Abstract
Objective
There is a need to develop novel therapies for non-small cell lung cancer (NSCLC). Photodynamic therapy has been used successfully for endobronchial palliation of NSCLC and its role in early stages of disease is being explored. We hypothesized that a novel photosensitizer, PS1, would be more effective than the standard agent, Porfimer sodium (Photofrin® or PFII), in treating human lung cancer xenografts in mice.
Materials and methods
Patient-derived NSCLC xenografts were established subcutaneously in SCID mice. Two groups of 5 mice were injected with PS1 [3-(1’-m-iodobenzyloxy)ethyl-3-devinylpyropheophorbide-a], a chlorophyll-a derivative or PFII (a purified version of hematoporphyrin derivative) then treated with non-thermal laser light. 4 mice were treated with laser light without photosensitizer and 6 mice received no treatment at all. All mice were then observed for tumor growth. The tumor growth endpoint, time-to-1000mm3, was evaluated using standard Kaplan-Meier methods and the log-rank test. Tumor H&E and Caspase3 staining was done to evaluate necrosis and apoptosis.
Results
The median time-to-1000mm3 was 12, 12, 26 and 52 days for the control, light only, PF II and PS1 groups. There was a significant association between the tumor-growth endpoint and treatment (p<0.05). H&E staining revealed <1%, 0%, 67% and 80% necrosis, and Caspase3 positivity was 2%, <1%, 17% and 39%, respectively in the same four groups.
Conclusion
The mice treated with PS1 exhibited a longer time for tumor regrowth, showed more tumor necrosis and apoptosis compared to the other treatment groups. Thus, the novel photosensitizer, PS1, was demonstrated to be more effective than Porfimer sodium in this preclinical pilot study.
Introduction
Lung cancer is the most frequent cause of cancer death in both men and women in the United States and will account for about 27% of all estimated cancer deaths in 2014(1). It kills more people than breast, prostate and colon cancer combined. Given the high incidence and mortality from lung cancer, there is a need to develop novel therapies for this disease. In photodynamic therapy (PDT), systemic administration of a light-activatable drug (i.e. photosensitizer) is followed by illumination of the target tissue with visible light resulting in tumor cell death, microvasculature damage and local inflammation (2). Photodynamic therapy (PDT) has shown great promise in treating a variety of cancers, including lung cancer (2–4). This treatment modality has remained a promising modality in thoracic oncology for quite some time. It is an established therapy for obstructive or premalignant lesions in the tracheobronchial tree (5–7). The relative simplicity of this intervention and its highly reliable tumor ablative ability, make it attractive as a therapeutic modality for broader application in lung cancer. However, the common side effect of skin photosensitivity and the relatively superficial nature of tumor necrosis produced by this modality, have contributed to the limited application of PDT in lung cancer. Newer photosensitizers with long wavelength absorptions in the near infrared region (NIR) with enhanced tumor-specificity and limited skin phototoxicity are in development to address the limitations associated with Photofrin (8). This report describes a pilot preclinical study of one such agent, PS1. On the basis of our previous study with PS1 in other tumor models (9), we hypothesized that this novel photosensitizer would be more effective than the current clinical standard, Porfimer sodium (Photofrin® or PFII), in treating human lung cancer xenografts in mice. This is due to the longer wavelength of absorption which should result in deeper tumor necrosis when the activating light is delivered on the surface of the tumor. PS1 is still in preclinical trials and has not been used in humans yet.
Materials and Methods
Patient-derived non-small cell lung cancer (NSCLC) xenografts were established subcutaneously in 20 severe combined immune deficiency (SCID) mice. These mice were part of a larger cohort established from 85 patients who underwent surgical resection of NSCLC from June 2000 to June 2010. Samples of lung tumors of various histologies and stages were immediately implanted subcutaneously in NOD SCID mice (Charles River Laboratories International, Inc.) under an IACUC approved protocol. Patients with lung tumors proven to be metastases from other primary malignancies were excluded. Of note, the histologies of the primary tumors were adenocarcinoma 46%, squamous cell carcinoma 37% and others 17%. Also, 87% of the patients had stage I or II disease while 13% had stage III or IV disease. Surgical specimens of patients' lung tumors were received shortly after resection through the Tissue Procurement Facility and cut into 2 mm × 2 mm pieces in tissue culture medium (RPMI 1640) under sterile conditions. SCID mice were then anesthetized with isoflurane and individual tumor pieces were implanted subcutaneously in the abdominal wall of three mice (1st passage) and monitored for growth. The mice used in all experiments were 7–8 weeks old CB17 SCID mice with an average weight of 18–20g. Tumor specimens that grew were surgically retrieved and subsequently passaged into recipient mice (2nd passage) and were considered to have successfully engrafted when these tumors grew. Pathological diagnosis of patient specimens and evaluation of engrafted/ passaged tumors was performed in collaboration with a staff pathologist.
There were four groups of mice - two treatment and two control groups. The mice had tumors which were all 4–6 mm in size at the beginning of the experiment and had been established from an adenocarcinoma patient. Two groups of five mice were injected intravenously with PS1 [3-(1’-m-iodobenzyloxy)ethyl-3-devinylpyropheophorbide-a], a chlorophyll-a derivative] or PFII (a purified version of hematoporphyrin derivative). The dose of PS1 was 1.5 micromole/kg based on previous dose optimization studies (9) while the dose of PFII was 6mg/kg based on the dose used clinically in humans. Twenty-four hours later, the subcutaneous tumors in these mice were treated with non-thermal laser light at a wavelength of 630nm for PFII and 665nm for PS1. These wavelengths of light were chosen based on the specific characteristics of those photosensitizers. The light source consists of tunable dye lasers (375; Spectra-Physics, Mt. View, CA) pumped by an argon-ion laser (either 171 or 2080; Spectra-Physics). The tumor-bearing mice were restrained without anesthesia in Plexiglas holders, designed to expose to light only the tumor and a 4–5 mm margin of skin. Four mice were treated with laser light with no photosensitizer (sham treatment – two at a wavelength of 630nm and two at a wavelength of 665nm) and six mice received no treatment at all. The mice were then observed for 60 days for tumor growth prior to euthanasia. The tumor volume was calculated by the following formula: tumor volume (mm3) = ½ × (long diameter) × (short diameter)2.
One representative tumor in each group was cut and stained with hematoxylin and eosin (H&E) and Caspase3 to evaluate necrosis and apoptosis, respectively. All slides were analyzed by one dedicated pathologist. Caspase3 positive cells were identified using a modified Aperio image analysis cytoplasmic algorithm. Results were reported as percent of tumor necrosis and percent of cells positive for Caspase3.
The tumor volume was modeled as a function of treatment and time (in days) using standard time-series modeling. This type of model will account for the auto-correlated nature of the data, as observations were taken serially from the same mice. Bonferroni adjusted contrast was used to compare the tumor growth rates between treatment groups. The tumor growth endpoint, time-to-1000mm3, was evaluated using standard Kaplan-Meier methods and compared between groups using the log-rank test. All analyses were conducted in SAS v9.3 (Cary, NC) at a significance level of 0.05.
Results
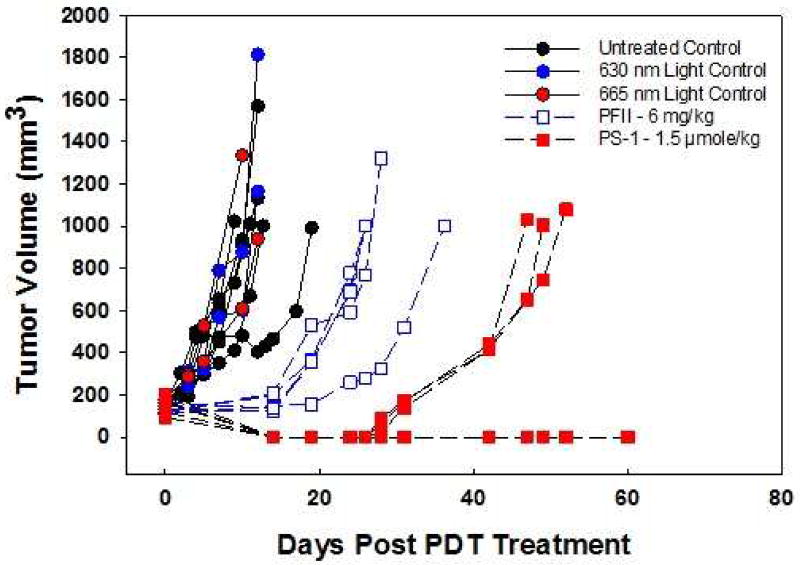
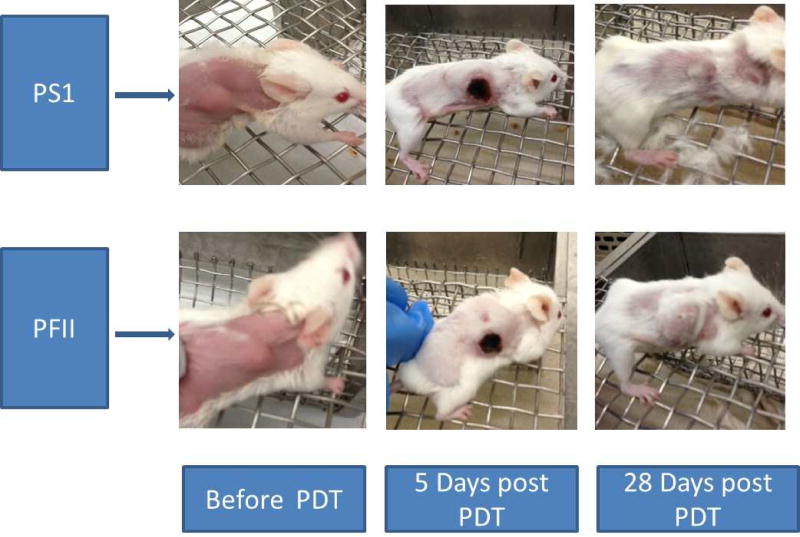
Tumor re-growth pattern in the SCID mice is illustrated in figure 1. The median time-to-1000mm3 was 12, 12, 26 and 52 days for the control, light only, PF II and PS1 groups. Pair-wise comparisons (using a Bonferroni adjustment) of tumor growth rate between the different treatment groups was performed. The PFII and PS1 groups differed from both control groups (all p<0.001). Additionally, the tumor growth rate differed between the PFII and PS1 groups (p=0.028). Figure 3 shows two mice that were photographed before, 5 days and 28 days after PDT with PS1 or PFII. The skin overlying the tumor got dark and ecchymotic, but these changes seen were transient. By the 28th day post-PDT, the tumor growth was obviously much slower in the group of mice that received PS1 as its photosensitizer.
Figure 1.
PDT efficacy of PS1 and Photofrin (PFII) on human NSCLC xenograft in SCID mice. Compared to Photofrin (5 mice), the PS1 (5 mice) showed enhanced long-term tumor control. Light alone at the long wavelength absorption of PFII (630 nm - 2 mice) or PS1 (665 nm - 2 mice) did not show any cure. Neither did the untreated controls (6 mice).
Figure 3.
SCID mice before and after PDT with PS1 (upper panel) and PFII (lower panel)
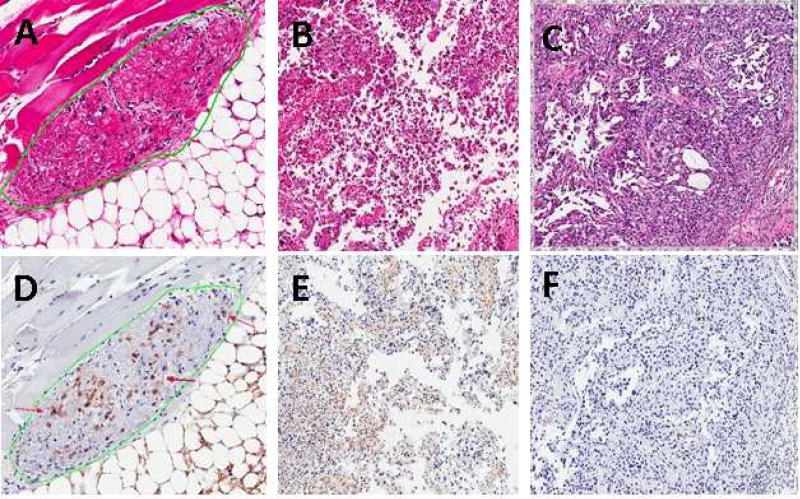
H&E analysis revealed <1%, 0%, 67% and 80% necrosis, respectively in representative samples from the control, light only, PF II and PS1 groups. Caspase3 positivity in these groups were 2%, <1%, 17% and 39% respectively. Examples of presence and absence of necrosis and apoptosis are shown in figure 2. The mice treated with PS1 exhibited a longer time for tumor regrowth, showed more tumor necrosis and apoptosis compared to the other treatment groups.
Figure 2.
High power photomicrographs of A) H&E staining showing tumor (green circle) with extensive necrosis after PDT with PS1. B) H&E staining showing tumor with necrosis after PDT with PFII. C) H&E staining showing absence of necrosis after PDT in a control tumor. D) Caspase3 staining of apoptotic cells (red arrows) after PDT with PS1. E) Caspase3 staining of apoptotic cells after PDT with PFII. F) Caspase3 staining showing absence of necrosis after PDT in a control tumor.
Discussion
Non-small Cell Lung Cancer remains a formidable public health challenge in the United States. There have been demonstrable improvements in surgery, radiation therapy and chemotherapy, which are the three main treatment modalities that are available to patients. Nevertheless, there has been limited change in lung cancer survival in decades. Molecularly targeted therapy is promising, but only a small fraction of patients have targetable mutations. Thus, the need for additional therapeutic modalities remains high. Photodynamic therapy (PDT) can be applied at various stages of lung cancer. It has been used successfully for endobronchial palliation of advanced disease (6) and investigated for the management of early lung cancer (10). The fact that PDT works well in combination with other oncologic procedures and therapies (11– 14) positions PDT as an important tool in the modern multidisciplinary approach to thoracic malignancies.
This pilot preclinical study showed that the novel photosensitizer, PS1, was more effective than Porfimer sodium in treating human lung cancer xenografts in SCID mice. Porfimer Sodium (Photofrin®) is the most commonly used photosensitizer for PDT in the United States. It is an effective drug, but suffers from several limitations. It is a complex chemical mixture of porphyrin monomers and higher oligomers. The oligomers tend to be retained in the skin for a long time causing significant skin phototoxicity. Therefore, the patients are advised to be away from sunlight for 6–8 weeks after the treatment. In contrast, PS1 has limited skin photosensitivity which would make PDT more acceptable to patients. This was demonstrated in a skin phototoxicity foot response experiment with 5–7 week old Swiss mice where PS1 resulted in much less edema than PFII after photosensitizer injection and controlled sunlight exposure 24, 48, 72 and 96 hours later [Pandey R.K. - Personal Communication].
PS1 also has deeper tissue penetration which should result in greater tumor necrosis. Every photosensitizer has its optimal light activation wavelength thus the two photosensitizers in this study were activated with non-thermal laser light at different wavelengths. As anticipated, the mice treated with PS1 exhibited a longer time for tumor regrowth, showed more tumor necrosis and apoptosis compared to the other treatment groups. The median time-to-1000mm3 was twice as long in the PS1 group relative to the PFII group (p=0.028). However, this was only a pilot trial in a small number of experimental animals and additional study of this agent is warranted. Only one representative tumor from each group was cut and stained due to limited funding. Now that a National Cancer Institute grant (1R21CA176154-01A1 has been obtained to fund this project, a larger study of 60 mice with pathologic analysis of all tumors is underway. The effectiveness of this agent in different histologic types of NSCLC also needs to be determined. If it is confirmed that PS1 and other related photosensitizers are more effective in the treatment of lung cancer, there will be several opportunities for translational studies. Clinical trials have been published exploring PDT for the treatment of diffuse malignant pleural mesothelioma (15). PDT may be effective for treatment of peripheral tumors in patients unable to tolerate surgery especially since it can be administered multiple times in contrast to radiation therapy which can typically be administered only once. The immunologic mechanism of action (16, 17) also requires further characterization.
In conclusion, a novel photosensitizer, PS1, was demonstrated to be more effective than Porfimer sodium in inducing tumor necrosis and apoptosis following photodynamic therapy of human NSCLC xenografts in SCID mice. This agent deserves further study to explore its potential translational value.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Drs. Nwogu and Pandey were responsible for the concept and design of the study and for data interpretation. The photosensitizer was synthesized in Dr. Pandey’s laboratory. P. Pera carried out the experiments in the lab and acquired the data. Dr. Bshara performed all the pathologic analysis while Dr. Attwood conducted the statistical analysis. Dr. Nwogu drafted the manuscript and all authors reviewed and approved the final version.
Author Disclosure Statement: Dr. Pandey is the Founder and Chief Scientific Officer of Photolitec, LLC, a spin-off of Roswell Park Cancer Institute Buffalo, NY 14263. The other authors have no relevant disclosures.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: An update. CA Cancer J Clin. 2011;61:250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty TJ, Cooper MT, Mang TS. Cutaneous phototoxic occurrences in patients receiving photofrin. Lasers Surg Med. 1990;10:485–8. doi: 10.1002/lsm.1900100514. [DOI] [PubMed] [Google Scholar]
- 4.Grossman CE, Pickup S, Durham A, Wileyto EP, Putt ME, Busch TM. Photodynamic therapy of disseminated non-small cell lung carcinoma in a murine model. Lasers Surg Med. 2011;43:663–75. doi: 10.1002/lsm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghissi K, Dixon K. Is bronchoscopic photodynamic therapy a therapeutic option in lung cancer? Eur Respir J. 2003;22:535–41. doi: 10.1183/09031936.03.00005203. [DOI] [PubMed] [Google Scholar]
- 6.Loewen GM, Pandey R, Bellnier D, Henderson B, Dougherty T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med. 2006;38:364–70. doi: 10.1002/lsm.20354. [DOI] [PubMed] [Google Scholar]
- 7.Usuda J, Kato H, Okunaka T, et al. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol. 2006;1:489–93. [PubMed] [Google Scholar]
- 8.Pandey RK, Goswami LN, Chen Y, et al. Nature: A rich source for developing multifunctional agents. tumor-imaging and photodynamic therapy. Lasers Surg Med. 2006;38:445–67. doi: 10.1002/lsm.20352. [DOI] [PubMed] [Google Scholar]
- 9.Pandey SK, Gryshuk AL, Sajjad M, et al. Multimodality agents for tumor imaging (PET, fluorescence) and photodynamic therapy. A possible "see and treat" approach. J Med Chem. 2005;48:6286–95. doi: 10.1021/jm050427m. [DOI] [PubMed] [Google Scholar]
- 10.Okunaka T, Kato H, Tsutsui H, Ishizumi T, Ichinose S, Kuroiwa Y. Photodynamic therapy for peripheral lung cancer. Lung Cancer. 2004;43:77–82. doi: 10.1016/j.lungcan.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Jiang F, Lilge L, Belcuig M, et al. Photodynamic therapy using photofrin in combination with buthionine sulfoximine (BSO) to treat 9L gliosarcoma in rat brain. Lasers Surg Med. 1998;23:161–6. doi: 10.1002/(sici)1096-9101(1998)23:3<161::aid-lsm5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Crescenzi E, Chiaviello A, Canti G, Reddi E, Veneziani BM, Palumbo G. Low doses of cisplatin or gemcitabine plus photofrin/photodynamic therapy: Disjointed cell cycle phase-related activity accounts for synergistic outcome in metastatic non-small cell lung cancer cells (H1299) Mol Cancer Ther. 2006;5:776–85. doi: 10.1158/1535-7163.MCT-05-0425. [DOI] [PubMed] [Google Scholar]
- 13.Nowak-Sliwinska P, Karocki A, Elas M, Pawlak A, Stochel G, Urbanska K. Verteporfin, photofrin II, and merocyanine 540 as PDT photosensitizers against melanoma cells. Biochem Biophys Res Commun. 2006;349:549–55. doi: 10.1016/j.bbrc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario A, Von Tiehl K, Wong S, Luna M, Gomer CJ. Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy-mediated tumor response. Cancer Res. 2002;62:3956–61. [PubMed] [Google Scholar]
- 15.Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2012;93:1658–67. doi: 10.1016/j.athoracsur.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belicha-Villanueva A, Riddell J, Bangia N, Gollnick SO. The effect of photodynamic therapy on tumor cell expression of major histocompatibility complex (MHC) class I and MHC class I-related molecules. Lasers Surg Med. 2012;44:60–8. doi: 10.1002/lsm.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gollnick SO. Photodynamic therapy and antitumor immunity. J Natl Compr Canc Netw. 2012;10(Suppl 2):S40–3. doi: 10.6004/jnccn.2012.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]