Abstract
Nicotine administration induces many effects on animal behavior. In wild-type Caenorhabditis elegans, gustatory plasticity results in reduced chemotaxis toward NaCl of otherwise attractive concentrations after pre-exposure to 100 mM NaCl in the absence of food. However, acute nicotine administration during a 15 min pre-exposure period inhibits gustatory plasticity, whereas chronic nicotine administration during worm development facilitates the plasticity. To investigate the relationship between the duration of nicotine administration and its effects, we exposed worms to nicotine for various periods during development. The modulatory effect of nicotine on gustatory plasticity was gradually switched from inhibition to facilitation with increased duration of nicotine administration. Moreover, inhibition of plasticity was sustained after relatively short-term chronic administration, with effects lasting for 45 h after the removal of nicotine. Similar to the acute inhibitory effect after 15 min nicotine pre-exposure, the inhibitory effect after short-term chronic administration was dependent on the nicotinic acetylcholine receptor subunit genes lev-1 and unc-29, and genes involved in serotonin biosynthesis bas-1 and tph-1. The impaired inhibition in bas-1 and tph-1mutants was recovered by exogenous serotonin, demonstrating that serotonin plays an important role in the long-lasting inhibitory effects of short-term chronic nicotine exposure.
Keywords: Ceanorhabditis elegans, Nicotine, Behavioral plasticity, Serotonin, Chemotaxis, Learning
Highlights
-
•
We analyzed gustatory plasticity of C. elegans after nicotine administration.
-
•
Nicotine modulates gustatory plasticity in various ways.
-
•
Nicotine inhibits gustatory plasticity after short-term chronic administration.
-
•
The inhibitory effect was long-lasting even after removal of nicotine.
1. Introduction
Nicotine is the main addictive and psychoactive ingredient in tobacco, and its acute administration affects many aspects of animal behavior in vertebrates including locomotor activities, grooming, feeding, attention, and mental flexibility [1], [2], [3]. Chronic nicotine administration also influences animal behaviors, although the effects on rodent learning behaviors remain controversial because improvement [4], impairment [5] and no apparent effects [6], [7] on learning have been reported. To see the effects of nicotine on learning behavior, we chose Caenorhabditis elegans as a simple model system.
Because of its simple nervous system comprised of 302 neurons and abundance of genetic informations available, C. elegans is one of the suitable model organisms to study neuroscience. C. elegans senses the environmental cues via the amphid, which is a major sensory organ that detects various volatile and water-soluble chemicals and ambient temperatures using defined chemosensory and thermosensory neurons [8], [9]. In particular, C. elegans is capable of learning depending on their previous experiences, in both associative [10], [11], [12] and non-associative manner [13], [14]. One of such associative learning is called gustatory plasticity [15], [16], [17] or salt chemotaxis learning [18], [19]. In standard experimental conditions, worms cultured with NaCl in the presence of abundant food show strong chemoattraction toward the salt. However, worms cultured with NaCl in the presence of repulsive sensory cues, such as the absence of food or presence of heavy metals, show reduced attraction toward the salt. During this behavioral alteration, worms associate low food availability (or repulsive sensory information) and the other chemicals (in this case, NaCl) within their nervous system, and modify chemotaxis behaviors accordingly.
C. elegans is also used as a model system to evaluate the effects of addictive chemicals on behavior. The effects of nicotine [20], [21], cocaine [22], and amphetamine [23] on locomotion and the effects of ethanol on gustatory plasticity [24] have been studied. In our previous studies, we examined the effects of nicotine on gustatory plasticity and revealed two types of nicotine-dependent modulatory mechanisms [25], [26]. Specifically, under an acute nicotine exposure for a 15 min conditioning period [nicotine (+) conditioning: NaCl(+)/food(−) in the presence of nicotine], gustatory plasticity was not observed. Moreover, the inhibitory effect of acute nicotine exposure was abolished in tph-1 mutant with defective serotonin biosynthesis, which showed normal plasticity even after nicotine (+) conditioning [25]. In contrast, under a chronic nicotine exposure during worm development (from egg to young adult), gustatory plasticity was enhanced, whereas cat-2 mutant worms with defective dopamine biosynthesis showed no enhancement in plasticity [26]. In the present study, we cultured worms in the presence of nicotine for various periods during worm development and examined gustatory plasticity to determine the effects of various durations of chronic nicotine exposure on the switch from acute inhibitory effects to chronic facilitatory effects. These experiments indicate the presence of the third modulatory mechanism that produces long-lasting inhibitory effects after relatively short-term chronic nicotine exposure.
2. Materials and methods
2.1. Strains and culture
The wild-type strain of C. elegans (Bristol N2) and CB211 lev‐1(e211)IV, RB2355 lev-1(ok3201)IV, CB1072 unc‐29(e1072)I, LC33 bas-1(tm351)III, MT7988 bas‐1(ad446)III, CB1112 cat‐2(e1112)II, and GR1321 tph‐1(mg280)II mutants were obtained from the Caenorhabditis Genetics Center of the University of Minnesota. A transgenic strain, pha‐1(e2123)III; rgEx387[Punc-29::unc-29::YFP+pha-1(+)] [27] were kindly provided by Dr. Luis Rene Garcia of Texas A&M University. Nematodes were cultured under standard conditions on nematode growth medium (NGM) agar plates at 20 °C [28].
Synchronously staged young adult (YA) hermaphrodites were used in all assays. To obtain synchronously staged nematodes, about 50 gravid hermaphrodites were transferred to fresh NGM plates with or without 0.3 mM nicotine and were incubated for 3 h at 25 °C to lay eggs. Subsequently, gravid nematodes were removed from culture plates and the remaining eggs were incubated at 20 °C.
2.2. Nicotine and serotonin treatments
Wild-type and mutant nematodes were cultured on NGM plates containing 0.3 mM nicotine for specific periods from larval to YA stages, and the effects of short-term chronic nicotine exposure on gustatory plasticity were investigated. We used 0.3 mM because nicotine at this concentration was most effective to modulate the gustatory plasticity in our previous study [26]. Larval stages were defined as time from hatching [29]. Larval worms were collected and washed three times with wash buffer containing 5 mM KH2PO4 (pH 6.0), 1 mM CaCl2, 1 mM MgSO4, and 0.005% Tween20 and were transferred from nicotine (+) to nicotine (−) culture plate or from nicotine (−) to nicotine (+) culture plates.
To investigate the roles of serotonin in nicotine-dependent alterations of gustatory plasticity, wild-type, bas-1, and tph-1 mutants were treated with serotonin for approximately 75 h (from hatching to the YA stage). Aliquots (10 μL) of 200 mM serotonin creatinine sulfate solution were spread onto nicotine (+) or nicotine (−) culture plates. Concentrations of serotonin were determined according to Nuttley et al. [15].
2.3. Gustatory plasticity assays
Pre-exposure to NaCl was performed using the methods described by Hukema et al. [17]. Briefly, worms were cultured on nicotine (+) or nicotine (−) plates and the well-fed young adult worms were collected into 1.5 mL tubes and washed three times in wash buffer. Worms were then conditioned by pre-exposure to wash buffer with (conditioned) or without (mock-conditioned) 100 mM NaCl. 1.5 mL tubes were leaved for 15 min at 20 °C in the absence of food. Note that in this conditioning period, nicotine was not included in the buffer. After pre-exposure, worms were washed once using wash buffer without NaCl and were collected by centrifugation at approximately 450 × g for 60 s to eliminate NaCl from body surfaces.
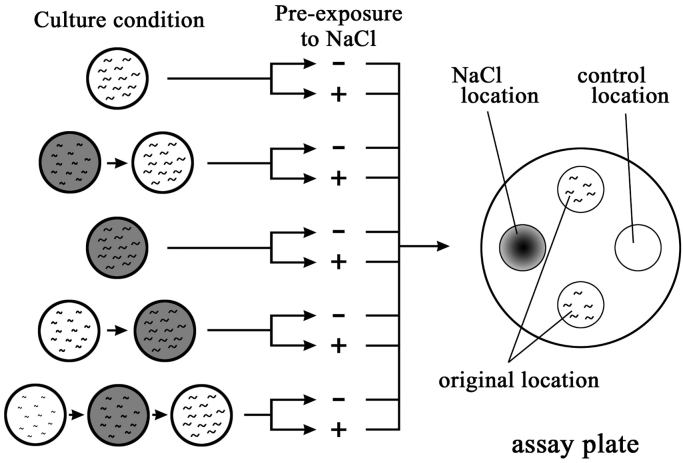
Gustatory plasticity was evaluated by chemotaxis assays toward NaCl. In these chemotaxis assays, tissue culture dishes (9 cm in diameter) containing 5 mM KH2PO4 (pH 6.0), 1 mM CaCl2, 1 mM MgSO4, and 17 g/L agar were used. To obtain a concentration gradient of NaCl, 7 μL of 100 mM NaCl solution was spotted on the surfaces of assay plates (NaCl locations are shown in Fig. 1A) at 18 h and 3 h before the start of the assay [25]. Subsequently, 1 μL aliquates of 0.5 M sodium azide were spotted onto the same locations shortly before the chemotaxis assay to anesthetize the nematodes. As a control, 1 μL of 0.5 M sodium azide was spotted onto a control location. Subsequently, approximately 30 conditioned or mock-conditioned worms were placed at original locations, which were equidistant (2.8 cm) from NaCl and control spots (Fig. 1A), and the wash buffer remaining at original location was absorbed, and worms were separated by gently touching them with a Kimwipe. Worms were allowed to move freely on the assay plate for 90 min. Numbers of worms at NaCl and control locations (circle with a 1-cm radius) were counted every 10 min using a dissecting microscope (Olympus SZ40), and chemotaxis indices were calculated according to the following equation: chemotaxis index=(number of worms at the NaCl location−number of worms at the control location)/(total number of worms on the plate). In this study, we used chemotaxis indices at 90 min because the indices were increased during initial 60 min period then became constant after 60–90 min period [25], [26]. Assays were performed during the daytime in a room maintained at approximately 20 °C, and all measurements were repeated at least three times.
Fig. 1.
Experimental procedures for nicotine administration and gustatory plasticity assays. A schematic of experimental procedures; wild-type and mutant worms were cultured in the presence (gray) or absence (white) of nicotine for various periods during larval development. Worms were then conditioned in the absence of food with (+) or without (−) 100 mM NaCl for 15 min. After brief washing, approximately 30 worms were placed at original locations on the assay plates and were allowed to move freely for 90 min to analyze chemotaxis behaviors.
2.4. UNC-29::YFP expression analysis
Transgenic strain harboring Punc‐29::UNC29::YFP reporter gene [27] was cultured in the presence of 0.3 mM nicotine for a defined period at 20 °C and observed at young adult stages. To examine the acute effect of nicotine treatment, the transgenic worms were cultured in the absence of nicotine until the young adult stage, and then treated with 3 mM nicotine in wash buffer as described in our previous study [25]. Fluorescence images were obtained using Nikon C2 conforcal laser scanning microscopy.
2.5. Statistical analysis
Differences in chemotaxis indices between mock and NaCl-conditioned nematodes and between nematodes cultured with and without nicotine were analyzed using student's t-test. Values are presented as means±standard errors of the mean, and differences were considered significant when P<0.05.
3. Results
3.1. Duration of nicotine exposure affects gustatory plasticity in Caenorhabditis elegans
As noted above, our previous studies of gustatory plasticity in C. elegans [25], [26] revealed an acute inhibitory effect of nicotine exposure for a 15 min conditioning period and a chronic facilitatory effect after 75 h nicotine exposure during worm development. These results led us to hypothesize that the effects of nicotine on gustatory plasticity switch from inhibition (acute) to facilitation (chronic) depending on the duration of nicotine exposure. Or alternatively, these effects may switch at specific developmental stages. To address this issue, we treated worms with nicotine for various times during worm development and performed chemotaxis assays after conditioning in the presence [NaCl(+)/food(−); conditioned] or absence [NaCl(−)/food(−); mock-conditioned] of NaCl (Fig. 1). As described previously[26], nicotine exposure did not affect the behavior of mock-conditioned animals.
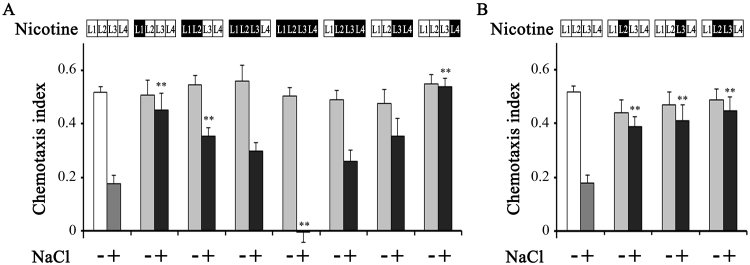
Nicotine exposure from the egg to the end of L1 stage (30 h) led to similar inhibition of gustatory plasticity to the inhibition following acute nicotine exposure. This inhibitory effect was gradually decreased with the duration of nicotine exposure and eventually switched to facilitation after chronic nicotine exposure during all developmental stages (75 h; Fig. 2). Although worm locomotion is known to be affected by high concentrations of nicotine [30], our previous studies demonstrated that 0.3 mM nicotine did not affect the worm locomotion [25], [26]. Therefore, low chemotaxis indices after chronic nicotine exposure were not due to defect in locomotion. Duration-dependent changes from inhibition to facilitation of the plasticity were not stage-specific. Worms treated with nicotine during the single larval stage always showed reduced gustatory plasticity, whereas those treated with nicotine for two to three larval stages showed only mild inhibition (Fig. 2A, B). Hence there is no specific developmental stage which is sufficient to switch the effects from inhibition to facilitation. These results suggest that the duration of exposure is the key determinant of the effects of nicotine. Interestingly, the inhibitory effects of relatively short-term chronic nicotine exposure were long-lasting. Worms cultured in the presence of nicotine at L1 (30 h, from egg to the end of L1), L2 (10 h) or L3 (10 h) larval stages exhibited very weak gustatory plasticity at 45 h, 35 h or 25 h after transfer to nicotine free culture plates (Fig. 2A, B). Thus, in further experiments we investigated the mechanisms behind this long-lasting inhibitory effect after short-term chronic nicotine exposure using mutant nematodes.
Fig. 2.
Gustatory plasticity of worms cultured in the presence of nicotine for various periods. (A) (B) Chemotaxis indices of wild-type worms toward 100 mM NaCl; worms were cultured in the presence (gray and black) or absence (white and dark gray) of nicotine for defined larval stage(s) as indicated above. Larval stages were defined as L1 (from egg to the end of L1; 30 h), L2 (10 h), L3 (10 h) and L4 (from L4 to young adult; 25 h). The presence (+) and absence (−) of NaCl during the 15 min conditioning periods is indicated below. Average chemotaxis indices are presented with standard errors of the mean (SEM); **P<0.01 (n≥12 assays).
3.2. Nicotinic acetylcholine receptor (nAchR) mediates the long-lasting inhibitory effect of nicotine
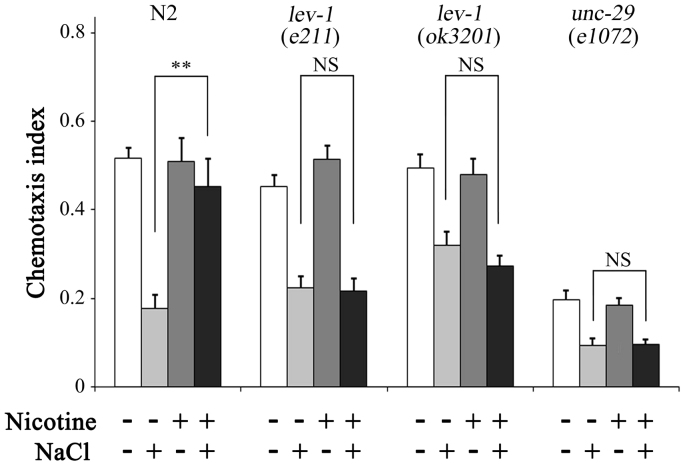
Modulatory effects of acute nicotine exposure for a 15 min conditioning period and the facilitatory effect of long-term chronic nicotine exposure were mediated by non-alpha subunits of nAchR, LEV-1, and UNC-29 [25], [26]. To see whether these nAchRs contribute to the long-lasting inhibitory effects after short-term chronic nicotine exposure or not, gustatory plasticity of lev‐1(e211), lev‐1(ok3201), and unc‐29(e1072) mutants was determined after nicotine exposure from egg to the end of L1 (L1 exposure; Fig. 3). Gustatory plasticity was not impaired in these mutants, indicating that the long -lasting modulatory effects after short-term nicotine exposure requires LEV-1 and UNC-29 nAchRs as observed after acute and long-term chronic nicotine exposures.
Fig. 3.
Gustatory plasticity of nAChR mutants cultured in the presence of nicotine during L1 stage. Long-lasting inhibitory effects of nicotine exposure during the L1 stage were examined in lev‐1(e211), lev‐1(ok3201), and unc-29 (e1072) mutants. Worms were cultured in the presence (+) or absence (−) of nicotine from the egg to the end of L1. Presence (+) or absence (−) of NaCl during 15 min conditioning periods was indicated below. Average chemotaxis indices are presented with standard errors of the mean (SEM); **P<0.01 (n≥16 assays).
3.3. Nicotine exposure affects UNC-29::YFP expression in head neurons
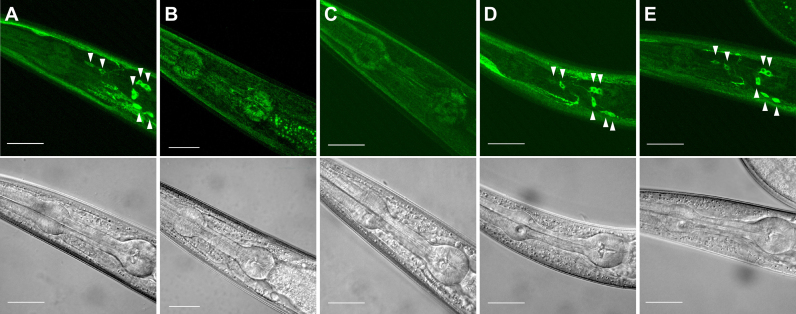
unc-29 gene is reported to be expressed in vulval muscles, body wall muscles, and a small subset of head neurons [27], [31], [32]. Since, worm chemotaxis behavior and its plasticity were regulated predominantly by head neurons, we observed the effect of nicotine exposure on the abundance of UNC-29 receptor protein in these neurons. Expression of UNC-29 protein fused with YFP at its C-terminal (UNC-29::YFP) was driven by its own promoter [27]. In the transgenic worms cultured in the absence of nicotine, detectable amount of the UNC-29::YFP fusion protein was observed in the head neurons (Fig. 4A). The abundance of the fusion protein was obviously reduced when we treated the transgenic worms with 0.3 mM nicotine for 30 h (from egg to the end of L1) or 25 h (L4 to young adult) (Fig. 4B, C). In the transgenic worms treated with nicotine for 75 h (from egg to young adult), the abundance of the fusion protein was comparable to that of untreated worms (Fig. 4D). The amount of UNC-29 receptor might recover after the sustained nicotine exposure.
Fig. 4.
Expression of UNC-29::YFP fusion protein after nicotine exposure. Transgenic worms harboring unc-29p::UNC-29::YFP were observed under conforcal laser scanning microscope. Worms cultured in the absence of nicotine (A) or in the presence of 0.3 mM nicotine for 30 h (from egg to the end of L1) (B), 25 h (from L4 to young adult) (C), 75 h (from egg to young adult) (D) were shown. A worm cultured in the absence of nicotine and exposed to 3 mM nicotine for 15 min in the presence of NaCl and in the absence of food [nicotine (+), NaCl (+), food (−) conditioning] was also shown (E). Fluorescence images (top) and bright field images (bottom) were shown. Arrow heads indicate cell body of head neurons. Scale bar 25 µm.
We also examined the effect of brief nicotine exposure during the conditioning period. Young adult transgenic worms were treated with 3 mM nicotine solution for 15 min in the presence of NaCl and in the absence of food. In these worms, the abundance of the UNC-29::YFP fusion protein was similar to that before nicotine treatment (Fig. 4E). Although we cannot conclude definitely, because we do not know whether the turnover rate of the fusion protein is the same as that of endogenous receptor, it is likely that the abundance of the receptor do not alter during the acute nicotine treatment.
3.4. Serotonin is required for long-lasting inhibitory effects of short-term chronic nicotine exposure
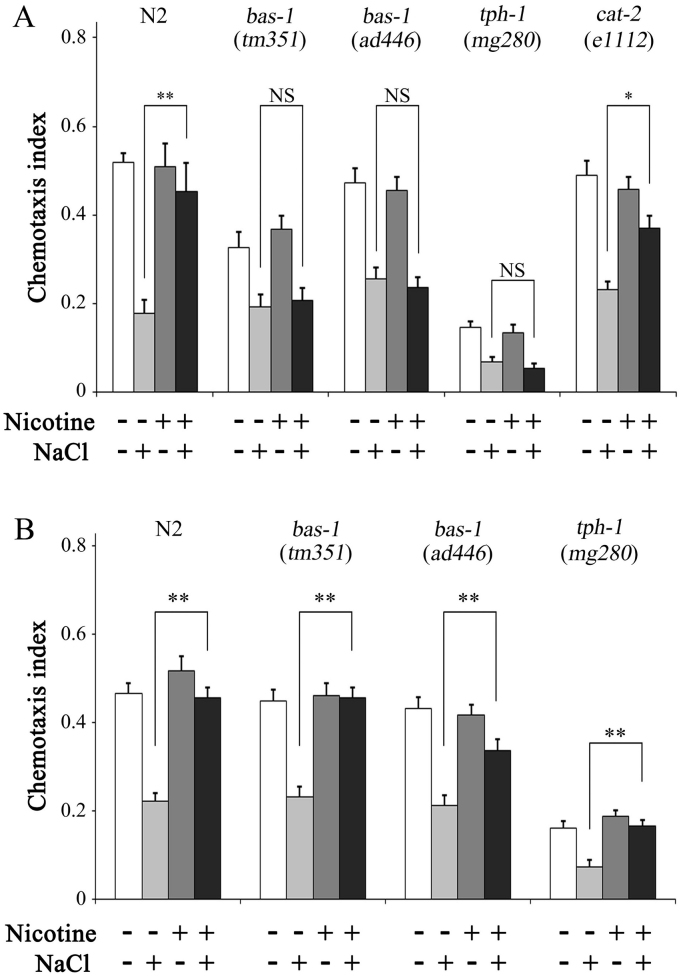
Serotonin and dopamine play important roles in various experience-dependent modulation of behaviors in C. elegans [14], [15], [33], [34], [35], [36]. Previously we showed that the inhibitory effect of acute nicotine exposure on gustatory plasticity requires serotonin [25] and that the facilitatory effect of chronic nicotine exposure requires dopamine [26]. Thus, to examine the roles of these neurotransmitters in long-lasting inhibitory effects of short-term chronic nicotine exposure, we analyzed gustatory plasticity of bas‐1(tm351), bas-1(ad446), tph-1(mg280), and cat‐2(e1112) mutants after exposure during the L1 stage. In these experiments, bas‐1(tm351) and bas‐1(ad446) mutants were defective in both serotonin and dopamine, whereas tph‐1(mg280) mutant was defective in serotonin. Gustatory plasticity was not inhibited even after nicotine exposure during the L1 stage. In contrast, cat‐2(e1112) mutant defective in dopamine showed normal inhibition under the same conditions (Fig. 5A). Furthermore, 75 h serotonin treatments of bas‐1(tm351), bas‐1(ad446), and tph‐1(mg280) mutants led to almost complete recovery of gustatory plasticity after nicotine exposure during L1 stage (Fig. 5B). Taken together, these observations indicate that serotonin but not dopamine is required for the long-lasting inhibition of gustatory plasticity after short-term chronic nicotine exposure.
Fig. 5.
Gustatory plasticity of mutant worms with defective serotonergic and/or dopaminergic neurotransmission after exposure to nicotine during the L1 stage. (A) Long-lasting inhibitory effects of nicotine exposure during the L1 stage were examined in bas‐1(tm351)(bas‐1(ad446)), tph‐1(mg280)(ad446), tph-1(mg280), and cat-2(e1112) mutants. Worms were cultured in the presence (+) or absence (−) of nicotine from the egg to the end of L1. Presence (+) or absence (−) of NaCl during 15 min conditioning periods was indicated below. (B) Long-lasting inhibitory effects of nicotine exposure during the L1 stage were observed in bas-1(tm351), bas-1(ad446), and tph-1(mg280) mutants cultured in the presence of serotonin for 75 h. Worms were cultured in the presence (+) or absence (−) of nicotine from the egg to the end of L1. Presence (+) or absence (−) of NaCl during 15 min conditioning periods was indicated below. Average chemotaxis indices are presented with standard errors of the mean (SEM); *P<0.05, **P<0.01 (n≥18 assays).
4. Discussion
4.1. Nicotine modulates worm gustatory plasticity via three mechanisms
In our previous studies, we revealed two nicotine-dependent regulatory pathways that affect gustatory plasticity in C. elegans. Specifically, acute exposure to nicotine for a 15 min conditioning period inhibited plasticity [25], whereas. chronic administration of 0.3 mM nicotine for 75 h facilitated plasticity [26]. These acute and chronic effects were subsequently shown to require serotonin and dopamine, respectively. In this study, we observed long-lasting inhibition of the plasticity after relatively short-term (10–30 h) chronic exposure to nicotine during defined larval stages, with reduced plasticity even after 45 h cultivation in the absence of nicotine. Similar to the acute inhibitory effect, this short-term effect required serotonin.
Difference in concentrations and durations of nicotine exposure preclude easy comparisons of the effects of nicotine on worm gustatory plasticity with those observed in worm egg-laying [31] and locomotion [20]. However, in all worm behaviors examined, acute and chronic nicotine administration caused distinct symptoms that were comparable to the effects of nicotine in vertebrates. In addition, the long-lasting effects of nicotine on egg-laying were observed in wild-type worms incubated overnight (16 h) with 30 mM nicotine with long-lasting resistance to stimulation of egg-laying by levamisole, even after 24 h cultivation in the absence of nicotine [31]. In this context, with the simple procedure for quantitative evaluation of the chemotaxis behavior, experimental system that we report here could be a suitable model system to study the effect of nicotine on animal behavior especially in associative learning. It should be noted that mutation in tph-1 gene is associated with abnormalities in behavior and metabolism that are coupled with the sensation and ingestion of food, and the rates of feeding and egg-laying are decreased [37]. Gustatory plasticity may also be a suitable to elucidate the relationship between nicotine and biogenic amine transmitters.
Gustatory plasticity was inhibited by acute nicotine exposure during conditioning (15 min), and short-term chronic exposure during larval development (10–30 h), raising a question whether these two types of modulation differ or not. Despite its toxicity, nicotine acts as an attractive sensory cue (taste) for C. elegans [25], and because gustatory plasticity of C. elegans is established by associations of NaCl taste and negative sensory cues such as starvation [38], apparent inhibition of plasticity might reflect masking of negative sensory information (starvation) by attractive nicotine taste. Furthermore, nicotine administration during the assays did not inhibit plasticity, supporting the idea that nicotine affects the integration of sensory information. Therefore, the inhibition of gustatory plasticity following short-term (10–30 h) chronic nicotine exposure without nicotine conditioning likely differs from that caused by acute nicotine exposure. Long-lasting modulatory effects of nicotine have been observed in egg-laying behaviors [31], with reduced UNC-29 nAchR expression in vulval muscles following prolonged nicotine exposure (16 h). In this study, we also observed the downregulation of the UNC-29::YFP fusion protein in head neurons of young adult worms experienced short-term (25–30 h) chronic nicotine exposure. While acute 15 min nicotine exposure was not sufficient to downregulate the fusion protein, suggesting direct activation of nAchR by nicotine or sensation of nicotine as an attractive taste play a role in the inhibition of the gustatory plasticity after acute nicotine exposure. Our expression analysis strongly suggests that the inhibition of gustatory plasticity after acute nicotine exposure is different from that after short-term (10–30 h) chronic nicotine exposure. However, nAchRs (lev-1, unc-29) and serotonergic signaling genes (bas-1, tph-1) played similar roles in both modes of modulation. It became important to see whether the acute inhibition of the plasticity is long-lasting or not.
4.2. Duration-dependent switch of modulatory effect by nicotine from inhibition to facilitation
Although the molecular mechanisms behind the effects of long-term administration of nicotine on animal behaviors remain unclear, C. elegans gustatory plasticity was inhibited or facilitated depending on the duration of chronic nicotine exposure. Moreover, our results indicated that serotonin and dopamine are required for inhibition and facilitation, respectively [26] [this study]. Hence, after binding the nAchR, nicotine leads to the release of these two biogenic amine transmitters from neurons. There are several possible explanations for the role of these two transmitters on the switching from inhibition to facilitation: 1) these transmitters counteract with each other and the level of gustatory plasticity was determined by balance of these two signaling pathways, or 2) these transmitters act independently from each other and the switching depending on amount or timing of release of these transmitters. Since, mutants having a defect in biosynthesis of one of these transmitters did not show opposed phenotype, we think that they act mutually independent manner. In this case, serotonin may be released following nicotine administration and the release sustained for a certain period until inhibition of gustatory plasticity is established. The inhibitory state may be lasting for a longer period even after nicotine removal. In addition, nAchR activation by nicotine may cause consecutive release of dopamine from unidentified dopaminergic neurons, which may thereby facilitate gustatory plasticity during extended periods of nicotine exposure and the effects may eventually override the inhibitory effects of serotonin. Indeed, abundance of UNC-29::YFP fusion protein was recovered after prolonged nicotine exposure. It might contribute to dopaminergic signaling during chronic nicotine exposure. However, the roles of serotonin and dopamine on gustatory plasticity remain confusing. Hukema et al. [38] showed reduced gustatory plasticity in tph-1 and bas-1 mutants with reduced serotonin levels, and in mod-5 mutant with increased serotonin level at synapses due to defects in serotonin reuptake transporter. Thus, gustatory plasticity is reduced under conditions of elevated and decreased serotonin. These authors also described similar observations for dopamine. Both reduced and elevated level of dopamine affects similarly to gustatory plasticity. C. elegans has more than 30 nAchR genes [39], 5 serotonin receptor genes, and 6 dopamine receptor genes in its genome [40]. Many of them were expressed in head neurons. Therefore, it is possible to think that neuron-specific or receptor subtype-specific effects that are differentially modulated as the function of duration of nicotine exposure contribute to the regulation of the plasticity. More detailed genetic and behavioral analyses are required to unravel the mechanisms of nicotine- and biogenic amine- dependent regulation of gustatory plasticity in C. elegans.
Acknowledgments
Nematode strains were provided by the Caenorhabditis Genetics center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Dr. Luis Rene Garcia of Texas A&M University for providing us a Punc-29::UNC-29::YFP strain. We thank Dr. Yoshio Yamamoto of Iwate University for his help in microscopic observation. This work was partially supported by a grant-in-aid for scientific research to T.M. from the Japan Society for the Promotion of Science (JP22570071).
Footnotes
Transparency data associated with this article can be found in the online version at 10.1016/j.bbrep.2016.08.008.
Appendix A. Transparency document
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Scheufele P.M., Faraday M.M., Grunberg N.E. Nicotine administration interacts with housing conditions to alter social and non-social behaviors in male and female Long-Evans rats. Nicotine Tob. Res. 2000;2:169–178. doi: 10.1080/713688133. [DOI] [PubMed] [Google Scholar]
- 2.Levin E.D., McClernon F.J., Rezvani A.H. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 3.Allison C., Shoaib M. Nicotine improves performance in an attentional set shifting task in rats. Neuropharmacology. 2013;64:314–320. doi: 10.1016/j.neuropharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 4.Levin E.D., Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology. 1996;123:88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- 5.Hambsch B., Keyworth H., Lind J., Otte D.M., Racz I., Kitchen I. Chronic nicotine improves short-term memory selectively in a G72 mouse model of schizophrenia. Br. J. Pharmacol. 2014;171:1758–1771. doi: 10.1111/bph.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicens P., Carrasco M.C., Redolat R. Effects on early training and nicotine treatment on the performance of male NMRI mice in the water maze. Neural Plast. 2003;10:303–317. doi: 10.1155/NP.2003.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach P.T., Cordero K.A., Gould T.J. The effects of acute nicotine, chronic nicotine, and withdrawal from chronic nicotine on performance of a cued appetitive response. Behav. Neurosci. 2013:303–310. doi: 10.1037/a0031913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bargmann C.I., Horvitz R.H. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 9.Bargmann C.I., Hartwieg E., Horvitz R.H. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 10.Hedgecock E.M., Russell R.L. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori I., Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 12.Kunitomo H., Sato H., Iwata R., Satoh Y., Ohno H., Yamada K. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat. Commun. 2013;4:2210. doi: 10.1038/ncomms3210. [DOI] [PubMed] [Google Scholar]
- 13.Rankin C.H., Beck C.D.O., Chiba C.M. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav. Brain Res. 1990;37:89–92. doi: 10.1016/0166-4328(90)90074-o. [DOI] [PubMed] [Google Scholar]
- 14.Kimura K.D., Fujita K., Katsura I. Enhancement of odor avoidance regulated by dopamine signaling in Caenorhabditis elegans. J. Neurosci. 2010;30:16365–16375. doi: 10.1523/JNEUROSCI.6023-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuttley W.M., Atkinson-Leadbeater K.P., Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2002;99:12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen G., Weinkove D., Plasterk R.H. a. The G-protein ?? subunit gpc-1 of the nematode C.elegans is involved in taste adaptation. EMBO J. 2002;21:986–994. doi: 10.1093/emboj/21.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hukema R.K., Rademakers S., Dekkers M.P.J., Burghoorn J., Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 2006;25:312–322. doi: 10.1038/sj.emboj.7600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeki S., Yamamoto M., Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J. Exp. Biol. 2001;1764:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- 19.Tomioka M., Adachi T., Suzuki H., Kunitomo H., Schafer W.R., Iino Y. The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron. 2006;51:613–625. doi: 10.1016/j.neuron.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Feng Z., Li W., Ward A., Piggott B.J., Larkspur E.R., Sternberg P.W. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127:621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobkowiak R., Kowalski M., Lesicki A. Concentration- and time-dependent behavioral changes in Caenorhabditis elegans after exposure to nicotine. Pharmacol. Biochem. Behav. 2011;99:365–370. doi: 10.1016/j.pbb.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Ward A., Walker V.J., Feng Z., Xu X.Z.S. Cocaine modulates locomotion behavior in C. elegans. PLoS One. 2009;4:4–8. doi: 10.1371/journal.pone.0005946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvelli L., Matthies D.S., Galli A. Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol. Pharmacol. 2010;78:151–156. doi: 10.1124/mol.109.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Tang L., Feng X., Du W., Liu B.F. Ethanol interferes with gustatory plasticity in Caenorhabditis elegans. Neurosci. Res. 2011;71:341–347. doi: 10.1016/j.neures.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura T., Miura H., Nishino A. Inhibition of gustatory plasticity due to acute nicotine exposure in the nematode Caenorhabditis elegans. Neurosci. Res. 2013;77:155–161. doi: 10.1016/j.neures.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura T., Urushihata T. Chronic nicotine exposure augments gustatory plasticity in Caenorhabditis elegans: involvement of dopamine signaling. Biosci. Biotechnol. Biochem. 2015;79:462–469. doi: 10.1080/09168451.2014.980220. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., LeBeouf B., Guo X., Correa P.A., Gualberto D.G., Lints R. A cholinergic-regulated circuit coordinates the maintenance and bi-stable states of a sensory-motor behavior during Caenorhabditis elegans male copulation. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsh D., Oppenheim D., Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- 30.Gottschalk A., Almedom R.B., Schedletzky T., Anderson S.D., Yates J.R., Schafer W.R. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J. 2005;24:2566–2578. doi: 10.1038/sj.emboj.7600741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waggoner L.E., Dickinson K. a, Poole D.S., Tabuse Y., Miwa J., Schafer W.R. Long-term nicotine adaptation in Caenorhabditis elegans involves PKC-dependent changes in nicotinic receptor abundance. J. Neurosci. 2000;20:8802–8811. doi: 10.1523/JNEUROSCI.20-23-08802.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherlekar A.L., Janssen A., Siehr M.S., Koo P.K., Caflisch L., Boggess M. The C. elegans male exercises directional control during mating through cholinergic regulation of sex-shared command interneurons. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colbert H.A., Bargmann C.I. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn. Mem. 1997;4:179–191. doi: 10.1101/lm.4.2.179. [DOI] [PubMed] [Google Scholar]
- 34.Sawin E.R., Ranganathan R., Horvitz H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 35.Chao M.Y., Komatsu H., Fukuto H.S., Dionne H.M., Hart A.C. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc. Natl. Acad. Sci. USA. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohri A., Kodama E., Kimura K.D., Koike M., Mizuno T., Mori I. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics. 2005;169:1437–1450. doi: 10.1534/genetics.104.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sze J.Y., Victor M., Loer C., Shi Y., Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- 38.Hukema R.K., Rademakers S., Jansen G. Gustatory plasticity in C. elegans involves integration of negative cues and NaCl taste mediated by serotonin, dopamine, and glutamate. Learn. Mem. 2008;15:829–836. doi: 10.1101/lm.994408. [DOI] [PubMed] [Google Scholar]
- 39.Holden-Dye L., Joyner M., O’Connor V., Walker R.J. Nicotinic acetylcholine receptors: a comparison of the nAChRs of Caenorhabditis elegans and parasitic nematodes. Parasitol. Int. 2013;62:606–615. doi: 10.1016/j.parint.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Altun Z.F. Neurotransmitter receptors in C. elegans. Wormatlas. 2011 〈http://www.wormatlas.org/NTRmainframe.htm〉 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material