Abstract
Lymphocytic esophagitis (LE) is a histologic pattern with no established clinical correlates in the majority of patients. The goal of this study was to evaluate the association between non-achalasia primary esophageal motility disorders (PMED) and LE. Sixty-nine patients with PMED and esophageal biopsies, including 22 with nutcracker esophagus, 33 with ineffective motility and 14 with diffuse spasm, constituted the study group. The control group consisted of 70 patients with severe dysmotility-negative gastroesophageal reflux disease (GERD) requiring referral for Nissen fundoplication. To improve the criteria for LE, a lymphocyte reference range at different esophageal levels was first established in 17 healthy volunteers. The cutoffs for normal intraepithelial lymphocytes (IEL), defined as lymphocyte levels not exceeding mean level + 2 standard deviations, were set at 62, 46, and 41 lymphocytes per high power field at 0 to 2 cm, 5 cm, and 10 cm above the gastroesophageal junction, respectively. Predominantly focal peripapillary LE was observed in approximately 40% of patients with nutcracker esophagus or diffuse spasm and 20% of patients with ineffective motility, in comparison to 4% of patients with dysmotility-negative GERD (P < 0.035 versus any subtype of PMED). Overall, LE was strongly associated with PMED in multivariate analysis (adjusted odds ratio, 7.93; 95% CI: 2.26, 27.9; P=0.001). LE had a chronic course in 56% of the patients with follow-up biopsies. In conclusion, LE has a strong association with PMED, suggesting utility of LE in raising the possibility of PMED.
INTRODUCTION
Non-achalasia primary esophageal motility disorders (PEMD), such as nutcracker esophagus, ineffective motility, and diffuse spasm, are defined as motility patterns that differ significantly from normal variants.(1) The etiology and pathogenesis of PEMD are largely unknown and clinical features vary. Moreover, histologic correlates for these conditions have not been described, resulting in controversy regarding their significance as specific clinicopathological entities.
Lymphocytic esophagitis (LE) is a histologic pattern characterized by high number of intraepithelial lymphocytes (IEL) and rare or absent intraepithelial granulocytes.(2–5) Peripapillary localization of lymphocytes and spongiosis are also characteristic of LE, and were used as additional criteria by some authors.(2, 3) Previous studies report associations of LE with achalasia, Crohn’s disease in children, post-ablation status in Barrett esophagus, common variable immunodeficiency, lichen planus and some other dermatological disorders.(3, 6–11) However, in the majority of adult patients with LE clinical correlates have not been established.(2–4) We have recently proposed a link between LE and esophageal dismotility patterns.(5) Yet, the significance of LE for PEMD, including the prevalence of LE, the strength of the association and the natural history of LE remained unknown. The goal of this study was to investigate a putative association of LE with PEMD.
Knowledge of the normal range of esophageal IEL is essential for accurate diagnosis of LE. However, it has not been yet reported in healthy adult subjects. Therefore, the specific aims of our study were, first, to establish normal values for esophageal IEL at different levels of the esophagus and, second, to evaluate the prevalence, morphologic features and natural history of LE in patients with PEMD.
METHODS
Case Material
A total of 146 cases of diffuse esophageal spasm, nutcracker esophagus and ineffective esophageal motility diagnosed using standard manometry were identified through electronic medical records of our institution from 2007–2014. Esophageal biopsies were available in a subgroup of 69 patients, including 22 with nutcracker esophagus, 33 with ineffective motility, and 14 with diffuse spasm.
The control group was derived from 233 patients with severe GERD undergoing endoscopy and manometry before referral for Nissen fundoplication at our institution between 2007 and 2014.The control group included patients with manometry-proven absence of esophageal motility disorders and available esophageal biopsies. Individuals with histologic esophagitis arising from causes other than GERD were excluded. Of the 77 patients with dysmotility-negative GERD and esophageal biopsies, 70 qualified for the study. Seven excluded patients consisted of 2 patients with mild non-classifiable motility abnormalities, 2 with features of Candida esophagitis, 2 with features compatible with eosinophilic esophagitis and 1 with pill esophagitis. The study was approved by the Committee for the Protection of Human Subjects at Dartmouth College.
Clinical information
Clinical data was extracted from the electronic medical records of Dartmouth-Hitchcock Medical Center and included: demographics, clinical diagnosis, complaints, past medical history, endoscopic and manometric findings.
Nutcracker esophagus was defined as a mean distal esophageal amplitude of contraction > 180 mm Hg. Ineffective motility was defined as hypocontraction in the distal part of the esophagus with at least 30% of wet swallows showing any combination of the following abnormalities: distal esophagus peristaltic wave amplitude < 30 mmHg; failed peristalsis in which the peristaltic wave does not traverse the entire length of the distal esophagus; or absent peristalsis. Diffuse esophageal spasm was defined as simultaneous contractions in the distal esophagus in ≥ 20% of wet swallows, with amplitude contraction of ≥ 30 mmHg, alternating with normal peristalsis.(1)
The vast majority of patients with motility disorders received treatment with one or more of the following: proton pump inhibitors, calcium channel blockers, peppermint tea, tricyclic antidepressants, botulinum toxin injections, anticholinergics, and/or fundoplication. None of the patients received steroids or immunomodulatory drugs.
To ensure proper recording of retrospective data, a uniform electronic data abstraction form with data codes and a protocol to resolve ambiguous or conflicting data was created. The abstractors (J.P. and M.L.) were blinded to the histologic and immunophenotypic data.
Evaluation of healthy volunteers
Thirty three volunteers, asymptomatic by GERD-Q questionnaire(12) and clinical evaluation, were recruited within the hospital staff. Further inclusion criteria included the absence of endoscopic or histologic esophagitis and normal esophageal acid exposure. Twenty eight subjects completed both unsedated transnasal esophagoscopy with biopsy and 48-hour wireless Bravo pH monitoring.(13) Normal acid exposure was defined as equal or less than 5.6% of total time with pH <4. Seventeen subjects satisfied the inclusion criteria. Biopsies were performed using pediatric forceps and consisted of 2 – 4 mucosal fragments taken between 0 and 2 cm (gastroesophageal junction), 5 cm (distal esophagus) and 10 cm (mid esophagus) from the Z-line. The number of intraepithelial lymphocytes was counted in the field of view (400×) with the highest density of lymphocytes using an Olympus BX 41 microscope with a field number 22 eyepiece, resulting in a 0.237 mm2 field of view. The upper limit of normal number of lymphocytes was calculated for each esophageal level as the mean count of lymphocytes + 2 standard deviations (SD).
Histologic Evaluation
Biopsies of approximately a half (49%) of PEMD patients were taken from the mid esophagus, largely to rule out eosinophilic esophagitis. Biopsies of another half (51%) of PEMD patients were taken from the distal esophagus. In patients with dysmotility-negative GERD, biopsies were taken from the distal esophagus in 97% of the cases. The following histologic features were evaluated in the biopsies: distribution, localization and number of IEL, the presence of spongiosis (i.e. intercellular edema) in the area of IEL, and the number of intraepithelial eosinophils and neutrophils. Basal hyperplasia and elongation of stromal papillae were noted in biopsies with adequate orientation.(14) LE was defined as a lymphocyte count exceeding the upper limit of the normal range (i.e. mean + 2 SD) for a given biopsy level and absent or rare intraepithelial granulocytes, arbitrarily defined as no more than, on average, one granulocyte for every two fields of view (400×). A single lymphoid infiltrate was also considered as LE. Reflux esophagitis was diagnosed on the basis of basal hyperplasia, elongated stromal papillae and multiple intraepithelial granulocytes (arbitrarily defined as ≥ 1 granulocyte/HPF). Other types of esophagitis, such as infectious esophagitis, eosinophilic esophagitis, pill esophagitis, and radiation/chemotherapy-induced esophagitis were excluded. Two authors (J.P. and M.L.) analyzed biopsies independently, and discrepancies were resolved by consensus. As in healthy volunteers, lymphocytes were counted in the field of view (400×) in the most affected area. Increased lymphocytes were considered focal when present in <50% of the biopsy and diffuse when present in ≥50%. They were deemed peripapillary when the lymphocytic infiltrate was centered on a stromal papilla and there was an abrupt transition of the density of lymphocytes from the peripapillary area to the mainly devoid of lymphocytes interpapillary area. Lymphocytes were considered peripapillary/interpapillary when they extended into the interpapillary space as a gradient with decreasing density. Lymphocytes were considered diffuse when no relation of lymphocytes to the stromal papillae was present. Spongiosis was defined as either irregular rounded dilatation or widening of the spaces between neighboring epithelial cells. Spongiosis was considered prominent when the space between the epithelial cells was equal to or greater than the width of the epithelial cell nucleus; it was considered moderate when the intercellular space was less than the width of the epithelial cell nucleus.
CD4 and CD8 Immunohistochemistry
Routine CD4 and CD8 immunohistochemistry was performed using Bond Polymer Refine Detection staining reagents (Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, UK) and Bond III autostainer (Leica Microsystems). The following primary antibodies were used: anti-CD4 antibody at a 1:100 dilution (clone SP35, Cell Marque, Rocklin, CA, USA) and anti-CD8 BondTM ready-to-use antibody (clone 4b11, Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, UK). CD4 T-cells and CD8 T-cells were counted in the same field of view (400×) where lymphocytes were counted. A CD4:CD8 ratio > 1 indicated a predominance of CD4 T-cells; a ratio ≤ 1 indicated a predominance of CD8 T-cells.
Statistics
The distributions of baseline characteristics were compared between the groups using non-parametric Wilcoxon rank-sum tests for continuous variables and χ2 tests for categorical variables. Multivariate logistic regression models were used to estimate odds ratios and the associated 95% confidence intervals (CIs). Potential confounding covariates included: age, sex, dysphagia, reflux, presence of normal esophagus, irregular Z-line/evidence of Barret esophagus, and ulcer/stricture. Only covariates that produced a >10% change in the odds ratio were included in the fully adjusted models.(15) Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) and STATA – version 12 (StataCorp LP, College Station, TX, USA). P values are from two-sided tests.
RESULTS
Lymphocyte counts in healthy volunteers
Seventeen asymptomatic volunteers had normal endoscopic and histologic findings and normal esophageal acid exposure (Table 1). The biopsies from these volunteers had a normal distribution of IEL. The mean ± SD of lymphocyte counts were: 26 ± 18 at 0 – 2 cm (gastroesophageal junction), 24 ± 11 at 5 cm (distal esophagus) and 23 ± 9 at 10 cm (mid-esophagus). The cut-offs for high IEL (i.e. mean + 2 SD) were estimated as: 62, 46 and 41 at 0 to 2 cm, 5 cm, and 10 cm from the gastroesophageal junction, respectively. In 12 patients, CD4 and CD8 immunohistochemistry was performed on biopsies at 10 cm. CD8 T-cells outnumbered CD4 T-cells in 83% (10/12) of the subjects and the mean CD4:CD8 ratio was 0.56 ± 0.43, indicating that esophageal IEL are CD8-predominant in healthy individuals.
Table 1.
Demographics, pH-metry data and intraepithelial lymphocyte counts in healthy volunteers.
| Volunteer | Sexa | Age | BMI | Total % time pH <4 |
Intraepithelial lymphocytes |
CD4:CD8 Ratio |
||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| (years) | (kg/m2) | GEJ −2 cm | 5 cm | 10 cm | ||||
| 1 | F | 37 | 19.8 | 0.5 | n.d. | 32 | 22 | 0.74 |
| 2 | M | 26 | 33.7 | 1.7 | n.d. | 20 | 21 | 0.69 |
| 3 | F | 30 | 17.9 | 0.1 | n.d. | 25 | 26 | 0.25 |
| 4 | F | 42 | 20.4 | 0.1 | 12 | 32 | 23 | 0.5 |
| 5 | F | 51 | 28.3 | 4.1 | 18 | 26 | 16 | 1.58 |
| 6 | M | 23 | 18.7 | 4.4 | 46 | 21 | 27 | 0.31 |
| 7 | M | 46 | 24.4 | 3.3 | 58 | 57 | 45 | 0.25 |
| 8 | M | 31 | 27 | 2.6 | 14 | 14 | 25 | 0.21 |
| 9 | M | 32 | 26.4 | 0.6 | n.d. | 16 | 34 | 1.1 |
| 10 | M | 41 | 29.9 | 2.7 | n.d. | 20 | 13 | 0.11 |
| 11 | M | 35 | 25.1 | 4.2 | 18 | 20 | 21 | 0.27 |
| 12 | F | 26 | 22.6 | 0 | 12 | 9 | 11 | 0.71 |
| 13 | F | 52 | 27.2 | 2.3 | 20 | 19 | 19 | n.d. |
| 14 | F | 26 | 23.2 | 1 | 22 | 16 | 15 | n.d. |
| 15 | F | 25 | 37.1 | 4.6 | 16 | 34 | n.d. | n.d. |
| 16 | F | 32 | 37.4 | 5.6 | 60 | 20 | 20 | n.d. |
| 17 | F | 25 | 32.4 | 5.5 | 16 | 20 | 32 | n.d. |
| Total | M:F=1:1.4 | 34 ± 9 | 26.6 ± 6.0 | 2.5 ± 2.0 | 26 ± 18 | 24 ± 11 | 23 ± 9 | 0.56 ± 0.43 |
, F - female, M - male.
Abbreviations: BMI, body mass index; GEJ; gastroesophageal junction; n.d., no data.
Clinico-endoscopic features in patients with PEMD and dysmotility –negative GERD
Table 2 presents demographic, clinical and endoscopic characteristics of the patients. Patients with PEMD were more likely than the controls with dysmotility-negative GERD to experience dysphagia, 51% (35/69) versus 11% (8/70), and present with a normal esophagus during endoscopy, 45% (31/69) versus 21% (15/70). However, PEMD patients were less likely than GERD controls to report reflux/heartburn symptoms, 35% (24/69) versus 56% (39/70), and have endoscopic evidence of an irregular Z-line/possible Barrett esophagus, 26 % (18/69) versus 46% (32/70), or ulcer/stricture, 0 versus 8% (6/70). Case and control patients were similar with regard to age, sex, prevalence of chest/epigastric pain, and endoscopic evidence of rings/furrows, esophagitis, and hiatal hernia/Schatzki ring. Overall, the data demonstrate significant clinico-endoscopic differences between patients with PEMD and dysmotility-negative GERD.
Table 2.
Demographic and endoscopic characteristics of patients with non-achalasia primary motility disorders and dysmotility-negative gastroesophageal reflux disease.
| Nutcracker esophagus, n=22, (%) |
Ineffective motility, n=33 (%) |
Diffuse spasm, n=14, (%) |
All PEMD, n=69, (%) |
DN-GERD, n=70, (%) |
P-valuea | |
|---|---|---|---|---|---|---|
| Age (years) | 54 ± 11 | 56 ± 14 | 62 ± 11 | 56 ± 12 | 56 ± 10 | 1.0 |
| M:F ratio | 1 : 1 | 1 : 1.5 | 1 : 1.3 | 1 : 1.3 | 1 : 1.7 | .49 |
| Main symptom | ||||||
| Dysphagia | 10 (45) | 14 (42) | 11 (79) | 35 (51) | 8 (11) | < .0001 |
| Reflux/heartburn | 7 (32) | 15 (45) | 2 (14) | 24 (35) | 39 (56) | .017 |
| Chest/epigastric pain | 4 (18) | 1 (3) | 0 (0) | 5 (7) | 8 (11) | .57 |
| Other (regurgitation/nausea/cough) | 1 (5) | 3 (10) | 1 (7) | 5 (7) | 15 (22) | .028 |
| Endoscopyb | ||||||
| Normal esophagus | 9 (41) | 13 (39) | 9 (64) | 31 (45) | 15(21) | .004 |
| Rings and/or furrows | 5 (26) | 2 (6) | 1 (7) | 8 (12) | 3 (4) | .13 |
| Esophagitis | 4 (18) | 5 (15) | 1 (7) | 11 (16) | 16 (23) | .40 |
| Hiatal hernia and/or Schatzki ring | 1 (5) | 6 (18) | 1 (7) | 8 (12) | 9 (13) | 1.0 |
| Irregular Z-line and/or possible BE | 7 (32) | 10 (30) | 1 (7) | 18 (26) | 32 (46) | .021 |
| Ulcer or stricture | 0 | 0 | 0 | 0 | 6 (8) | .028 |
, all PEMD versus DN-GERD
, multiple abnormalities may be seen in one patient
Abbreviations: BE, Barrett esophagus; DN-GERD, dysmotility-negative gastroesophageal reflux disease; PEMD, non-achalasia primary motility disorders.
LE shows strong association with PEMD
LE was observed in 45% (10/22), 21% (7/33) and 36% (5/14) of patients with nutcracker esophagus, ineffective motility and diffuse esophageal spasm, respectively (Fig. 1). The rest of the patients with high IEL had a pattern of reflux esophagitis: 9% (2/22) with nutcracker esophagus, 18% (6/33) with ineffective motility, and 14% (2/14) with diffuse spasm (Table 3). In contrast, LE was seen only in 6% (4/70) of patients with dysmotility–negative GERD (P < 0.035, versus any type of the PMED) (Table 3). LE was strongly associated with PEMD (odds ratio, 7.72, 95% CI: 2.50, 23.9; Table 4). The association remained significant upon further adjustment for age, sex, dysphagia, reflux, and presence of normal esophagus (adjusted odds ratio, 7.93, 95% CI: 2.26, 27.9). The associations between LE and specific PEMD (nutcracker esophagus, ineffective motility, or diffuse spasm) were also positive, albeit with wide confidence intervals due to the small sample sizes, and the association was strongest for the subset of patients with nutcracker esophagus (Table 4).
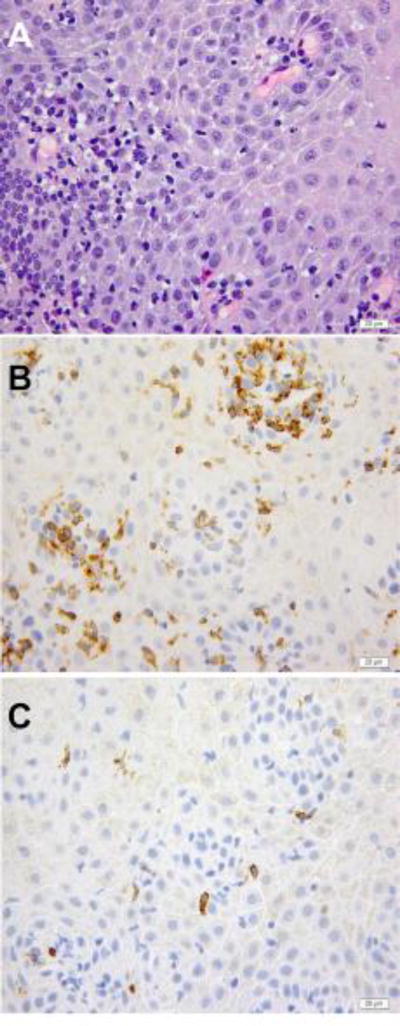
Figure 1.
A – C, Lymphocytic esophagitis in a biopsy from a patient with nutcracker esophagus. A, hematoxylin and eosine stain. B, immunohistochemistry demonstrating CD4 T-cells. C, immunohistochemistry demonstrating CD8 T-cells.
Table 3.
Prevalence of patterns of inflammation with increased intraepithelial lymphocytes in non-achalasia primary motility disorders and dysmotiltiy-negative gastroesophageal reflux disease.
| Nutcracker esophagus, n=22, (%) |
Ineffective motility, n=33 (%) |
Diffuse spasm, n=14 (%) |
All PEMD n=69, (%) |
DN-GERD, n=70 (%) |
P-valuea | |
|---|---|---|---|---|---|---|
| All cases with increased IEL | 12 (55) | 13 (39) | 7 (50) | 32 (46) | 7 (10) | <.001 |
| LE pattern | 10 (45) | 7 (21) | 5 (36) | 22 (32) | 4 (6) | <.0001 |
| RE + increased IEL pattern | 2 (9) | 6 (18) | 2 (14) | 10 (14) | 3 (4) | <.045 |
, all PEMD vs. DN-GERD
Abbreviations: IEL, intraepithelial lymphocytes; LE, lymphocytic esophagitis; PEMD, non-achalasia primary motility disorders; DN-GERD, dysmotiltiy-negative gastroesophageal reflux disease; RE, reflux esophagitis.
Table 4.
Associations of primary esophageal motility disorders with lymphocytic esophagitis.
| No. of Patients | Unadjusted Odds Ratio (95% CI) relative to patients with DN-GERD |
P-value | Adjusted Odds Ratio (95% CI)a |
P-value | |
|---|---|---|---|---|---|
| Nutcracker Esophagus | 22 | 13.7 (3.70, 51.1) | <0.001 | 24.6 (4.44, 136.4) | <.001 |
| Ineffective Motility | 33 | 4.44 (1.20, 16.5) | 0.026 | 3.63 (0.86, 15.3) | .08 |
| Diffuse Spasm | 14 | 9.17 (2.07, 40.6) | 0.004 | 4.82 (0.66, 35.3) | .12 |
| All PEMD | 69 | 7.72 (2.50, 23.9) | <0.001 | 7.08 (1.96, 25.6) | .003 |
Odds ratios were adjusted for age, sex, dysphagia, reflux, presence of normal esophagus, irregular Z-line/evidence of Barrett esophagus, and ulcer/stricture.
Abbreviations: CI, confidence intervals; DN-GERD, dysmotility-negative gastroesophageal reflux disease; PEMD, non-achalasia primary motility disorders.
Morphologic and immunophenotypic features of LE
LE was focal in 82% (18/22) and peripapillary in 68% (15/22) of patients with PMED (Table 5). Prominent spongiosis was present in a minority of patients, 32% (7/22). Similarly, in control patients with dysmotility-negative GERD, LE was focal in 75% (3/4) of the patients and was peripapillary in 50% (2/4). However, there were no cases (0/4) with prominent spongiosis.
Table 5.
Microscopic characterization of lymphocytic esophagitis in patients with non-achalasia primary motility abnormalities and dysmotiltiy-negative gastroesophageal reflux disease.
| Nutcracker esophagus, n=10, (%) |
Ineffective motility, n=7, (%) |
Diffuse spasm, n=5, (%) |
All PEMD, n=22, (%) |
DN-GERD, n=4, (%) |
P-value (all PEMD versus DN-GERD) |
|
|---|---|---|---|---|---|---|
| Lymphocytes, median (25th; 75th percentile) | 65 (58; 150) | 69 (75; 88) | 67 (73; 142) | 72 (63; 271) | 67 (61; 88) | |
| Distribution of increased IEL | ||||||
| Focal | 9 (90) | 6 (86) | 3 (60) | 18 (82) | 3 (75) | 1.0 |
| Diffuse | 1 (10) | 1 (12) | 2 (40) | 4 (18) | 1 (25) | 1.0 |
| Localization of increased IEL | ||||||
| Peripapillary | 9 (90) | 4 (57) | 3 (60) | 15 (68) | 2 (50) | .59 |
| Peripapillary/interpapillary | 1 (10) | 3 (43) | 2(40) | 6 (27) | 1 (25) | 1.00 |
| Diffuse | 0 | 0 | 0 | 1 (3) | 1 (25) | .29 |
| Prominent spongiosis | 3 (30) | 2 (29) | 2 (40) | 7 (32) | 0 | .55 |
| Cases with CD4-predominant IEL | 5 (50) | 6 (86) | 3 (60) | 14 (64) | 1 (25) | .28 |
| Cases with CD8-predominant IEL | 5 (50) | 1 (14) | 2 (40) | 8 (36) | 3(75) | .28 |
| CD4:CD8 ratio for CD4-predominant cases | 1.7 ± 0.3 | 3.4 ± 2.1 | 3.0 ± 1.8 | 2.7 ± 1.7 | 2.1 | - |
| CD4:CD8 ratio for CD8-predominant cases | 0.5 ± 0.2 | 0.1 | 0.3 ± 0.1 | 0.4 ± 02 | 0.1 ± 0.1 | .013 |
Abbreviations: IEL, intraepithelial lymphocytes; DN-GERD, dysmotility-negative gastroesophageal reflux disease; PEMD, non-achalasia primary motility disorders.
LE demonstrated CD4-predominant IEL in 50% (5/10), 86% (6/7) and 60% (3/5) of patients with nutcracker esophagus, ineffective motility and diffuse spasm, respectively. Thus, the majority of patients with PMED, 64% (14/22), had CD4-predominant T-cells (Table 5). In contrast, only 25% (1/4) patients with LE and dysmotility–negative GERD had CD4-predominant IEL.
Follow up data in patients with PMED
Six of 22 (27%) patients with nutcracker esophagus had follow-up biopsies (mean ± SD follow up period 4.0 ± 4.1 years). Three of the six followed patients had LE on initial biopsy, and one of three (33%) demonstrated the same 7 years after the index biopsy. This patient was treated with a calcium channel blocker diltiazem and a proton pump inhibitor esomeprazole. The other two patients, in whom LE was not present on follow up, received proton pump inhibitors omeprazole and pantoprazole. The three patients who had initial lymphocyte counts in the normal range continued to have normal lymphocyte levels at follow-up.
Ten of 33 (30%) patients with ineffective motility had follow-up biopsies (mean ± SD follow up period 2.5 ± 2.0 years). In 5 of the followed patients who had LE on initial biopsy, 3 (60%) demonstrated LE after 0.5, 1 and 5 years of follow-up, respectively. All 3 latter patients received esomeprazole, and, in addition, one received botulinum toxin injections and one received peppermint. The other two patients, in whom LE was not present on follow up, received proton pump inhibitors esomeprazole and pantoprazole. In 5 of the followed patients with normal numbers of IEL on the original biopsy, one patient had LE 4 years after the index biopsy. The patient received sublingual nitroglycerin.
One patient with diffuse spasm and LE had a follow-up biopsy, which also demonstrated LE 8 years after the index biopsy. The patient was taking omeprazole during the follow up period. Overall, the data suggest that 56% (5/9) of patients with PEMD and LE continued to have LE on follow up. Thirteen percent (1/8) of patients with PMED and no evidence of LE on index biopsy demonstrated LE on follow up. These data suggests a chronic course of LE in a significant proportion of patients with PMED.
DISCUSSION
Despite wide acceptance of the term “LE” its histologic criteria remain insufficiently defined. Previously published data on normal numbers of IEL were derived from patients with upper gastrointestinal symptoms and may not be generalizable for the broader patient population.(16, 17) Thus, either qualitative assessment or arbitrary quantitative lymphocyte thresholds are in use. The qualitative assessment can reliably detect only prominent forms of LE. Therefore, knowledge of the normal range of IEL is essential for comprehensive study of the spectrum of LE.
In this study, we first evaluated the range of IEL in healthy volunteers and for the first time, to our knowledge, established upper cut-offs for normal content of the IEL at different levels of the esophagus in adult subjects. We then applied this data to identify LE in patients with PEMD and found that nearly half of the patients with PMED had high IEL in comparison to only 10% of patients with dysmotility-negative GERD. More specifically, about 40% of the patients with nutcracker esophagus or diffuse spasm and approximately 20% of patients with inefficient motility demonstrated LE characterized predominantly by focal (sometimes single) peripapillary lymphoid infiltrates. The true proportion of patients with LE is likely higher, because randomly taken biopsies may miss focal lymphoid infiltrates. Importantly, in multivariate analyses, patients with PMED had approximately eight times the odds of having LE relative to patients without motility disorders, suggesting a strong association.
More than a half (56%) of patients with PEMD and LE continued to have LE on follow up, consistent with a chronic condition. In addition, one of the patients with PMED demonstrated LE only on the follow up biopsy. It remains uncertain whether LE in this patient was newly developed. Focal LE could disappear or manifest in serially performed biopsies due the tendency to be missed in randomly performed biopsies. Alternatively, LE could wax and wane.
Nearly two thirds of all patients with PMED and LE had a predominance of CD4 T-cells. We also noted an association between CD4-predominant LE and motility abnormalities in a previous study, however the significance of CD4-predominant lymphocytes remains unclear.(5) There is limited literature on CD4 T-lymphocytes in the esophageal mucosa. In normal conditions, CD4 T-cells reside mainly in the lamina propria.(18) In pathologic conditions, CD4-predominant LE was recently reported in achalasia,(19) and high levels of intraepithelial CD4-predominant T-cells were observed in Candida esophagitis.(20) In contrast, IEL are predominantly CD8 T-cells in normal individuals,(18) which is supported by our findings of intraepithelial CD8-predominant T-lymphocytes in 83% of studied healthy volunteers.
The etiological relevance of LE in PMED warrants further consideration. First, it could be speculated that to cause motility disturbances, lymphocytic inflammation should involve esophageal smooth muscle, either directly or through its innervation, and that LE is too superficial to have such an effect. Because LE is associated with achalasia (and its myenteric lymphocytic inflammation),(8, 19) it is possible that LE of PMED is also an indicator of deeper inflammatory process. That the pathogenesis of PMED and achalasia may be related, is supported by transition of nutcracker esophagus(21, 22) and diffuse spasm(23, 24) to achalasia and by myenteric lymphocytic inflammation in the distal esophagus of 23% (3/13) of patients with nutcracker esophagus.(25)
A second consideration is the fact that LE was associated with both hypocontractile (i.e. ineffective motility) and hypercontractile motility patterns (i.e. diffuse esophageal spasm and nutcracker esophagus). While the heterogeneity of motility patterns is noteworthy, there are examples of a single entity, such as eosinophilic esophagitis, demonstrating both hypercontractile and hypocontractile motility patterns.(26) Therefore, heterogeneity of motility patterns does not preclude compatibility with LE. Finally, reverse causality, where PMED cause LE, remains a possibility.
Our study has several limitations. The study is retrospective and selection bias cannot be excluded. Because endoscopic biopsies are not standard of care in GERD or PMED, not all patients had histologic evaluation, which could also introduce bias. Motility abnormalities were evaluated only with the help of standard manometry. In addition, due to the difficulty of obtaining follow-up biopsies, we were unable to fully investigate the extent to which LE remained chronic. These deficits could be corrected in future studies that employ prospective designs and high resolution manometry. Finally, because the pathophysiological mechanisms underlying LE and PMED are uncertain, residual confounding by an unmeasured marker remains possible.
In conclusion, our data suggest a strong association between PMED and LE. LE has a chronic course in many patients with PMED. Presence of LE in biopsies may be helpful in raising the possibility of PMED.
Acknowledgments
Grant support: Intradepartmental
The authors are grateful to Dr Jonathan Marotti for the critical review of the manuscript.
Footnotes
Disclosure/Conflict of Interest
No conflicts of interest to declare.
References
- 1.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61:1108–1114. doi: 10.1136/gutjnl-2011-301014. [DOI] [PubMed] [Google Scholar]
- 3.Rubio CA, Sjodahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. American journal of clinical pathology. 2006;125:432–437. [PubMed] [Google Scholar]
- 4.Purdy JK, Appelman HD, Golembeski CP, et al. Lymphocytic esophagitis: a chronic or recurring pattern of esophagitis resembling allergic contact dermatitis. American journal of clinical pathology. 2008;130:508–513. doi: 10.1309/D3PCF6D6YYMQRX9A. [DOI] [PubMed] [Google Scholar]
- 5.Xue Y, Suriawinata A, Liu X, et al. Lymphocytic Esophagitis With CD4 T-cell-predominant Intraepithelial Lymphocytes and Primary Esophageal Motility Abnormalities: A Potential Novel Clinicopathologic Entity. The American journal of surgical pathology. 2015;39:1558–1567. doi: 10.1097/PAS.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 6.Ebach DR, Vanderheyden AD, Ellison JM, et al. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn's disease. Inflammatory bowel diseases. 2011;17:45–49. doi: 10.1002/ibd.21347. [DOI] [PubMed] [Google Scholar]
- 7.Sutton LM, Heintz DD, Patel AS, et al. Lymphocytic Esophagitis in Children. Inflammatory bowel diseases. 2014;20:1324–1328. doi: 10.1097/MIB.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 8.Lehman MB, Clark SB, Ormsby AH, et al. Squamous mucosal alterations in esophagectomy specimens from patients with end-stage achalasia. The American journal of surgical pathology. 2001;25:1413–1418. doi: 10.1097/00000478-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Quispel R, van Boxel OS, Schipper ME, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187–193. doi: 10.1055/s-0028-1119590. [DOI] [PubMed] [Google Scholar]
- 10.Daniels JA, Lederman HM, Maitra A, et al. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. The American journal of surgical pathology. 2007;31:1800–1812. doi: 10.1097/PAS.0b013e3180cab60c. [DOI] [PubMed] [Google Scholar]
- 11.Kissiedu J, Thota PN, Gohel T, et al. Post-ablation lymphocytic esophagitis in Barrett esophagus with high grade dysplasia or intramucosal carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016 doi: 10.1038/modpathol.2016.50. In press. [DOI] [PubMed] [Google Scholar]
- 12.Jonasson C, Wernersson B, Hoff DA, et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Alimentary pharmacology & therapeutics. 2013;37:564–572. doi: 10.1111/apt.12204. [DOI] [PubMed] [Google Scholar]
- 13.Lacy BE, O'Shana T, Hynes M, et al. Safety and tolerability of transoral Bravo capsule placement after transnasal manometry using a validated conversion factor. The American journal of gastroenterology. 2007;102:24–32. doi: 10.1111/j.1572-0241.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 14.Ismail-Beigi F, Horton PF, Pope CE., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58:163–174. [PubMed] [Google Scholar]
- 15.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 16.Wang HH, Mangano MM, Antonioli DA. Evaluation of T-lymphocytes in esophageal mucosal biopsies. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1994;7:55–58. [PubMed] [Google Scholar]
- 17.Resnick MB, Finkelstein Y, Weissler A, et al. Assessment and diagnostic utility of the cytotoxic T-lymphocyte phenotype using the specific markers granzyme-B and TIA-1 in esophageal mucosal biopsies. Human pathology. 1999;30:397–402. doi: 10.1016/s0046-8177(99)90114-4. [DOI] [PubMed] [Google Scholar]
- 18.Geboes K, De Wolf-Peeters C, Rutgeerts P, et al. Lymphocytes and Langerhans cells in the human oesophageal epithelium. Virchows Arch A Pathol Anat Histopathol. 1983;401:45–55. doi: 10.1007/BF00644788. [DOI] [PubMed] [Google Scholar]
- 19.Muller KE, Putra J, Gabbard S, et al. Frequency and nature of increased intraepithelial lymphocytes in biopsies from patients with achalasia. Modern Pathology. 2014;27:194A. [Google Scholar]
- 20.Martin I, Liu X, Suriawinata AA, et al. Mucosal Inflammation in Candida Esophagitis (CE) Has Distinct Features. Modern pathology. 2015;28:393A. doi: 10.1038/s41379-018-0060-4. [DOI] [PubMed] [Google Scholar]
- 21.Anggiansah A, Bright NF, McCullagh M, et al. Transition from nutcracker esophagus to achalasia. Digestive diseases and sciences. 1990;35:1162–1166. doi: 10.1007/BF01537590. [DOI] [PubMed] [Google Scholar]
- 22.Paterson WG, Beck IT, Da Costa LR. Transition from nutcracker esophagus to achalasia. A case report. Journal of clinical gastroenterology. 1991;13:554–558. doi: 10.1097/00004836-199110000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Khatami SS, Khandwala F, Shay SS, et al. Does diffuse esophageal spasm progress to achalasia? A prospective cohort study. Digestive diseases and sciences. 2005;50:1605–1610. doi: 10.1007/s10620-005-2903-3. [DOI] [PubMed] [Google Scholar]
- 24.Fontes LH, Herbella FA, Rodriguez TN, et al. Progression of diffuse esophageal spasm to achalasia: incidence and predictive factors. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2013;26:470–474. doi: 10.1111/j.1442-2050.2012.01377.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim HS, Park H, Lim JH, et al. Morphometric evaluation of oesophageal wall in patients with nutcracker oesophagus and ineffective oesophageal motility. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20:869–876. doi: 10.1111/j.1365-2982.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 26.Lucendo AJ, Pascual-Turrion JM, Navarro M, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765–771. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]