Abstract
Multimorbidity, the presence of multiple coexisting diseases or conditions, afflicts the majority of older adults, and is associated with increased mortality and healthcare utilization. In addition, multimorbidity negatively impacts quality of life and increases symptom burden. Yet, there is a dearth of evidence on how to best manage symptoms in patients with multimorbidity. Research in this area has been hampered by inconsistent definitions of multimorbidity and challenges in outcome measurement. Investigations of symptom management strategies in specific disease states, like cancer, typically exclude medically complex patients. In the absence of evidence, the American Geriatrics Society’s recommendations for the care of adults with multimorbidity provide a useful starting point for clinicians. We present a case to demonstrate how the AGS recommendations can be tailored to the situation of symptom management in patients with multimorbidity. We also present suggestions for future research directions.
Keywords: Multimorbidity, Symptom Management, Palliative care
Introduction
Though medical practice has traditionally focused on the diagnosis and treatment of individual diseases, adults are frequently burdened by more than one illness or condition, particularly as they age1. The negative effect of multiple comorbid illnesses on health is not simply additive; diseases compound each other, leading to medication-related problems2, functional impairment3, and negative impact on health-related quality of life4. The concept of multimorbidity, defined by Boyd et al as “the total burden of biological dysfunction or physiologic dysregulation,”5 captures this problem, and a growing body of literature has accumulated in the past decade to characterize the population of patients with multimorbidity.
Many studies have examined the prevalence of multimorbidity6, as well as the impact of multimorbidity on health outcomes such as hospital admission7 or death8, and on system outcomes, like healthcare utilization9. As methodology to assess prevalence has varied across studies, estimates range widely, but the Centers for Medicare and Medicaid Services reported in 2012 that about two thirds of Medicare beneficiaries have ≥2 chronic conditions10. A more recent study in the US Veterans Administration found that 29% of US veterans have ≥3 chronic conditions, while 9% have ≥5 chronic conditions9. A systematic review of studies done worldwide to describe multimorbidity prevalence found that before age 40 approximately 20% or less of primary care patients had ≥2 diseases, with an increase in multimorbidity incidence throughout middle age and a plateau at 75% among patients around 70 years of age 6.
Important efforts have been made to explore patient perspectives on living with multimorbidity11, and to examine the symptom burden experienced by patients with multimorbidity, though more research is needed12. A prospective study tracking older patients in their last years of life demonstrated that the prevalence of symptoms that restricted activity or function was higher in patients with multimorbidity than in those with zero or one disease, suggesting that multimorbidity negatively impacts not only length, but also quality of life. 13
Despite the appreciation that multimorbidity is a common problem in the aging population, and affects health-related quality of life and symptom burden, there is a dearth of evidence on how to manage symptoms in patients with multimorbidity. Among the symptom-focused studies done in patients with advanced illness and at the end of life, the most evidence exists for management of pain, and to a lesser degree, dyspnea, in cancer. There is a paucity of evidence for how to manage pain, dyspnea or depression in non-cancer illnesses, let alone in patients with combinations of diseases or conditions14. We will herein describe the challenges that hinder efforts to study symptom management in multimorbidity, and with a case, suggest ideas for approaching symptom management in patients with multimorbidity.
Challenges in multimorbidity symptom management research
The first hurdle in studying multimorbidity is to consistently define it, as there is heterogeneity in the criteria defining multimorbidity in epidemiologic research on multimorbidity to date. The most basic, working definition is the presence of ≥2 diseases, though some investigators have also evaluated for the presence of dyads or triads of diseases. The question of whether medical diagnoses should exclusively be counted in the morbidity tally, or if conditions, like sensory and functional impairments or symptoms, like chronic pain, should be included, creates more ambiguity in the definition.
Tools like the Charlson Comorbidity Index and the Cumulative Illness Rating Scale (CIRS) have been used to measure burden of multimorbidity in research studies, primarily to adjust for the impact of multimorbidity on mortality. The Functional Comorbidity Index (FCI) was developed with health-related quality of life as an outcome,15 though in a comparison study of the Charlson, the CIRS and the FCI, the CIRS explained the most variation in health-related quality of life when validated against the SF-36 questionnaire16. These indices of disease burden are all distinct from measures of acute illness severity such as the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, a clinical tool that uses physiologic markers, comorbidity and other factors to predict hospital death17.
Another reason that minimal data exist about patients with multimorbidity is that most research studies that are focused on single diseases exclude patients with significant comorbid conditions18. By definition, patients with multimorbidity are complex and diverse, but it is exactly that complexity that is unattractive in research studies, which aim to isolate the effect of one variable, or select a population with the best likelihood of response to a treatment (and lowest likelihood of harm). Comorbidity indices like the Charlson do not capture the diversity or range of symptom severity caused by the illnesses of which they are comprised, and also do not take into account mental illnesses, which can detract significantly from quality of life19. Heterogeneity in the population of multimorbidity patients also affects the generalizability of study findings.
There are many challenges inherent in measurement of symptoms in patients with multimorbidity. Tools used for rating symptoms are not validated for patients with multimorbidity. In particular, depression is difficult to identify in patients with multimorbidity, as somatic symptoms of depression can be hard to distinguish from symptoms of other chronic illness20. Another challenge of symptom management research in the context of multiple conditions relates to the dynamic nature of symptoms and the difficulty of capturing the patient’s symptom experience when assessment intervals are often infrequent, and assessments do not fully capture the multiple factors (and conditions) influencing symptoms at any one time12.
In addition to the need for inclusion of patients with multimorbidity into treatment trials, more data about how to prognosticate for patients with multiple conditions is necessary to guide treatment choices and eventually, the timing of a shift in focus to predominantly symptom-focused care21. Other important future research directions include better understanding of the relationship between symptom burden and quality of life in patients with multimorbidity, as well as how to best apply shared decision-making to the complex risk-benefit calculus in patients with multimorbidity.
Ideas for approaching symptom management in patients with multimorbidity
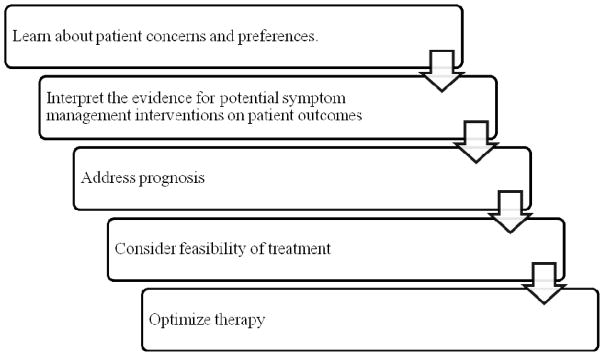
Though the evidence is sparse, and no guidelines yet exist for the care of patients with multimorbidity, the American Geriatrics Society (AGS) has put forth a general approach to the overall care of older adults with multimorbidity22 that we will draw on and supplement to suggest an approach to symptom management in these patients. A modified schema of the steps in the AGS guiding principles are presented in Figure 1. Though these are arguably important steps in any patient-centered encounter, they are nevertheless valuable considerations for patients with multimorbidity. To illustrate these ideas in action, we present the case of Barbara, a patient with multiple comorbid conditions seen in an outpatient palliative care clinic. Each step of the case represents a step from the AGS guiding principles.
Case: Barbara is a 55 year-old woman with history of Stage II ovarian cancer, diabetes and depression. She underwent surgery and has completed adjuvant treatment with carboplatin and paclitaxel, and has had no evidence of disease for two years. Her diabetes is poorly controlled, however, and she experiences persistent depressed mood despite counseling and treatment with a selective serotonin reuptake inhibitor (SSRI). She is referred to outpatient palliative care for symptom management. At her first clinic visit, she says that she is bothered most by burning, numbness and tingling in her hands and feet.
Figure 1.
Approach to choosing symptom management therapy in patients with multimorbidity, adapted from American Geriatrics Society “Guiding Principles for Care of Older Adults with Multimorbidity”22
Step one: Learn about patient concerns and preferences
In symptom management, more so than any other aspect of medicine, the patient experience is the major focus and driver of treatment choices, and patients dictate the self-administration of as-needed medications. Thus, close collaboration between patients and their healthcare providers in treatment decisions is critical. A long-term qualitative study by Morris et al. suggests that patients with multimorbidity are constantly reassessing their prioritization of conditions, as they shift between experiencing disruption by the condition and finding the ability to accommodate to the condition’s challenges23. Physicians often approach problem lists sequentially, and can be slower to appreciate the dynamic nature of the patient experience.
Prior to delving into a discussion of the possible treatment choices to address patient complaints, it is beneficial to have a sense of the patient’s values and approach to his or her own health. Fried et al demonstrated that patients felt comfortable prioritizing broad health outcomes, such as “keeping me alive” versus “maintaining independence” and “reducing pain and other symptoms,” and suggested using these questions to open conversations with patients about goals and preferences24. Inquiring about values and goals prior to presenting options can avoid overwhelming patients with extensive discussion of options that are unlikely to meet their treatment goals and allow providers to make a patient-centered recommendation.
To determine the best course of action for Barbara, her palliative care doctor asked about Barbara’s goals of treatment. Barbara felt that her pain was so bothersome that minimizing the discomfort she feels is her top priority. Pain interferes with her sleep, and she hopes that relief from pain will also allow her to get back to work. She does not mind taking medications more than once during the day if it might help, and is willing to chance side effects, like sedation, if she can get relief. She has concerns about the cost of any proposed treatment.
Step two: Interpret the evidence for potential interventions
As described above, the evidence is lacking for symptom management in general, and for patients with multimorbidity in particular. Nevertheless, the existing literature can serve as a starting point for evaluating possible therapies. In examining the evidence for a proposed treatment in a single disease or condition, an important first step is to critically evaluate the methods, specifically the inclusion/exclusion criteria, as well as study design. Randomized clinical trials are the gold standard but are often most stringent in patient selection and thus least generalizable to patients with multimorbidity. Any benefits seen should be cautiously interpreted and weighed against the risk of harm, ideally reported as changes in absolute risk so that number needed to treat and number needed to harm may be calculated22.
Barbara agrees to a trial of gabapentin for neuropathic pain, suggested by her palliative care doctor on the basis of a recent meta-analysis of gabapentin in chronic neuropathic pain that demonstrated that 43% of patients can expect at least modest benefit from gabapentin, while there was an absolute difference of 0.15 in the proportion of patients reporting adverse events from gabapentin over placebo, leading to a number need to harm (NNH) of 6.625.
If available, a very relevant metric for patients with multimorbidity is the time to benefit (or harm) from an intervention18. The most classic examples of delayed time to benefit are cancer screening, or preventive medicine like statins for elevated cholesterol or tight glucose management in diabetes. In symptom management, with the exception of medications like antidepressants that need to be increased slowly to therapeutic dose, effects of medications are usually immediate, so time to benefit is less relevant. However, in patients with limited prognosis, the calculus regarding time to harm may shift, and treatments that are less acceptable in patients with a long life expectancy, like opioids for chronic pain because of risk of dependence, may be more reasonable to consider.
Another important consideration in patients with multimorbidity is medication interactions. Not only should clinicians consider the effect of one drug on the metabolism of another, but in addition, expected side effects of one medication may make the experience of another condition worse12, such as corticosteroids used for a COPD flare potentiating underlying psychotic illness or complicating glucose control in diabetes. The lack of care coordination between the many subspecialist providers who serve patients with multimorbidity also contributes to medication complications2. The US Department of Health and Human Services has therefore identified care coordination as a top priority in a strategic framework to improve the care of patients with multimorbidity26.
Step three: Address prognosis
A patient’s ability to make informed decisions is predicated on her understanding of her medical conditions, including prognosis. For patients with cancer and comorbid conditions, survival estimates based on outcomes from treatment trials over-estimate survival benefits for the overall population of cancer patients, because of strict exclusion criteria that selects against cancer patients with poor performance status27. Though disease-specific information is a useful starting point, this strong caveat, that patients with comorbid conditions and decreased functional status live less long than patients who are fit enough for trials, should be taken into consideration in discussions of prognosis. Outside of cancer, non-disease-specific prognostic tools are largely driven by functional status28, and therefore this may be a more relevant factor in prognosis estimation. These tools are synthesized into an accessible user interface on the website eprognosis.ucsf.edu, which allows providers to choose a prognostic tool tailored to the patient’s clinical setting.
For patients with multimorbidity, the end of life can be difficult to recognize, but the Palliative Performance Scale (PPS)29, a particular functional based prognostic tool developed for use in hospice patients, may be most useful for that purpose. A study by Wharton et al found that the PPS is highly predictive of mortality in an outpatient home-based primary care practice, and therefore identified patients for whom palliative care consultation (and thus increased attention to symptom management) may be beneficial30. Another study of inpatients referred to palliative care consultation in Singapore found that change in PPS was highly predictive of mortality31.
Discussion of prognosis is always challenging, but providers with the luxury of a long relationship with a patient, like primary care doctors and outpatient primary care and palliative care doctors, can “cultivate prognostic awareness” over time by following patient cues about readiness to receive information32 Communication experts Anthony Back and Robert Arnold provided the following recommendations for oncologists, though they are useful for any provider approaching a prognosis conversation: first gauge how much patients want to know about prognosis prior to sharing the information, then acknowledge patient emotions in reaction to the news and check for understanding33. The website eprognosis.ucsf.edu also has a series of videos demonstrating how to individualize counseling about prognosis for patients.
Step four: Consider feasibility of treatment
Case: Barbara returned to clinic and reported that the burning pain in her hands and feet had improved on gabapentin, despite feeling a little sleepier at times. However, on a subsequent visit, she mentioned that sensation in her feet was diminished, and she had fallen a few times at home. She also admitted to drinking wine on occasion to deal with her stress, and shared that taking pills three times a day was difficult. The palliative care pharmacist suggested consolidating her antidepressant and neuropathic pain regimen into monotherapy with a serotonin-norepinephrine reuptake inhibitor (SNRI), which may have benefit for both depression and neuropathic pain. After discussion of the risks and benefits, Barbara agreed to taper off gabapentin and switch to an SNRI.
In addition to the potential harms of a proposed treatment, it is important to consider the burden of any proposed therapy. In this context, the concept of burden encompasses not only the financial cost, but also the inconvenience incurred by a complex treatment option. In a qualitative study, Eton and colleagues explored the experience of patients with multiple chronic conditions who were enrolled in a pharmacist-led medication therapy management program. They found that patients felt burdened by the work of self-care, particularly the time-consuming nature of adhering to medication regimens and clinic appointments, as well as remaining vigilant about their health, and staying abreast of new research and treatment options34.
In the case presented above, both the harms of treatment (sedation from gabapentin increasing risk of falls) and the feasibility of treatment (the necessity of thrice daily pill administration) prompted re-evaluation of Barbara’s neuropathic pain regimen and rotation to an alternative treatment. Though her primary objective, relief from pain, had been met by the medication, the new risks and burdens were too great to justify its continuation.
Step five: Optimize therapy
In summary, choosing the optimal therapy for a symptomatic complaint of patient with multimorbidity involves first considering patient preferences and priorities, then matching treatments suggested by the evidence with those preferences, counterbalanced against the possible harms and burdens of treatments. As results can be unpredictable, it is important to build into any treatment plan a timeline and set of parameters for assessing response to treatment, and have flexibility to try something different if the first treatment is unsuccessful by predetermined metrics. Setting realistic expectations on which providers and patients can agree prior to initiating therapy maximizes likelihood of successful treatment. In addition, clinicians should consider whether medications can be discontinued as a routine part of the medication prescribing process at every patient encounter, to minimize risk of harm from adverse effects or drug-drug interactions, or simply to test whether medication is still needed.35
Future directions
Future studies might evaluate the comparative effectiveness of symptom treatments in complex patients who might otherwise be excluded from research18,36. An idea with potential for studying patients with multimorbidity is the concept of an “N-of-1 trial,” in which treatments are randomly allocated and assessed in a single subject using objective criteria, and the patient serves as his own control37. Given that it would take an enormous amount of resources to test the benefits (and harms and feasibility) of every treatment in every complex patient, N-of-1 trials are more efficient, if performed rigorously, and can be combined into meta-analyses that offer population-level insight.
Conclusion
Multimorbidity is an incredibly common and overlooked problem in our healthcare system, and only stands to increase in relevance as patients live longer and have the opportunity to accrue a greater burden of chronic illness. At present, there is a dearth of evidence about how to manage symptoms in patients with multimorbidity. However, as suggested by the American Geriatrics Society, a comprehensive approach to patients with multimorbidity includes focusing on patient preferences, carefully interpreting the available evidence (including both the benefits and potential harms), and thinking critically about the burden of any treatment, are important first steps. Taking time to elicit patient goals and preferences, and apprise patients of their prognosis if they want to know, are especially important in symptom management discussions with patients with multimorbidity. Another key is partnering with multidisciplinary team members, including pharmacists, nurses, social workers and other ancillary providers to create a comprehensive care plan that is safe, streamlined, and meets the dynamic needs of patients with multimorbidity.
Acknowledgments
Funding sources:
Laura Petrillo: Veterans Affairs Quality Scholars Fellowship
Christine Ritchie: National Institute of Nursing Research
Agency for Healthcare Research and Quality
Commonwealth Fund
Retirement Research Foundation
California Healthcare Foundation
Tideswell at UCSF
Footnotes
Conflicts of Interest:
Christine Ritchie: Editor for UptoDate and President, American Academy of Hospice and Palliative Medicine
References
- 1.Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of Multimorbidity Among Adults Seen in Family Practice. Ann Fam Med. 2005;3(3):223–8. doi: 10.1370/afm.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roughead EE, Vitry AI, Caughey GE, Gilbert AL. Multimorbidity, care complexity and prescribing for the elderly. Aging Health. 2011;7(5):695–705. [Google Scholar]
- 3.Kadam UT, Croft PR Group NSGPC. Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam Pract. 2007;24(5):412–9. doi: 10.1093/fampra/cmm049. [DOI] [PubMed] [Google Scholar]
- 4.Fortin M, Bravo G, Hudon C, et al. Relationship Between Multimorbidity and Health-Related Quality of Life of Patients in Primary Care. Qual Life Res. 2006;15(1):83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 5.Boyd C, Ritchie C, Tipton E, Studenski S, Wieland D. From Bedside to Bench: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Comorbidity and Multiple Morbidity in Older Adults. Aging Clin Exp Res. 2008;20(3):181–8. doi: 10.1007/bf03324775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortin M, Stewart M, Poitras M-E, Almirall J, Maddocks H. A Systematic Review of Prevalence Studies on Multimorbidity: Toward a More Uniform Methodology. Ann Fam Med. 2012;10(2):142–51. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condelius A, Edberg A-K, Jakobsson U, Hallberg IR. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Arch Gerontol Geriatr. 2008;46(1):41–55. doi: 10.1016/j.archger.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.St John PD, Tyas SL, Menec V, Tate R. Multimorbidity, disability, and mortality in community-dwelling older adults. Can Fam Physician. 2014;60(5):e272–80. [PMC free article] [PubMed] [Google Scholar]
- 9.Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2015-007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lochner KA, Cox CS. Prevalence of Multiple Chronic Conditions Among Medicare Beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61. doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liddy C, Blazkho V, Mill K. Challenges of self-management when living with multiple chronic conditions: Systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchie C. Symptom burden : In need of more attention and more evidence. JAMA Intern Med. 2013;173(16):1541–2. doi: 10.1001/jamainternmed.2013.6583. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhry S, Murphy T, Gahbauer E, Sussman L, HGA, TMG Restricting symptoms in the last year of life: A prospective cohort study. JAMA Intern Med. 2013;173(16):1534–40. doi: 10.1001/jamainternmed.2013.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenz KA, Lynn J, Dy SM, et al. Evidence for Improving Palliative Care at the End of Life: A Systematic Review. Ann Intern Med. 2008;148(2):147–59. doi: 10.7326/0003-4819-148-2-200801150-00010. [DOI] [PubMed] [Google Scholar]
- 15.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Fortin M, Hudon C, Dubois M-F, Almirall J, Lapointe L, Soubhi H. Comparative assessment of three different indices of multimorbidity for studies on health-related quality of life. Health Qual Life Outcomes. 2005;3(1):1–7. doi: 10.1186/1477-7525-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 18.Ritchie CS, Zulman DM. Research Priorities in Geriatric Palliative Care: Multimorbidity. J Palliat Med. 2013;16(8):843–7. doi: 10.1089/jpm.2013.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ording AG, Sørensen HT. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol. 2013;5:199–203. doi: 10.2147/CLEP.S45305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meader N, Mitchell AJ, Chew-Graham C, et al. Case identification of depression in patients with chronic physical health problems: a diagnostic accuracy meta-analysis of 113 studies. Br J Gen Pract. 2011;61(593):e808–20. doi: 10.3399/bjgp11X613151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burge F, Lawson B, Mitchell G. How to move to a palliative approach to care for people with multimorbidity. BMJ. 2012:345. doi: 10.1136/bmj.e6324. [DOI] [PubMed] [Google Scholar]
- 22.Ickowicz E. Guiding principles for the care of older adults with multimorbidity: An approach for clinicians: American Geriatrics Society expert panel on the care of older adults with multimorbidity. J Am Geriatr Soc. 2012:60. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris RL, Sanders C, Kennedy AP, Rogers A. Shifting priorities in multimorbidity: a longitudinal qualitative study of patient’s prioritization of multiple conditions. Chronic Illn. 2011;7(2):147–61. doi: 10.1177/1742395310393365. [DOI] [PubMed] [Google Scholar]
- 24.Fried T, Tinetti M, Iannone L, O’Leary J, Towle V, Van Ness P. HEalth outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171(20):1856–8. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;(3):CD007938–CD007938. doi: 10.1002/14651858.CD007938.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parekh AK, Goodman RA, Gordon C, Koh HK Conditions THHSIW on MC. Managing Multiple Chronic Conditions: A Strategic Framework for Improving Health Outcomes and Quality of Life. Public Health Rep. 2011;126(4):460–71. doi: 10.1177/003335491112600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger JM, Barlow WE, Martin DP, et al. Comparison of Survival Outcomes Among Cancer Patients Treated In and Out of Clinical Trials. JNCI J Natl Cancer Inst. 2014;106(3):dju002. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LCY, SJL, MAS, EWW, AKS Prognostic indices for older adults: A systematic review. JAMA. 2012;307(2):182–92. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson F, Downing G, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12(1):5–11. [PubMed] [Google Scholar]
- 30.Wharton T, Manu E, Vitale CA. Enhancing Provider Knowledge and Patient Screening for Palliative Care Needs in Chronic Multimorbid Patients Receiving Home-Based Primary Care. Am J Hosp Palliat Med. 2015;32(1):78–83. doi: 10.1177/1049909113514475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan E-Y, Wu H-Y, Chan Y-H. Revisiting the Palliative Performance Scale: Change in scores during disease trajectory predicts survival. Palliat Med. 2013;27(4):367–74. doi: 10.1177/0269216312451613. [DOI] [PubMed] [Google Scholar]
- 32.Jackson Va, Jacobsen J, Greer Ja, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16:894–900. doi: 10.1089/jpm.2012.0547. [DOI] [PubMed] [Google Scholar]
- 33.Back AL, Arnold RM. Discussing prognosis: “how much do you want to know? ” talking to patients who are prepared for explicit information. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(25):4209–13. doi: 10.1200/JCO.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Eton DT, Ramalho de Oliveira D, Egginton JS, et al. Building a measurement framework of burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Relat Outcome Meas. 2012;3:39–49. doi: 10.2147/PROM.S34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bain KT, Holmes HM, Beers MH, Maio V, Handler SM, Pauker SG. Discontinuing Medications: A Novel Approach for Revising the Prescribing Stage of the Medication-Use Process. J Am Geriatr Soc. 2008;56(10):1946–52. doi: 10.1111/j.1532-5415.2008.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinetti ME, Studenski SA. Comparative Effectiveness Research and Patients with Multiple Chronic Conditions. N Engl J Med. 2011;364(26):2478–81. doi: 10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- 37.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Pers Med. 2011;8(2):161–73. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]