Abstract
Objective
To determine whether granulocyte colony-stimulating factor (G-CSF), stem cell factor (SCF), or vascular endothelial growth factor (VEGF) improve the outcome of ovarian grafting.
Design
Experimental animal study.
Setting
Tertiary care hospital, animal facilities.
Animal(s)
Young adult (6- to 8-week-old) C57BL/6 female mice.
Intervention(s)
Orthotopic transplantation of the frozen-thawed ovary. Group 1 (n = 6) received VEGF (8 g/kg/day); group 2 (n = 6) received VEGF and G-CSF (50 g/kg/day), group 3 (n = 6) received G-CSF and SCF (100 g/kg/day), and group 4 (n = 5) received saline (vehicle controls). All injections were given once daily for 5 days starting the day after surgery. Ovaries were collected 2 weeks after transplantation.
Main Outcome Measure(s)
Number of nonatretic immature (primordial, primary, and small preantral) follicles.
Result(s)
Transplanted ovaries in mice injected with VEGF concurrently with G-CSF maintained a statistically significantly larger pool of primordial follicles compared with transplanted ovaries in saline-injected controls. Follicle numbers (total immature and primordial) in transplanted ovaries showed no statistically significant difference in mice injected with VEGF alone or G-CSF plus SCF compared with saline-injected controls.
Conclusion(s)
After ovarian transplantation, mice treated with VEGF and G-CSF maintain a significantly greater number of primordial follicles compared with the transplanted ovaries in control animals, suggesting that the combination of G-CSF and VEGF minimizes ischemic damage and thus improves the viability and function of the ovarian graft.
Keywords: Atresia, follicle, granulocyte colony-stimulating factor, oocyte, stem cell factor, vascular endothelial growth factor
Recent advances in cancer therapy have resulted in an increased number of long-term cancer survivors. Over the past 25 years, the 5-year relative survival rate for all cancers combined in women improved from 56% to 64% (1). High-dose chemotherapy and radiotherapy have dramatically increased long-term survival in female patients with cancers that occur during reproductive age, but major side effects of these treatments are premature ovarian failure and infertility. Because quality of life is an important issue for cancer survivors, young women with cancer are concerned about their fertility after cancer therapy. For example, a recent survey about fertility issues in young women with a history of early stage breast cancer revealed that fertility after treatment is a major concern (2). Moreover, it is challenging for many patients to live with an early onset of postmenopausal health problems, including osteoporosis, hot flashes, loss of tissue elasticity, and vascular problems. Although there are several options for preserving female fertility, none are as reliable as sperm banking in men, and all require invasive procedures and drugs.
Current clinical and experimental strategies to preserve fertility in women include embryo cryopreservation, oocyte cryopreservation for future in vitro fertilization, ovarian tissue cryopreservation for future transplantation, ovarian tissue or follicle cryopreservation for in vitro growth and maturation (3–9), ovarian transposition before radiotherapy (10, 11), hormonal protection with gonadotropin-releasing hormone (GnRH) analogs (12, 13), and pharmacologic protection with antiapoptotic agents, such as sphingosine-1-phospate (14). Ovarian tissue cryopreservation and transplantation are experimental procedures that have been introduced to preserve fertility in women whose reproductive potential is threatened (15). To date, ovarian tissue has been successfully cryopreserved and transplanted in rodents, rabbits, sheep, monkeys, and humans (16–19). Transplantation of cryopreserved ovarian tissue poses several challenges. Probably the most important is tissue ischemia. Revascularization ischemia is a major limiting factor that has been shown to negatively affect primordial follicle numbers in transplanted ovarian tissue (20). Although traditional thinking is that this reduction in follicle numbers is due to follicle degeneration (atresia), recent studies in mice indicate that oocyte-producing stem cells exist in adult ovarian tissue (21–24). Hence, the decline in follicle numbers after transplantation could also represent impaired renewal of primordial follicles in the ischemic tissue. Irrespective of the mechanism underlying this follicle depletion, identification of approaches to alleviate this problem would have considerable impact on further refinements in ovarian cryopreservation and transplantation.
In this regard, increasing interest has been directed at defining the role that various growth factors may play in improving graft survival after transplantation. Vascular endothelial growth factor (VEGF) is the focus of intensive research in many laboratories because of its role in blood vessel formation (angiogenesis and vasculogenesis) in a variety of physiologic and pathologic biological processes (25–29). In the female reproductive tract, VEGF is thought to play a crucial role in follicular growth and development, endocrine function of the corpus luteum (30, 31), ovarian hyperstimulation syndrome (32, 33), and development of endometriosis (34). Previous studies of mice with mutations at the Steel (stem cell factor or SCF) gene locus and c-kit (SCF receptor) gene locus have demonstrated that activity of this growth factor-receptor complex is critical for normal gametogenesis (35–38), and SCF is known to exert potent antiapoptotic effects in germ cells (39). Finally, granulocyte colony-stimulating factor (G-CSF) is a cytokine known for its beneficial effects on adult stem and progenitor cells, and is often used as a preconditioning agent to mobilize stem cells for autologous and allogeneic bone marrow transplantation (40). Recently, studies in mice have shown that transplantation of bone marrow-derived cells into adult female mice conditioned with cyclophosphamide and busulfan rescues ovarian function and sustains long-term fertility (41). Our study tested whether VEGF, SCF, and G-CSF, given alone or in combination after ovarian transplantation, improved ovarian graft survival as reflected by maintenance of follicle numbers.
MATERIALS AND METHODS
Animals
All studies were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic. Young adult (6- to 8-week-old) C57BL/6 female mice were purchased from Charles River Laboratories (Wilmington, MA). Animals were kept in light and temperature controlled conditions (12 hours of light; 12 hours of darkness; 22 ± 2°C) and were given chow pellets and water ad libitum.
Ovariectomies
Animals were anesthetized by intraperitoneal injection using a xylazine-ketamine mixture: 1 mL of ketamine (100 mg/mL), 0.1 mL of xylazine (100 mg/mL), and 8.9 mL of sterile water. Skin of the abdominal wall was incised in the V pattern, and the incision carried through the subcutaneous tissue, fascia, and muscles to enter the peritoneal cavity. Ovaries were then identified on both ends of the uterus, excised, and transferred to M2 medium (Sigma-Aldrich, St. Louis, MO) containing 0.25 g/L of calcium chloride CaCl2.2H2O, 0.16 g/L of magnesium sulfate (anhydrous), 0.35 g/L of potassium chloride, 0.16 g/L of monobasic potassium phosphate, 0.35 g/L of sodium bicarbonate, 5.53 g/L of sodium chloride, 4.0 g/L albumin (bovine fraction V), 1.0 g/L of D-glucose, 0.036 g/L of pyruvic acid, 0.01 g/L of 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, 0.06 g/L of potassium penicillin-G, and 0.05 g/L of streptomycin sulfate. The mice were killed by carbon dioxide inhalation followed by cervical dislocation.
Cryopreservation
After being removed from the ovarian bursa, whole ovaries were placed in M2 medium, held at room temperature for 10 minutes, and placed into cryovials (one ovary per vial) containing 200 μL of cryoprotectant (1.5 mol/L dimethyl sulfoxide [DMSO] in M2 medium supplemented with 10% fetal bovine serum). Cryovials were placed in a programmable freezer at 20°C and cooled at a rate of 2°C per minute to −6.5°C and held 10 minutes for seeding. The temperature was then decreased by 0.3°C per minute to −34°C and held several minutes, after which the cryovials were transferred directly into liquid nitrogen (−196°C) and stored for at least 96 hours until thawing and grafting. After at least 96 hours, the cryovials were thawed at room temperature, and ovaries were transferred into 200 μL of fresh M2 medium and were rehydrated for 10 minutes before grafting.
Ovarian Transplantation
Ovaries were transplanted back into the ovarian bursa (orthotopic transplantation) of female C56BL/6 mice after bilateral ovariectomy, essentially as already described. In brief, one of the thawed intact ovaries was placed in the vacated right ovarian bursa (to maintain consistency) and was secured with one stitch of 7-0 Prolene suture. The body wall was then sutured in an interrupted pattern using 6-0 Vicryl, and the skin incision was reapproximated with interrupted suture of 6-0 Vicryl. Intraperitoneal buprenorphine injections were used for postoperative analgesia.
VEGF, SCF, and G-CSF Treatments
The dosages of VEGF, SCF and G-CSF were established based on previously published data regarding use of these agents in vivo (42–44). Mice were divided into four groups. Group 1 received intraperitoneal (IP) injections of VEGF-164 (R&D Systems, Minneapolis, MN) for 5 days (8.0 μg/kg/day). Group 2 received subcutaneous (SC) injections of SCF (R&D Systems) (100 μg/kg/day) and SC G-CSF (R&D Systems) (50 μg/kg/day) for 5 days. Group 3 received IP injections of VEGF (8.0 μg/kg/day) and SC injections of G-CSF (50 μg/kg/day) for 5 days. Group 4 received SC injections of saline for 5 days (vehicle controls). Animals were killed by carbon dioxide inhalation followed by cervical dislocation 14 days after the first injection.
Follicle Counts
The number of nonatretic immature (primordial, primary and preantral) follicles per ovary was determined by serial section histomorphometry, as detailed previously elsewhere (45).
Statistical Analysis
All statistical tests were performed using R version 2.9.1 statistical software (http://cran.r-project.org). One-way analysis of variance (ANOVA) was done for each type of follicle (i.e., primordial, primary, preantral, and total), followed by multiple comparisons using Tukey’s and Dunnett’s methods for adjustment. P<.05 was considered statistically significant.
RESULTS
As anticipated, 2 weeks after grafting frozen-thawed ovaries into the ovarian bursas of recipient females injected with saline, there occurred a near 80% decline (P<.05) in primordial follicle numbers when compared with primordial follicle numbers in age-matched nontransplanted ovaries. Injection of transplanted mice with VEGF alone had no effect on the degree of primordial follicle loss in the transplanted ovaries. In contrast, a combination of VEGF and G-CSF significantly (P =.003) increased primordial and total (P = .012) follicle numbers over those detected in transplanted ovaries of saline-injected control mice. Although primordial follicle numbers in transplanted ovaries of mice injected with VEGF and G-CSF were approximately 50% lower than those of nontransplanted ovaries, this difference was not statistically significant (P = .058) (Fig. 1). A similar, but not statistically significant, beneficial effect of injecting G-CSF with SCF on the maintenance of primordial follicle numbers in transplanted ovaries was also observed (see Fig. 1). With regard to primary and preantral follicle numbers, no statistically significant effects were noted across any of the treatment groups (Fig. 2). Statistically significant differences in ovarian histology were noted between the four treatment groups. There were almost no follicles in VEGF-treated group, mostly preantral follicles in placebo-treated group, and many follicles at different developmental stages in the G-CSF–treated ovaries (Fig. 3).
FIGURE 1.
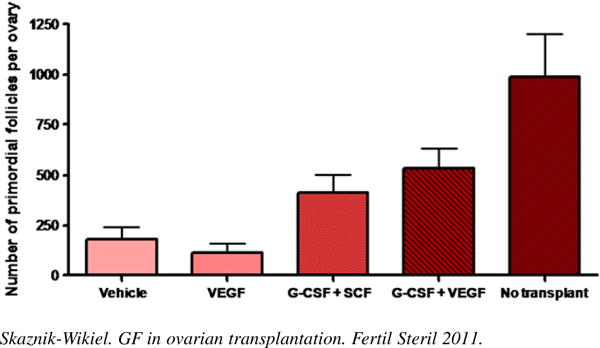
Granulocyte colony-stimulating factor (G-CSF) in conjunction with vascular endothelial growth factor (VEGF) significantly increases the number of primordial follicles in transplanted ovaries by 160% over placebo (P = .003). Primordial follicle numbers in transplanted ovaries of mice injected with VEGF and G-CSF are not statistically significantly different from those of nontransplanted ovaries. Animals per group: n = 5–6. Mean number of follicles: VEGF only: 112 ± 45; vehicle: 182 ± 60; G-CSF/SCF (stem cell factor): 411 ± 93; G-CSF/VEGF: 534 ± 95.
FIGURE 2.
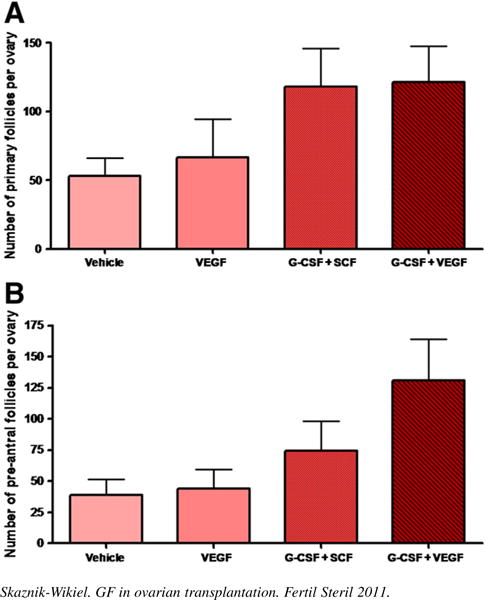
Granulocyte colony-stimulating factor (G-CSF), vascular endothelial growth factor (VEGF), and stem cell factor (SCF) did not influence the number of (A) primary or (B) preantral follicles in the transplanted ovary (not statistically significant).
FIGURE 3.
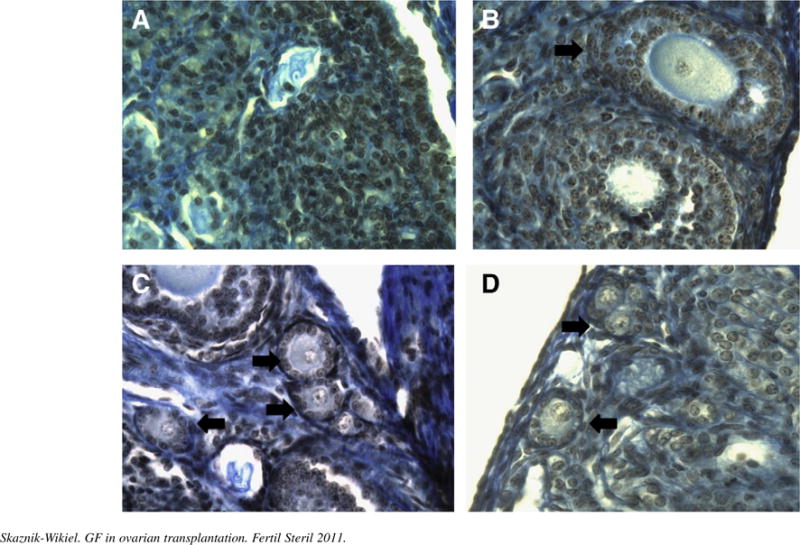
Histologic sections illustrate the appearance of a grafted frozen and thawed ovary in different treatment groups (arrows indicate resting and early growing follicles). (A) Vascular endothelial growth factor (VEGF). (B) Vehicle. (C) VEGF + granulocyte colony-stimulating factor (G-CSF). (D) G-CSF + stem cell factor (SCF). Notice the absence of primordial follicles in VEGF-only and vehicle-treated groups.
DISCUSSION
Our study has established that VEGF in conjunction with G-CSF maintains primordial follicles in transplanted mouse ovaries. This is the first study that we are aware of that has assessed the influence of growth factors on the number of follicles after ovarian transplantation.
To date, little is known about factors that orchestrate the maintenance and, possibly the renewal, of oocytes in postnatal ovaries. Numerous cytokines and growth factors including VEGF, transforming growth factor-β (TGF-β), SCF, and growth differentiation factor-9 have been implicated in oocyte maturation, follicular development, ovulation, and corpus luteum formation (30, 35–39, 46). A crucial angiogenic factor, VEGF plays an important role in cell proliferation and sex steroid–dependent angiogenesis in the ovary during the estrous cycle and pregnancy (30, 31). Matrix metalloproteinase-9 mediated stem cell factor (SCF; Kit ligand) processing is essential for cell mobilization induced by chemokines/cytokines, VEGF, placental growth factor (PlGF), and stromal cell derived factor-1 (47).
Stem cell factor was originally characterized because of its ability to influence stem cell growth and differentiation. Previous studies on mice with mutations at the Steel gene locus and c-kit (SCF receptor) locus have demonstrated deficient gametogenesis (38, 39). In situ hybridization and immunocytochemistry for c-kit with normal ovarian sections revealed high levels of expression in developing oocytes. Analysis of SCF expression revealed high levels of SCF in granulosa cells (37).
Genetic evidence and localization studies have suggested an important interaction between granulosa cells and oocytes via SCF. Stem cell factor was also found to induce the primordial to primary follicle transition (37–39). In combination with cytokines, SCF results in a synergistic enhancement of the proliferation, differentiation, and survival of various hematopoietic lineages. Its synergy with G-CSF is of particular importance in normal hematopoiesis (46). Granulocyte colony-stimulating factor is an effective mobilizer of peripheral blood progenitor cells and is used to mobilize and provide a source of stem cells for autologous and allogeneic bone marrow transplantation (40). In vivo, the combination of SCF and G-CSF has been shown to increase the mobilization of peripheral blood progenitor cells over that seen with G-CSF alone (47).
Several possible ovarian growth factors have been proposed in the past. Yang et al. (48) demonstrated that the messenger RNA (mRNA) and protein expression of VEGF isoforms associated with angiogenesis increased in rat ovarian grafts after transplantation. The VEGF-188 mRNA level increased already 2 days after transplantation, while VEGF-120 and VEGF-164 did not rise significantly until day 30 after transplantation. It is interesting that gonadotropin treatment significantly increased VEGF-188 isoform expression. These findings suggest that different isoforms of VEGF may play different role during angiogenesis (48). In our experiment, we used VEGF-164 isoform and did not achieve follicle preservation in the group treated with VEGF only. However, when combined with G-CSF, a statistically significant effect was achieved.
Similarly, Maltaris et al. (49) examined the effect of gonadotropin stimulation on the primordial follicle pool of cryopreserved human ovarian tissue after xenotransplantation in SCID mice. It is interesting that gonadotropin administration after grafting stimulated the primordial follicles to maturity but prolonged stimulation caused accelerated depletion of the ovarian follicular reserve. The findings suggest that gonadotropin administration may be beneficial during the initial neovascularization period as suggested by Yang et al. (48) but may be harmful with prolonged use (49).
The results of our study reveal that loss of primordial follicles due to transplantation is prevented by VEGF and G-CSF in mice. These findings suggest that these growth factors may play a crucial role in regulating germ cell survival in the ovary. However, only the combination of VEGF and G-CSF statistically significantly impacted the number of primordial follicles. No effect was observed with VEGF alone, and a borderline effect was noticed with combination of G-CSF and SCF.
We postulate that the increased number of the primordial follicles in the VEGF and G-CSF cotreated group was mostly related to a reduction in the incidence of ischemia-induced primordial follicle loss. Only the resting follicle pool was increased 2 weeks after treatment, but the number never exceeded the starting number before transplantation. No influence was observed on early growing follicles compared with those receiving the vehicle alone.
The main focus of this preliminary study was to assess ovarian histology in transplanted ovaries exposed to cytokines. A long-term follow-up and assessment of function of the transplanted ovaries is still needed to ascertain whether the maintenance of primordial follicle numbers in transplanted animals receiving VEGF and G-CSF is functionally important. Nevertheless, our study suggests that treatment of mice after ovarian transplantation with VEGF and G-CSF maintains primordial follicle numbers in transplanted ovaries. Our findings suggest that growth factors may play a crucial role in preventing or minimizing ischemia-induced follicle loss and possibly may enhance oogenesis. However, the exact mechanisms underlying these findings are not clearly understood and need further evaluation. Future directions should include defining the mechanism by which growth factors maintain primordial follicle numbers and an assessment of the long-term function of the transplanted ovaries, such as their hormonal function and fertility.
Footnotes
M.E.S-W. has nothing to disclose. R.K.S. has nothing to disclose. K.S. has nothing to disclose. H-J.L. has nothing to disclose. J.L.T. has nothing to disclose. T.F. has nothing to disclose.
References
- 1.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 2.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey on fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Bassil S. Indications for cryopreservation of ovarian tissue. Hum Reprod Update. 1998;4:413–20. doi: 10.1093/humupd/4.3.248. [DOI] [PubMed] [Google Scholar]
- 4.Oktay K, Newton H, Aubard Y, Salha O, Gosden RG. Cryopreservation of immature human oocytes and ovarian tissue: an emerging technology? Fertil Steril. 1998;69:1–7. doi: 10.1016/s0015-0282(97)00207-0. [DOI] [PubMed] [Google Scholar]
- 5.Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–40. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Dolmans MM, Martinez-Madrid B, Demylle D, Van Langendonckt A. The role of cryopreservation for women prior to treatment of malignancy. Curr Opin Obstet Gynecol. 2005;17:333–8. doi: 10.1097/01.gco.0000175348.72566.47. [DOI] [PubMed] [Google Scholar]
- 7.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342:1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 8.Torrents E, Boiso I, Barri PN, Veiga A. Applications of ovarian tissue transplantation in experimental biology and medicine. Hum Reprod Update. 2003;9:471–81. doi: 10.1093/humupd/dmg036. [DOI] [PubMed] [Google Scholar]
- 9.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–66. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 10.Williams RS, Mendenhall N. Laparoscopic oophoropexy for preservation of ovarian function before pelvic node irradiation. Obstet Gynecol. 1992;80:541–3. [PubMed] [Google Scholar]
- 11.Williams RS, Littell RD, Mendenhall NP. Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin’s disease. Cancer. 1999;86:2138–42. [PubMed] [Google Scholar]
- 12.Ataya KM, McKanna JA, Weintraub AM, Clark MR, LeMaire WJ. A luteinizing hormone-releasing hormone agonist for the prevention of chemotherapy-induced ovarian follicular loss in rats. Cancer Res. 1985;61:861–5. [PubMed] [Google Scholar]
- 13.Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52:365–72. doi: 10.1095/biolreprod52.2.365. [DOI] [PubMed] [Google Scholar]
- 14.Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–14. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 15.Oktay K, Buyuk E. The potential of ovarian tissue transplant to preserve fertility. Expert Opin Biol Ther. 2002;2:361–70. doi: 10.1517/14712598.2.4.361. [DOI] [PubMed] [Google Scholar]
- 16.Candy CJ, Wood MJ, Whittingham DG. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum Reprod. 1995;10:2334–8. doi: 10.1093/oxfordjournals.humrep.a136295. [DOI] [PubMed] [Google Scholar]
- 17.Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15:1300–4. doi: 10.1093/humrep/15.6.1300. [DOI] [PubMed] [Google Scholar]
- 18.Salle B, Demirci B, Franck M, Berthollet C, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril. 2002;77:403–8. doi: 10.1016/s0015-0282(01)02960-0. [DOI] [PubMed] [Google Scholar]
- 19.Almodin CG, Minguetti-Camara VC, Meister H, Ferreira JO, Franco RL, Cavalcante AA, et al. Recovery of fertility after grafting of cryopreserved germinative tissue in female rabbits following radiotherapy. Hum Reprod. 2004;19:1287–93. doi: 10.1093/humrep/deh246. [DOI] [PubMed] [Google Scholar]
- 20.Falcone T, Attaran M, Bedaiwy MD, Goldberg JM. Ovarian function preservation in the cancer patient. Fertil Steril. 2004;81:243–57. doi: 10.1016/j.fertnstert.2003.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 22.Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80:2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 24.Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–70. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 26.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32A:2413–22. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 30.Phillips HS, Hains J, Leung DW, Ferrara N. Vascular endothelial growth factor is expressed in rat corpus luteum. Endocrinology. 1990;127:965–77. doi: 10.1210/endo-127-2-965. [DOI] [PubMed] [Google Scholar]
- 31.Ravidranath N, Little-Ihring L, Phillips HS, Ferrara N, Zeleznik AJ. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology. 1992;131:254–60. doi: 10.1210/endo.131.1.1612003. [DOI] [PubMed] [Google Scholar]
- 32.McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV, Jr, et al. Vascular endothelial growth factor as a capillary permeability agent in ovarian hyperstimulation syndrome. Lancet. 1994;344:235–69. doi: 10.1016/s0140-6736(94)93001-5. [DOI] [PubMed] [Google Scholar]
- 33.Levin ER, Rosen GF, Cassidenti DL, Yee B, Meldrum D, Wisot A, et al. Role of vascular endothelial cell growth factor in ovarian hyperstimulation syndrome. J Clin Invest. 1998;102:1978–85. doi: 10.1172/JCI4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–8. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 35.Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157:100–9. doi: 10.1006/dbio.1993.1115. [DOI] [PubMed] [Google Scholar]
- 36.Manova K, Nocka K, Besmer P, Bachvarova RF. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057–69. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- 37.Manova K, Huang EJ, Angeles M, De Leon V, Sanchez S, Pronovost SM, et al. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol. 1993;157:85–99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]
- 38.Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c-kit receptor promotes oocyte growth. Dev Biol. 1994;161:194–205. doi: 10.1006/dbio.1994.1020. [DOI] [PubMed] [Google Scholar]
- 39.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–71. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- 40.Willis F, Pettengell R. Pegfilgrastim. Expert Opin Biol Ther. 2002;8:985–92. doi: 10.1517/14712598.2.8.985. [DOI] [PubMed] [Google Scholar]
- 41.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iijima K, Jiang J, Shimizu T, Miyabayashi K, Ogawa Y, Sasada H, et al. Acceleration of follicular development by administration of vascular endothelial growth factor in cycling female rats. J Reprod Dev. 2005;51:161–8. doi: 10.1262/jrd.51.161. [DOI] [PubMed] [Google Scholar]
- 43.Kanellakis P, Slater NJ, Du XJ, Bobik A, Curtis DJ. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res. 2006;70:117–25. doi: 10.1016/j.cardiores.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Deten A, Volz HC, Clamors S, Liblein S, Briest W, Marx G, et al. Hematopoietic stem cells do not repair the infarcted mouse heart. Cardiovasc Res. 2005;65:52–63. doi: 10.1016/j.cardiores.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Tilly JL. Ovarian follicle counts—not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11–2. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulich TR, Yi ES, Yin S, del Castillo J, McNiece I, Yung YP, et al. Hematologic effects of stem cell factor alone and in combination with G-CSF and GM-CSF in vivo and in vitro in rodents. Int Rev Exp Pathol. 1993;34:215–33. [PubMed] [Google Scholar]
- 47.Molineux G, Migdalska A, Szmitkowski M, Zsebo K, Dexter TM. The effects of hematopoiesis of recombinant stem cell factor (ligand for c-kit) administered in vivo in mice either alone or in combination with granulocyte colony stimulating factor. Blood. 1991;78:961–6. [PubMed] [Google Scholar]
- 48.Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90(Suppl):1550–8. doi: 10.1016/j.fertnstert.2007.08.086. [DOI] [PubMed] [Google Scholar]
- 49.Maltaris T, Beckmann MW, Mueller A, Hoffman I, Kohl J, Dittrich R. Significant loss of primordial follicles after prolonged gonadotropin stimulation in xenografts of cryopreserved human ovarian tissue in severe combined immunodeficient mice. Fertil Steril. 2007;87:195–7. doi: 10.1016/j.fertnstert.2006.05.058. [DOI] [PubMed] [Google Scholar]


