 2016;2016 doi: 10.1155/2016/6872890.6872890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamu M. A., Weck M. N., Gao L., Brenner H. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. European Journal of Epidemiology. 2010;25(7):439–448. doi: 10.1007/s10654-010-9482-0. [DOI] [PubMed] [Google Scholar]
- 5.Giannakis M., Chen S. L., Karam S. M., Engstrand L., Gordon J. I. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4358–4363. doi: 10.1073/pnas.0800668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco G., Corvalan A. H. Helicobacter pylori-induced chronic gastritis and assessing risks for gastric cancer. Gastroenterology Research and Practice. 2013;2013:8. doi: 10.1155/2013/393015.393015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue M., Tajima K., Matsuura A., et al. Severity of chronic atrophic gastritis and subsequent gastric cancer occurrence: a 10-year prospective cohort study in Japan. Cancer Letters. 2000;161(1):105–112. doi: 10.1016/S0304-3835(00)00603-0. [DOI] [PubMed] [Google Scholar]
- 8.Fang J. Y., Liu W. Z., Shi Y., Ge Z. Z., Xiao S. D. Consensus on chronic gastritis in China - second national consensus meeting on chronic gastritis (14–16 september 2006 Shanghai, China) Journal of Digestive Diseases. 2007;8(2):107–119. doi: 10.1111/j.1443-9573.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 9.Ching C.-K., Lam S.-K. Antacids: indications and limitations. Drugs. 1994;47(2):305–317. doi: 10.2165/00003495-199447020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hu F. L., Hu P. J., Liu W. Z. Third chinese national consensus report on the management of helicobacter pylori infection. Journal of Digestive Diseases. 2008;9(3):178–184. doi: 10.1111/j.1751-2980.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 11.Leontiadis G. I., Ford A. C. Helicobacter pylori eradication: gastric cancer prevention. BMJ Clinical Evidence. 2015;2015:p. 0406. [PMC free article] [PubMed] [Google Scholar]
- 12.Porcelli P., De Carne M. Non-fearful panic disorder in gastroenterology. Psychosomatics. 2008;49(6):543–545. doi: 10.1176/appi.psy.49.6.543. [DOI] [PubMed] [Google Scholar]
- 13.Du Y., Bai Y., Xie P., et al. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterology. 2014;14(1) doi: 10.1186/1471-230X-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zendehdel N., Nasseri-Moghaddam S., Malekzadeh R., Massarrat S., Sotoudeh M., Siavoshi F. Helicobacter pylori reinfection rate 3 years after successful eradication. Journal of Gastroenterology and Hepatology. 2005;20(3):401–404. doi: 10.1111/j.1440-1746.2005.03561.x. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y., Ma L. X., Yin S. J., An J., Wei Q., Yang J. X. Huangqi Jianzhong Tang for treatment of chronic gastritis: a systematic review of randomized clinical trials. Evidence-Based Complementary & Alternative Medicine. 2015;2015 doi: 10.1155/2015/878164.878164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H., Zhu J., Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. The Cochrane database of systematic reviews. 2014;12:p. CD010623. doi: 10.1002/14651858.CD010623.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.-C. Medicinal plant activity on Helicobacter pylori related diseases. World Journal of Gastroenterology. 2014;20(30):10368–10382. doi: 10.3748/wjg.v20.i30.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safavi M., Sabourian R., Foroumadi A. Treatment of helicobacter pylori infection: current and future insights. World Journal of Clinical Cases. 2016;4(1):5–19. doi: 10.12998/wjcc.v4.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin I.-S., Jeon W.-Y., Shin H.-K., Cha S.-W., Lee M.-Y. Banhabaekchulchunma-tang, a traditional herbal formula attenuates absolute ethanol-induced gastric injury by enhancing the antioxidant status. BMC Complementary and Alternative Medicine. 2013;13, article 170 doi: 10.1186/1472-6882-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson E. M., Lorenc A., Booker A., Robinson N. The rise of traditional Chinese medicine and its materia medica: a comparison of the frequency and safety of materials and species used in Europe and China. Journal of Ethnopharmacology. 2013;149(2):453–462. doi: 10.1016/j.jep.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Kang A., Guo J. R., Zhang Z., Wang X. L. Simultaneous quantification of ten active components in traditional chinese formula sijunzi decoction using a UPLC-PDA method. Journal of Analytical Methods in Chemistry. 2014;2014 doi: 10.1155/2014/570359.570359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu W., Lu B., Zhang H., Zhang Y., Yan J. Effects of the Sijunzi decoction on the immunological function in rats with dextran sulfate-induced ulcerative colitis. Biomedical Reports. 2016;5(1):83–86. doi: 10.3892/br.2016.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D., Shi W., Zhao Y., Zhong X. Adjuvant effects of Sijunzi decoction in chickens orally vaccinated with attenuated newcastle-disease vaccine. African journal of traditional, complementary, and alternative medicines: AJTCAM / African Networks on Ethnomedicines. 2012;9(1):120–130. [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong B., Qian H. Effects of Sijunzi decoction and Yupingfeng powder on expression of janus kinase-signal transducer and activator of transcription signal pathway in the brain of spleen-deficiency model rats. Journal of Traditional Chinese Medicine. 2013;33(1):78–84. doi: 10.1016/S0254-6272(13)60105-3. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y., Wang J.-T., Chen R.-X. Observation on therapeutic effect of acupuncture combined with drug for treatment of intestinal metaplasia of chronic atrophic gastritis. Zhongguo Zhen Jiu = Chinese acupuncture & moxibustion. 2005;25(7):457–459. [PubMed] [Google Scholar]
- 26.Zhong W. R., Huang Y. X., Cui J. P. Clinical study on modified sijunzi decoction in treating intestinal metaplasia of gastric mucosa. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine/Zhongguo Zhong xi yi jie he xue hui, Zhongguo Zhong yi yan jiu yuan zhu ban. 1997;17(8):462–464. [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PubMed] [Google Scholar]
- 28.Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney system. Proceedings of the International Workshop on the Histopathology of Gastritis; 1994; Houston, Texas, TX, USA. American Journal of Surgical Pathology; pp. 1161–1181. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J. P., Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5. 1. 0. the Cochrane Collaboration; 2009. [DOI] [Google Scholar]
- 30.Song F. L., Lin Y. F., Li H. R., et al. Effect of modified Sijunzi decoction based triple therapy on atrophic gastritis and expression of PCNA. Zhong Hua Zhong Yi Yao Za Zhi. 2009;24(3):367–369. [Google Scholar]
- 31.Hu Z. C. Forty-eight cases clinical study of modified Sijunzi decoction treatment for chronic atrophic gastritis. Zhong Wai Jian Kang Wen Zhai. 2011;8(27):p. 407. [Google Scholar]
- 32.Sun G. J., Zhang Y. P., Wu S. Thirty-six cases clinical study of modified Sijunzi decoction treatment for chronic atrophic gastritis with the syndrome of weakened spleen and stomach. Zhongguo Bao Jian Ying Yang. 2012;12, article 2504 [Google Scholar]
- 33.Li H. R. The clinical observation of modified Sijunzi decoction in the management of chronic atrophic gastritis. Zhongguo Xian Dai Yao Wu Ying Yong. 2013;7(21):123–124. [Google Scholar]
- 34.Zhang Z. M., Yang W. J., Ji B., Xu Z. B. Modified Sijunzi decoction combined with helicobacter pylori eradication therapy and folate treatment for chronic atrophic gastritis with the syndrome of weakened spleen and stomach: a clinical trial. Zhe Jiang Zhong Yi Za Zhi. 2016;51(6):393–394. [Google Scholar]
- 35.Shen G. F. Clinical efficacy evaluation of Chinese formula modified Sijunzi decoction treating for chronic atrophic gastritis. Clinical efficacy evaluation of Chinese formula modified Sijunzi decoction treating for chronic atrophic gastritis. 2016;28(5):110–111. [Google Scholar]
- 36.Guo Z. L., Su Z. N., Wang Z. F., Luo X. M. Meta-analysis of modified Sijunzi decoction for treating chronic atrophic gastritis. Zhongguo Shi Yan Fang Ji Xue Za Zhi. 2015;21(24):204–208. [Google Scholar]
- 37.Zhang W., Wang S., Zhang R. Erratum: evidence of Chinese herbal medicine Duhuo Jisheng decoction for knee osteoarthritis: A systematic review of randomised clinical trials (BMJ Open (2016) 6 (e008973)) BMJ Open. 2016;6(1) doi: 10.1136/bmjopen-2015-008973corr1.e008973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pengfei L., Xing L., Yanming X., Zhiguo W. The importance and necessity of establishing technical specifications for the registration of traditional chinese medicine clinical studies. Journal of Alternative and Complementary Medicine. 2014;20(1):65–66. doi: 10.1089/acm.2013.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D., Hopewell S., Schulz K. F., et al. Erratum: CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology. 2010;63(8):e1–e37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]