Abstract
Cluster of differentiation 69 (CD69), known as an early activation marker of lymphocytes, has been demonstrated to regulate inflammatory events in various disease models. Although the increased number of CD69-expressed T lymphocytes in the lungs of patients with chronic obstructive pulmonary disease (COPD) has been reported, a functional role of CD69 in the pathogenesis of COPD remains unknown. To address to this question, CD69-deficient (CD69KO) mice and wild-type (WT) mice were subjected to a mouse model of porcine pancreatic elastase (PPE)-induced pulmonary inflammation and emphysema. In the two genotypes, PPE increased counts of macrophages, neutrophils and lymphocytes in bronchoalveolar lavage fluid (BALF) and induced emphysematous changes in the lung, whereas those two pathological signs were significantly enhanced in CD69KO mice compared to WT mice. Moreover, the PPE-induced levels of IL-17 and IL-6 in BALF were significantly higher in CD69KO mice than in WT mice at the acute inflammatory phase. Immunofluorescent studies showed that IL-17 and IL-6 were predominantly expressed in CD4+ and γδ T cells and macrophages, respectively. Concomitant administration of IL-17- and IL-6-neutralizing antibodies significantly attenuated the PPE-induced emphysematous changes in the two genotypes. These findings suggest that CD69 negatively regulates the development of PPE-induced emphysema in part at least through modulating function of IL-17-producing T cells.
Keywords: CD69, Chronic obstructive pulmonary disease, IL-17, Th17, γδ T cell
Highlights
-
•
CD69 negatively modulated elastase-induced pulmonary inflammation and emphysema.
-
•
Disruption of the CD69 gene enhanced the elastase-induced IL-6 and IL-17 expression.
-
•
Th17 and γδ T cells affected the elastase-induced emphysema via producing IL-17.
-
•
Neutralization of IL-6&17 signals attenuated the elastase-induced emphysema.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a global health problem, which is predicted to be the third leading cause of death worldwide by 2020 [1]. COPD is characterized by chronic inflammation of the small airways and disruption of the alveolar walls, leading to small airway remodeling and airspace enlargement [2]. Although the main strategy for treating COPD is to control of chronic inflammation, current anti-inflammatory therapies are not successful [3]. Therefore, there is an emerging need for identification of new key inflammatory regulators in COPD.
CD69, a type II membrane protein with a C-type lectin biding domain [4], is known to be an early T lymphocyte activation marker that is upregulated in response to inflammatory stimuli [5]. The pathophysiological roles of CD69 in various inflammatory disease models have been studied using CD69-deficient (KO) mice [6], [7], [8], [9]. CD69 has been reported to be associated with JAK3/Stat5 protein and negatively control Th17 cell differentiation as a receptor for Galectin-1 on dendritic cells [10], [11]. These findings suggest that CD69 can function as a negative modulator in the pathogenesis of several autoimmune diseases [6]. In contrast, CD69 plays an active role in several animal models of inflammatory disease [7], [8], [9]. Hence, the controversial contribution of CD69-deficiency to inflammatory output of various disease models makes the CD69 function complicated. To employ CD69 as a feasible therapeutic option, information concerning functional role of CD69 in the different inflammatory diseases should be accumulated.
Recently, it has been revealed that CD69 expression on both lung CD4+ and CD8+ T cells correlates with COPD severity [12], [13], tempting us to consider that CD69 might be involved in the progression of COPD. However, the mechanism how CD69-expressed cells contribute to the pathogenesis of COPD, especially emphysema, is still unknown. Here, we investigated the role of CD69 in the pathogenesis of COPD by subjecting CD69KO and wild-type (WT) mice to a mouse model of elastase-induced emphysema.
2. Materials and methods
2.1. Mice and treatment
CD69KO mice (C57BL/6 background) were generated as previously described [8]. WT and CD69KO littermates aged 8–12 weeks were used for each experiment. All animal procedures conformed to the Japanese regulations for animal care and use, following the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science, and were approved by the Animal Care and Use Committee of Chiba University.
Mice under anesthesia were given a single intratracheal injection of porcine pancreatic elastase (PPE) (0.2 U/g, Elastin Products Company, Owensville, MO) dissolved in phosphate-buffered saline (PBS) by using a Microsprayer atomizer (Penn-Century, Philadelphia, PA). Control mice were treated with PBS. In some experiments, mice received intratracheal injection of anti-mouse IL-17A-neutralizing antibody (10 µg/body, Biolegend, San Diego, CA) and anti-mouse IL-6-neutralizing antibody (10 µg/body, Biolegend) concurrently with instillation of PPE. Control mice were treated with isotype control (rat IgG1κ, R&D Systems, Minneapolis, MN) in PBS.
2.2. Evaluation of cell populations and cytokines in BALF
At 1 day post-instillation (dpi), the trachea was exposed and lavaged three times with 1 ml PBS using a 20-gauge catheter. After centrifuging BALF at 400×g for 10 min, the resulting supernatants were stored as samples for ELISA at −80 °C, and the cell pellet was resuspended in PBS and subjected to the cell count using a hemocytometer in combination with Diff-Quick (Sysmex Corporation, Kobe, Japan) staining. The levels of IFN-γ, IL-17A, IL-6 and IL-23 in BALF were measured by ELISA kit (Biolegend) according to the manufacturer's protocols.
2.3. Histological examination
At 21 dpi, mice under anesthesia were intracardially perfused with ice-cold PBS, and lung tissue was inflated and fixed by infusion of 4% paraformaldehyde (PFA) through the tracheal cannula at a constant pressure of 20 cm of H2O. The left lung was dissected out, fixed, dehydrated and frozen. Freshly cut lung sections (5 µm thickness) placed on poly-L-lysine-coated slides were stained with hematoxylin-eosin (HE). Airspace enlargement was quantified by the mean linear intercept (MLI) calculations in 20 randomly selected fields of the lung tissue sections. MLI was obtained by dividing the total length of a line drawn across the lung section by the total number of intercepts encountered.
2.4. Immunofluorescent study
Freshly cut lung sections (5 µm thickness) from mice at 1 dpi were pretreated with FcR blocking agent (Miltenyi Biotec, Gladbach, Germany) for 15 min, and then reacted with various antibodies as follow: anti-mouse IL-6 antibody (Biolegend), anti-mouse IL-17A (Biolegend), anti-Iba1 antibody (WAKO, Osaka, Japan), anti-CD4 antibody (BIOSS ANTIBODIES, Boston, Massachusetts) and anti-mouse T-cell receptor (TCR)γδ antibody (Biolegend). After staining with each appropriate fluorescein-conjugated second antibody, the sections were observed under a fluorescence microscope (Axio ImagerA2, Zeiss, Oberkochen, Germany). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI). In case of counting IL-6-, IL-17-, Iba1-, CD4− and TCRγδ-like immunoreactivity (LI), two sections from each lung were randomly selected and subjected to immunofluorescent studies. Under 200× magnification, 3 fields in each section were randomly chosen, and fluorescent signal-expressing cells were counted and averaged (/0.1 mm2).
2.5. Intracellular IL-17 staining
At 1 and 3 dpi, single cell suspensions of lymph node cells (LNCs) were prepared from the cervical and axillary lymph nodes of WT and CD69KO mice and stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) plus 2 μg/ml ionomycin for 3 h in the presence of 2 µM monensin (Sigma, St. Louis, MO). Then, the cells were stained with allophycocyanin-conjugated CD4 antibody (Biolegend). After fixing and permeabilizing (Fix/Perm buffer, Biolegend), the cells were further stained with phycoerythrin-conjugated anti-IL-17 antibody and FITC-conjugated anti-IFN-γ antibody (BD Biosciences, San Jose, CA). Analysis was performed with a FACS CantII flow cytometer (BD Biosciences) and FlowJo software (Tree Star Inc., Ashland, OR, USA).
2.6. Evaluation of IL-17 production in γδ T cells
To purify γδ T cells, CD4+ T cells were first depleted from single cell suspensions of the LNCs derived from naïve WT and CD69KO mice by using anti-mouse CD4 magnetic microbeads (Miltenyi Biotec, Gladbach, Germany) in combination with MACS columns (Miltenyi Biotec). It has been demonstrated that activated γδ T cells amplify Th17 response [14]. Hence, this CD4+ cell-depletion step can minimize the participation of Th17-derived IL-17 in the assay. Then, the CD4− LNCs were incubated with FITC-conjugated anti-mouse TCRγδ antibody (eBioscience, Santa Clara, CA) followed by a reaction with anti-FITC magnetic microbeads (Miltenyi Biotec). The cells selected by MACS columns were used as γδ T cells and cultured in RPMI 1640 medium (Sigma) supplemented with 10% FBS, 500 µM 2-mercaptoethanol, penicillin (100 units/ml), and streptomycin (100 µg/ml) for 24 h at 37 °C in a humidified atmosphere (5% CO2). Then, according to a previous report [14], γδ T cells were stimulated with IL-1β (10 ng/ml) and IL-23 (10 ng/ml) for 72 h. The supernatants were subjected to enzyme-linked immunosorbent assay (ELISA) for mouse IL-17A (Biolegend). We confirmed that IL-17A levels in the supernatants from unstimulated γδ T cells of the two genotypes were below the detection limit.
2.7. Statistical analysis
Data are shown as mean±S.E.M. Statistical analysis was conducted using GraphPad Prism software (Version 6.0: GraphPad Software Inc., San Diego). Statistical significance was determined by one-way ANOVA, followed by the Tukey test. A p value <0.05 was considered to be significant.
3. Results and discussion
3.1. Deterioration of elastase-induced pulmonary emphysema in CD69KO mice
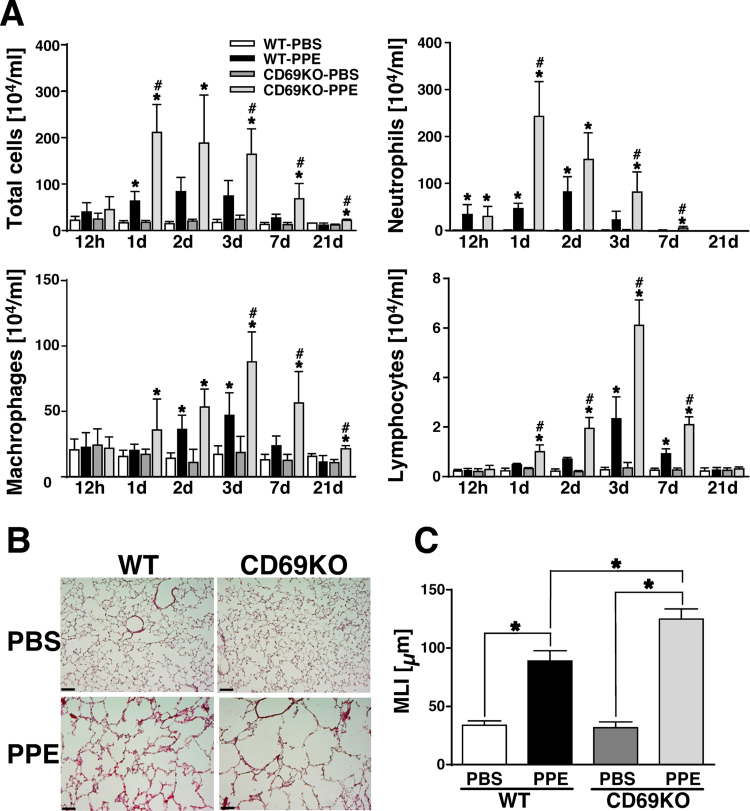
It has been clearly demonstrated that CD69 is expressed on activated lymphocytes, neutrophils and macrophages in various inflammatory diseases [5], [8], [9], [15]. Also in the PPE model, PPE apparently increased the numbers of CD69+/Iba1+, CD69+/Ly6G+, CD69+/CD4+, CD69+/TCRγδ+ cells in the lung at 1 dpi, indicating that PPE-mobilized inflammatory cells such as macrophages, neutrophils and T lymphocyte subsets upregulate CD69 expression (Supplemental Fig. 1). Hence, to elucidate how CD69 controls the progression of lung inflammation followed by emphysematous changes, WT and CD69KO mice were subjected to the PPE-induced COPD model. As shown in Fig. 1A, the numbers of total inflammatory cells, neutrophils, macrophages, and lymphocytes in BALF were significantly elevated by PPE in the both genotypes in a time-dependent manner with showing a peak level of each cell population. The increase in these cell populations in PPE-instilled mice was significantly enhanced in CD69KO mice. It has been reported that the initial inflammatory output induced by PPE correlates with severity of subsequent pulmonary emphysematous changes [16], [17]. As shown in Fig. 1B, PPE induced apparent emphysematous changes in the lung of both genotypes at 21 dpi, which were much severe in CD69KO mice compared with WT mice (Fig. 1B). Accordingly, the PPE-increased MLI was significantly higher in CD69KO mice than in WT mice (Fig. 1C). These results suggest that CD69 may play an inhibitory role in the elastase-induced pulmonary pathology.
Fig. 1.
Pathophysiological profiles of CD69KO mice in elastase-induced lung inflammation and subsequent emphysematous change. A) Effect of PPE-instillation on differential cell counts in WT and CD69KO mice. Time-dependent changes of differential cell counts in BALF were determined 1 d after the instillation of PPE (0.2 U/g body weight) or PBS. Data are shown as mean±S.E.M. (n=4–8). *P<0.05, significantly different from value of WT-PBS group (ANOVA followed by Tukey's test). The difference between WT-PPE and CD69KO-PPE groups was statistically significant (#P<0.05). B) Typical profiles of histopathological changes in the lung of WT and CD69KO mice. Lung sections from WT and CD69KO mice at 21 dpi were stained with hematoxylin and eosin. Scale bar represents 100 µm. C) Mean linear intercept (MLI), as a measure of interalveolar wall distance, was determined by light microscopy. Data are shown as mean±S.E.M. (n=8). *P<0.05, significantly different from value of WT-PBS group (ANOVA followed by Tukey's test).
Previous reports have demonstrated that elastase-induced lung injury leads production of various cytokines/chemokines, such as Eotaxin, MIP-1α, TNF-α, KC, IL-1β, IL-6, MIP-2 and IL-17. These cytokines are acutely expressed in response to the PPE administration, and some of them showed a peak expression level at 1 dpi [16], [17], [18]. Furthermore, TNF-α receptor plus IL-1β receptor-deficiency, IL-6-deficiency, MyD88 (IL-1R1 adapter protein)-deficiency or IL-17-deficiency can attenuate the PPE-induced acute lung inflammation and subsequent emphysema [16], [17], [18], [19]. Thus, the PPE-upregulated cytokines at the acute inflammatory phase positively contribute to the development of emphysema. Therefore, next, to elucidate the mechanisms underlying the enhanced signs of CD69KO mice, we investigated levels of various cytokines in BALF from WT and CD69KO mice at 1 dpi.
3.2. Involvement of IL-6 and IL-17 and their signals-associated cytokines in the enhanced signs of CD69KO mice
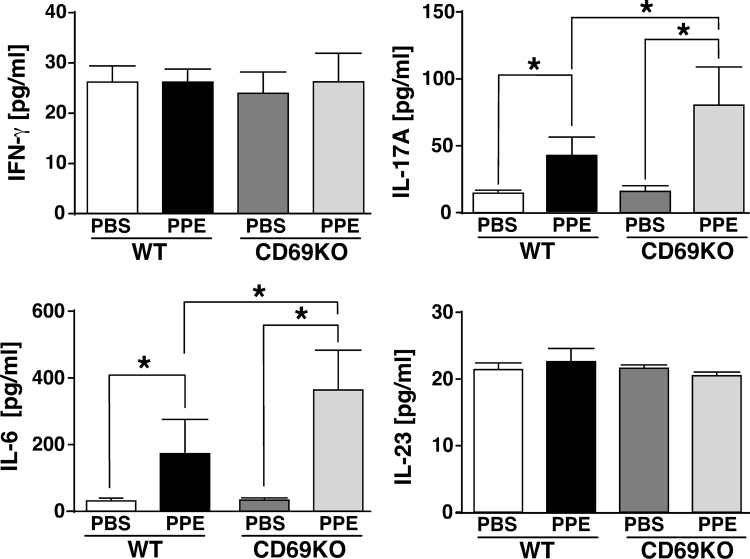
Lymphoid follicles composed of B-cell core, T cells in the periphery and dendritic cells presenting antigen are occasionally observed in the lungs of patients with COPD, indicating that T cells affect the pathogenesis of COPD [20]. Similarly, chronic inflammation mediated by Th1- and Th17-signals-associated cytokines has been shown to be associated with the development of COPD [21], [22], [23]. Therefore, we examined expression levels of IFN-γ and IL-17 that are key mediators of Th1- and Th17-related inflammation, respectively, in BALF from the two genotypes at 1 dpi. As shown in Fig. 2, the level of IL-17 induced by PPE was higher in CD69KO mice than WT mice. On the other hand, IFN-γ level in BALF was not induced by PPE in the two genotypes, suggesting that IL-17-producing T cells but not Th1 cells may be involved in the enhanced signs of CD69KO mice. It is well known that IL-6 and IL-23 are key mediators for the differentiation of Th17 and IL-17-producing γδ T cells [24], [25]. Then, the amounts of IL-6 and IL-23 in BALF were also examined. IL-6 was induced by the PPE challenge, and its level was significantly higher in CD69KO mice than WT mice. On the other hand, IL-23 was not induced by PPE in the two genotypes. These results provide the possibility that CD69 may modulate the development of COPD partly through regulating IL-6/IL-17-signaling axis.
Fig. 2.
Changes in cytokine levels in BALF from WT and CD69KO mice.
Concentrations of IFN-γ, IL-17A, IL-6 and IL-23 in BALF from the two genotypes at 1 dpi were determined by ELISA. Data are shown as mean±S.E.M. (n=6). *P<0.05, significantly different from value of WT-PBS group (ANOVA followed by Tukey's test).
In mouse models of COPD evoked by PPE and cigarette smoking (CS), various cytokines/chemokines sensitive to IL-17 or IL-6 coordinately contribute to the development of COPD. In IL-6-deficient mice, the PPE-induced MIP-1α is significantly suppressed in BALF compared to WT mice [18]. In the PPE and the CS models, the induction of MIP-2, IL-1β and G-CSF levels in BALF from IL-17-deficient mice was less than that from WT mice [17], [26]. Likewise, IL-17RA-deficiency failed to induce MCP-1, RANTES and IP-10 compared to WT mice [27]. Concerning these IL-6/IL-17-signals-associated cytokines/chemokines, we evaluated the effect of CD69-deficiency on their expression in the PPE model by performing a cytokine array. As expected, all of the PPE-induced levels of IL-6/IL-17-signals-associated cytokines/chemokines (G-CSF, IL-1β, IP-10, MCP-1, MIP-1α, MIP-2 and RANTES) in BALF were higher in CD69KO mice than WT mice (Supplemental Fig. 2). Hence, various cytokines sensitive to the upregulation of IL-6/IL-17 signaling coordinately contribute to the enhanced signs of CD69KO mice. Then, we investigated the sources of the key cytokines, IL-6 and IL-17 in the PPE-instilled lung.
3.3. 3.3. IL-6- and IL-17-producing cells in the PPE-instilled lung
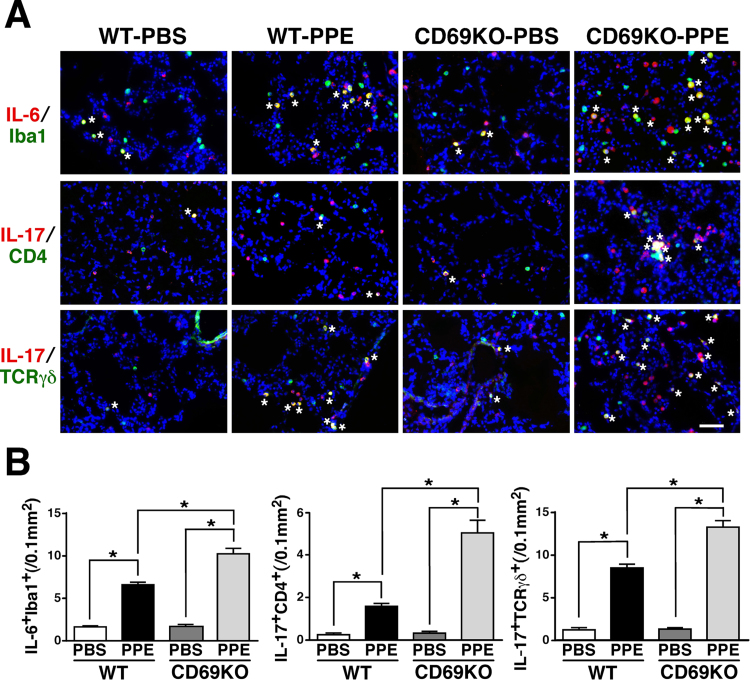
As shown in Fig. 3A, the PPE-induced IL-6-LI was predominantly observed in Iba1+ cells in the alveolar space at 1 dpi, which was apparent in the lung of CD69KO mice compared to WT mice. Accordingly, the number of IL-6+Iba1+ cells was increased in the alveolar space of the two genotypes instilled with PPE, whereas the increased number of IL-6+Iba1+ cells in CD69KO mice was significantly larger than that in WT mice (Fig. 3B). Although the PPE-induced IL-6-LI was also observed in SP-C+ alveolar epithelial cell type II and S100A4+ fibroblast as small populations, there was no significant difference in the numbers of IL-6+SP-C+ and IL-6+S100A4+ cells between the two genotypes (data not shown). These findings indicate that a predominant IL-6-producing cell is macrophage under our experimental condition and further suggest that the PPE-induced infiltration of macrophages producing IL-6 into the lung can be sensitive to the CD69-deficiency. On the other hand, IL-17-LI was mostly observed in CD4+ and TCRγδ+ lymphocytes in the lungs of the two genotypes after instillation of PPE (Fig. 3A). The PPE-increased numbers of IL-17+CD4+ and IL-17+TCRγδ+ cells in CD69KO mice were significantly larger than those in WT mice (Fig. 3B), indicating that PPE-mobilized IL-17-producing T cells could be enhanced by the CD69-deficiency. Although Th17 and regulatory T cells are recognized as IL-17-producing CD4+ cells [28], IL-17+Foxp3+ cells were merely observed in the PBS- or PPE-instilled mice (data not shown). Therefore, the IL-17+CD4+ cells were Th17 cells. These results suggest that Th17 and γδ T cells are predominant source of IL-17 under the PPE-provoked inflammatory environment in the lung and negatively modulated by CD69.
Fig. 3.
Immunofluorescent study for IL-6- and IL-17-producing cells in WT and CD69KO mice. A) Predominant IL-6- and IL-17-producing cells in the lung of the two genotypes instilled with PPE. Lung sections from WT and CD69KO mice at 1 dpi were reacted with the designated combination of antibodies. Asterisks indicate double-positive cells. Scale bar represents 50 µm. B) The PPE-increased and CD69-deficinecy-sensitive IL-6+Iba1+, IL-17+CD4+ and IL-17+TCRγδ+ cells. Data are shown as mean±S.E.M. (n=8). *P<0.05, significantly different from value of WT-PBS group (ANOVA followed by Tukey's test).
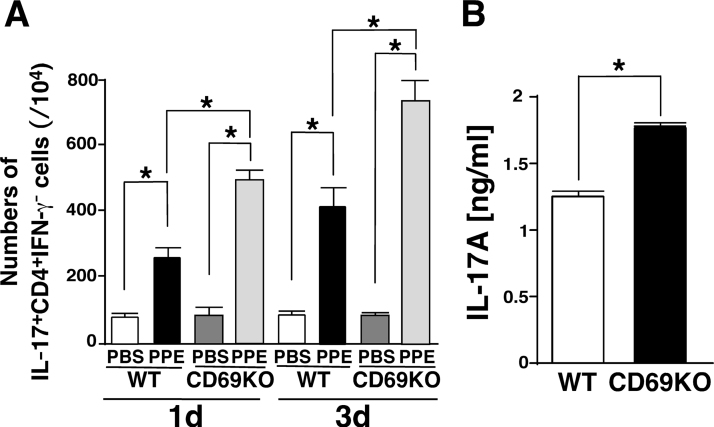
The question arose as to whether the ability of each cell in producing IL-6 or IL-17 might be regulated by CD69 signaling. Then, we evaluated the changes of following ratios: IL-6+Iba1+/Iba1+; IL-17+CD4+/CD4+ and IL-17+TCRγδ+/TCRγδ+ in four groups (WT-PBS, WT-PPE, CD69KO-PBS and CD69KO-PPE). As shown in Table 1, all of the ratios of IL-6+Iba1+/Iba1+, IL-17+CD4+/CD4+ and IL-17+TCRγδ+/TCRγδ+ significantly increased in response to PPE. The IL-6+Iba1+/Iba1+ ratio showed no significant difference between WT-PPE and CD69KO-PPE groups, suggesting that CD69 on macrophages may not function as a regulator for IL-6 production at least in the PPE model. In contrast, the ratios of IL-17+CD4+/CD4+ and IL-17+TCRγδ+/TCRγδ+ in CD69KO-PPE group were significantly elevated compared with those in WT-PPE group. This finding suggests that CD69 may be a negative regulator for IL-17-producing T cell subsets, especially Th17 and γδ T cells, in the PPE model. In fact, it has been demonstrated that CD69-mediated Jak3/Stat5 signaling negatively affects the differentiation from naïve CD4+ T cells into Th17 cells [11]. Moreover, it has been reported that the infiltration of CD4+ T cells producing IL-17 into the lung can be induced by the PPE instillation [17]. Then, we elucidated whether PPE might affect the CD69-deficiency-sensitive Th17 polarization. As shown in Fig. 4A, PPE increased the population of Th17 cells in the lymph nodes at 1 and 3 dpi, which was significantly higher in CD69KO mice than WT mice. These results indicate that PPE instillation can induce the CD69-deficiency-sensitive Th17 differentiation and support our present findings (Fig. 2, Fig. 3, and Table 1). On the other hand, the involvement of CD69 in IL-17-producing activity of γδ T cells has been still unknown. Then, according to the previous report [14], we evaluated IL-17 production from in vitro activated γδ T cells between the two genotypes. As shown in Fig. 4B, IL-1β plus IL-23 promoted IL-17 production in γδ T cells, which was significantly higher in CD69KO mice than WT mice. This finding suggests that CD69 negatively regulates IL-17 production from not only Th17 cells but γδ T cells.
Table 1.
Effect of PPE on activity of IL-6- and IL-17-producing cells in the lung of WT and CD69KO mice.
| WT-PBS | WT-PPE | CD69KO-PBS | CD69KO-PPE | |
|---|---|---|---|---|
| IL-6+Iba1+/Iba1+ | 17.8±1.5 | 40.3±2.7⁎ | 20.0±1.8 | 43.7±3.6⁎ |
| IL-17+CD4+/CD4+ | 10.6±3.2 | 23.7±1.8⁎ | 13.4±3.6 | 38.0±2.8⁎# |
| IL-17+TCRγδ+/ TCRγδ+ | 19.3±3.1 | 58.2±1.7⁎ | 19.4±2.3 | 68.0±3.0⁎# (%) |
Data represent mean±S.E.M. of eight independent lung samples.
P<0.05, significantly different from PBS group in each genotype by Student's t-test.
P<0.05, significantly different from WT-PPE group by Student's t-test.
Fig. 4.
IL-17-producing activity of Th17 and γδT cells from WT and CD69KO mice. A) The PPE-increased and CD69-deficinecy-sensitive Th17 differentiation. Single cell suspensions of LNCs from PPE-instilled WT and CD69KO mice at 1 and 3 dpi were stimulated with PMA+ionomycin or PBS for 3 h and subjected to surface staining for CD4 and intracellular staining for IL-17 and IFN-γ. Quantification of IL-17+CD4+IFN-γ− T cells was performed with FlowJo software. Data are shown as mean±S.E.M. (n=4–6). *P<0.05, significantly different from value of WT-PBS group (ANOVA followed by Tukey's test). B) IL-17 production in γδ T cells from the two genotypes. TCRγδ+ cells were prepared from the lymph nodes of naïve WT and CD69KO mice and stimulated with IL-1β+IL-23. The resulting supernatants were subjected to ELISA for mouse IL-17A. We confirmed that IL-17A levels in the supernatants from unstimulated γδ T cells of the two genotypes were below the detection limit. Data are shown as mean±S.E.M. (n=6). *P<0.05, significantly different from value of WT group by Student's t test.
Although a precise mechanism how CD69 controls IL-17-producing activity of γδ T cells is needed, γδ T cells are resident in the lung with a high population and recognized as primary cells producing IL-17 in autoimmune inflammatory disease [29]. Likewise, in addition to lung-resident γδ T cells, circulating γδ T cells are known to change their characteristics from an original phenotype as IFN-γ producers to potent IL-17 producers at sites of inflammation [25]. Although it has been reported that an increase of γδ T cell population in BALF or peripheral blood correlates with smokers but not COPD patients, γδ T cells have diverse subsets, and IL-17-producing γδ T cells show bidirectional effects on inflammation [30], [31]. Thus, further information concerning the relationship between IL-17-producing γδ T cells and COPD is expected.
Taken together, Th17- and γδ T cell-derived IL-17 in concert with macrophage-derived IL-6 may contribute to the enhanced emphysematous changes of CD69KO mice. Therefore, next, we examined the effect of their neutralizing antibodies on the PPE-induced emphysema.
3.4. Effect of IL-17- and IL-6-neutralization on elastase-induced pulmonary emphysema
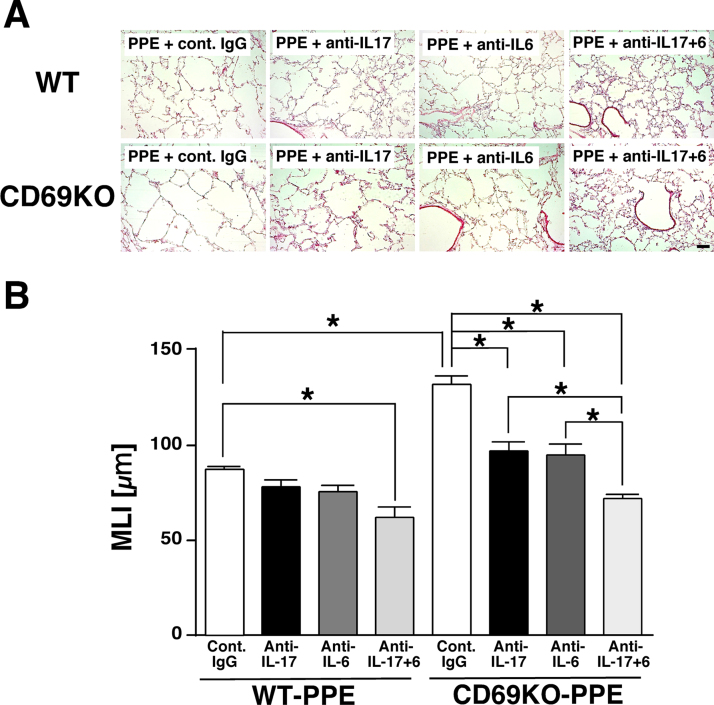
Typical histopathological changes in the lungs and MLI values were shown in Fig. 5A and B, respectively. In the lungs from WT mice, administration of IL-17-neutralizing antibody or IL-6-neutralizing antibody showed a tendency to inhibit the PPE-induced airspace enlargement at 21 dpi. However, the increase of MLI value was not significantly attenuated by each antibody. On the other hand, concomitant administration of IL-17-neutralizing antibody plus IL-6-neutralizing antibody markedly ameliorated the PPE-induced destruction of alveolar walls and significantly inhibited the increase of MLI value at 21 dpi. In the lungs from CD69KO mice, IL-17-neutralizing antibody or IL-6-neutralizing antibody significantly attenuated the PPE-increased MLI value at 21 dpi, wherefore concomitant administration of them further attenuated the PPE-induced emphysematous changes. These results indicate that the sensitivity of PPE-induced airspace enlargement to blockade of each cytokine could be higher in CD69KO mice than WT mice. Therefore, the CD69-deficiency-associated enhancement of IL-6 and IL-17 production may closely relate to the deterioration of emphysematous changes in CD69KO mice.
Fig. 5.
Effects of IL-6- and IL-17-neutralizing antibodies on elastase-induced lung emphysematous changes in WT and CD69KO mice. A) Typical histopathological changes of the lungs. Scale bar represents 100 µm. B) MLI, an index of enlargement of alveolar airspaces. Data are shown as mean±S.E.M. (n=4–6). *P<0.05, significantly different from value of WT-PBS group (ANOVA followed by Tukey's test).
IL-17 plays a crucial role in the pathogenesis of COPD mainly through recruitment of neutrophils [22]. Likewise, the degree of airflow obstruction in patients with COPD is correlated with sputum IL-17 concentrations [23]. Furthermore, the elevated IL-6 levels in the serum are recognized as one of prognostic factors in COPD [32]. Also in a mouse model of PPE-induced emphysema using IL17- and IL-6-deficient mice, each crucial role of IL-17 and IL-6 in the pathogenesis of lung emphysematous changes has been clearly demonstrated [17], [18]. However, the independent administration of each neutralizing antibody for IL-17 or IL-6 could not exert beneficial effect in the case of WT mice in which the PPE-induced emphysematous change was less severe compared with CD69KO mice (Fig. 5). Considering anti-cytokine strategy in COPD, blockade of both signals may be practical.
In an acute inflammatory model by short-term exposure of cigarette smoke (CS), we have previously demonstrated that infiltration of macrophages and neutrophils into the lung was attenuated in CD69KO mice, which is controversial to the case of this study [33]. In that model, emphysematous changes were not brought about because of short-term CS exposure, and infiltration of T lymphocytes was not observed. In particular, a cytokine array analysis showed that IL-17 was not mobilized in the BALF. Hence, the predominant involvement of IL-17-producing T cell may affect the fate of bidirectional role of CD69 in the development of pulmonary inflammation. Further study is needed for this point.
4. Conclusion
We demonstrated that CD69 plays an important role in the development of elastase-induced acute phase inflammation and subsequent emphysematous change of the lung. Genetic blockade of CD69 enhanced the elastase-induced emphysema, which was associated with both increased infiltration of inflammatory cells and upregulated levels of Th17- and γδ T cell-derived IL-17 and macrophage-derived IL-6. These results, for the first time, indicate a protective role of CD69 signaling in the development of pulmonary emphysema by PPE.
Authors' contributions
T.F., K. Tatsumi and Y.K. developed the concept and designed the experiments. T.F., K.Y., H.U., K. Tanaka, Y.N. and Y.K. performed the experiments. T.N. and M.H. gave conceptual advice. T.F. and Y.K. wrote the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research ((B), 24390137 to Y.K.) and for Challenging Exploratory Research (25670256 to Y.K.) from the Japan Society for the Promotion of Science, and by Visionary Research (to Y.K.) from the Takeda Science Foundation, Japan.
Footnotes
Transparency data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.07.010.
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.07.010.
Transparency document
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
References
- 1.Vestbo J., Hurd S.S., Agusti A.G., Jones P.W., Vogelmeier C., Anzueto A., Barnes P.J., Fabbri L.M., Martinez F.J., Nishimura M., Stockley R.A., Sin D.D., Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Hogg J.C., Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev. Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 3.Kim V., Rogers T.J., Criner G.J. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008;5:478–485. doi: 10.1513/pats.200802-014ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Cabrera M., Santis A.G., Fernandez-Ruiz E., Blacher R., Esch F., Sanchez-Mateos P., Sanchez-Madrid F. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J. Exp. Med. 1993;178:537–547. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cebrian M., Yague E., Rincon M., Lopez-Botet M., de Landazuri M.O., Sanchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J. Exp. Med. 1988;168:1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Amaro R., Cortes J.R., Sanchez-Madrid F., Martin P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol. Med. 2013;19:625–632. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizaki S., Kasuya Y., Kuroda F., Tanaka K., Tsuyusaki J., Yamauchi K., Matsunaga H., Iwamura C., Nakayama T., Tatsumi K. Role of CD69 in acute lung injury. Life Sci. 2012;90:657–665. doi: 10.1016/j.lfs.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Miki-Hosokawa T., Hasegawa A., Iwamura C., Shinoda K., Tofukuji S., Watanabe Y., Hosokawa H., Motohashi S., Hashimoto K., Shirai M., Yamashita M., Nakayama T. CD69 controls the pathogenesis of allergic airway inflammation. J. Immunol. 2009;183:8203–8215. doi: 10.4049/jimmunol.0900646. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi K., Kasuya Y., Kuroda F., Tanaka K., Tsuyusaki J., Ishizaki S., Matsunaga H., Iwamura C., Nakayama T., Tatsumi K. Attenuation of lung inflammation and fibrosis in CD69-deficient mice after intratracheal bleomycin. Respir. Res. 2011;12:131. doi: 10.1186/1465-9921-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Fuente H., Cruz-Adalia A., Del Hoyo G. Martinez, Cibrian-Vera D., Bonay P., Perez-Hernandez D., Vazquez J., Navarro P., Gutierrez-Gallego R., Ramirez-Huesca M., Martin P., Sanchez-Madrid F. The leukocyte activation receptor CD69 controls T cell differentiation through its interaction with galectin-1. Mol. Cell. Biol. 2014;34:2479–2487. doi: 10.1128/MCB.00348-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin P., Gomez M., Lamana A., Cruz-Adalia A., Ramirez-Huesca M., Ursa M.A., Yanez-Mo M., Sanchez-Madrid F. CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation. Mol. Cell. Biol. 2010;30:4877–4889. doi: 10.1128/MCB.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman C.M., Martinez F.J., Han M.K., Ames T.M., Chensue S.W., Todt J.C., Arenberg D.A., Meldrum C.A., Getty C., McCloskey L., Curtis J.L. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman C.M., Han M.K., Martinez F.J., Murray S., Liu L.X., Chensue S.W., Polak T.J., Sonstein J., Todt J.C., Ames T.M., Arenberg D.A., Meldrum C.A., Getty C., McCloskey L., Curtis J.L. Cytotoxic potential of lung CD8+ T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J. Immunol. 2010;184:6504–6513. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., Mills K.H.G. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T Cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Murata K., Inami M., Hasegawa A., Kubo S., Kimura M., Yamashita M., Hosokawa H., Nagao T., Suzuki K., Hashimoto K., Shinkai H., Koseki H., Taniguchi M., Ziegler S.F., Nakayama T. CD69-null mice protected from arthritis induced with anti-type II collagen antibodies. Int. Immunol. 2003;15:987–992. doi: 10.1093/intimm/dxg102. [DOI] [PubMed] [Google Scholar]
- 16.Couillin I., Vasseur V., Charron S., Gasse P., Tavernier M., Guillet J., Lagente V., Fick L., Jacobs M., Coelho F.R., Moser R., Ryffel B. IL-1R1/MyD88 signaling is critical for elastase-induced lung inflammation and emphysema. J. Immunol. 2009;183:8195–8202. doi: 10.4049/jimmunol.0803154. [DOI] [PubMed] [Google Scholar]
- 17.Kurimoto E., Miyahara N., Kanehiro A., Waseda K., Taniguchi A., Ikeda G., Koga H., Nishimori H., Tanimoto Y., Kataoka M., Iwakura Y., Gelfand E.W., Tanimoto M. IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir. Res. 2013;14:5. doi: 10.1186/1465-9921-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasaka S., Inoue K., Miyamoto K., Nakano Y., Kamata H., Shinoda H., Hasegawa N., Miyasho T., Satoh M., Takano H., Ishizaka A. Role of interleukin-6 in elastase-induced lung inflammatory changes in mice. Exp. Lung Res. 2010;36:362–372. doi: 10.3109/01902141003678590. [DOI] [PubMed] [Google Scholar]
- 19.Lucey E.C., Keane J., Kuang P.P., Snider G.L., Goldstein R.H. Severity of elastase-induced emphysema is decreased in tumor necrosis factor-alpha and interleukin-1beta receptor-deficient mice. Lab. Invest. 2002;82:79–85. doi: 10.1038/labinvest.3780397. [DOI] [PubMed] [Google Scholar]
- 20.Yadava K., Marsland B.J. Lymphoid follicles in chronic lung diseases. Thorax. 2013;68:597–598. doi: 10.1136/thoraxjnl-2012-203008. [DOI] [PubMed] [Google Scholar]
- 21.Hodge G., Nairn J., Holmes M., Reynolds P.N., Hodge S. Increased intracellular T helper 1 proinflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelial T cells of COPD subjects. Clin. Exp. Immunol. 2007;150:22–29. doi: 10.1111/j.1365-2249.2007.03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roos A.B., Sanden C., Mori M., Bjermer L., Stampfli M.R., Erjefalt J.S. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke-induced lymphoid neogenesis. Am. J. Respir. Crit. Care Med. 2015;191:1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 23.Maneechotesuwan K., Kasetsinsombat K., Wongkajornsilp A., Barnes P.J. Decreased indoleamine 2,3-dioxygenase activity and IL-10/IL-17A ratio in patients with COPD. Thorax. 2013;68:330–337. doi: 10.1136/thoraxjnl-2012-202127. [DOI] [PubMed] [Google Scholar]
- 24.Bettelli E., Korn T., Oukka M., Kuchroo V.K. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caccamo N., La Mendola C., Orlando V., Meraviglia S., Todaro M., Stassi G., Sireci G., Fournie J.J., Dieli F. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 26.Voss M., Wolf L., Kamyschnikow A., Wonnenberg B., Honecker A., Herr C., Lepper P.M., Wegmann M., Menger M.D., Bals R., Beisswenger C. Il-17A contributes to maintenance of pulmonary homeostasis in a murine model of cigarette smoke-induced emphysema. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L188–L195. doi: 10.1152/ajplung.00388.2014. [DOI] [PubMed] [Google Scholar]
- 27.Chen K., Pociask D.A., McAleer J.P., Chan Y.R., Alcorn J.F., Kreindler J.L., Keyser M.R., Shapiro S.D., Houghton A.M., Kolls J.K., Zheng M. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du R., Zhao H., Yan F., Li H. IL-17+Foxp3+ T cells: an intermediate differentiation stage between Th17 cells and regulatory T cells. J. Leukoc. Biol. 2014;96:39–48. doi: 10.1189/jlb.1RU0114-010RR. [DOI] [PubMed] [Google Scholar]
- 29.Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J. Exp. Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pons J., Sauleda J., Ferrer J.M., Barcelo B., Fuster A., Regueiro V., Julia M.R., Agusti A.G. Blunted gamma delta T-lymphocyte response in chronic obstructive pulmonary disease. Eur. Respir. J. 2005;25:441–446. doi: 10.1183/09031936.05.00069304. [DOI] [PubMed] [Google Scholar]
- 31.Patil R.S., Bhat S.A., Dar A.A., Chiplunkar S.V. The Jekyll and Hyde story of IL17-Producing gammadeltaT cells. Front. Immunol. 2015;6:37. doi: 10.3389/fimmu.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celli B.R., Locantore N., Yates J., Tal-Singer R., Miller B.E., Bakke P., Calverley P., Coxson H., Crim C., Edwards L.D., Lomas D.A., Duvoix A., MacNee W., Rennard S., Silverman E., Vestbo J., Wouters E., Agusti A. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012;185:1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 33.Tsuyusaki J., Kuroda F., Kasuya Y., Ishizaki S., Yamauchi K., Sugimoto H., Kono T., Iwamura C., Nakayama T., Tatsumi K. Cigarette smoke-induced pulmonary inflammation is attenuated in CD69-deficient mice. J. Recept. Signal Transduct. 2011;31:434–439. doi: 10.3109/10799893.2011.631929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material