Abstract
BACKGROUND & AIMS
For 4 decades, stigmata of recent hemorrhages in patients with non-variceal lesions have been used for risk stratification and endoscopic hemostasis. The arterial blood flow that underlies the stigmata is rarely monitored, but can be used to determine risk for rebleeding. We performed a randomized controlled trial to determine whether Doppler endoscopic probe monitoring of blood flow improves risk stratification and outcomes in patients with severe non-variceal upper gastrointestinal hemorrhage.
METHODS
In a single-blind study performed at 2 referral centers, we assigned 148 patients with severe non-variceal upper gastrointestinal bleeding (125 with ulcers, 19 with Dieulafoy’s lesions, and 4 with Mallory Weiss tears) to groups that underwent standard, visually guided endoscopic hemostasis (control, n=76) or endoscopic hemostasis assisted by Doppler monitoring of blood flow under the stigmata (n=72). The primary outcome was rate of rebleeding after 30 days; secondary outcomes were complications, death, and need for transfusions, surgery, or angiography.
RESULTS
There was a significant difference in rates of lesion rebleeding within 30 days of endoscopic hemostasis in the control group (26.3%) vs the Doppler group (11.1%) (P=.0214). The odds ratio for rebleeding with Doppler monitoring was 0.35 (95% CI, 0.143–0.8565) and number needed to treat was 7. There were also significant differences in rates of surgery and major complications (5.3% in the control group vs no patients in the Doppler monitoring group for each, P=.048)
CONCLUSIONS
In a randomized controlled trial of patients with severe upper gastrointestinal hemorrhage from ulcers or other lesions, Doppler probe-guided endoscopic hemostasis significantly reduced 30 day rates of rebleeding, surgery, and major complications compared to standard, visually guided hemostasis. Guidelines for non-variceal gastrointestinal bleeding should incorporate these results. ClinicalTrials.gov no: NCT00732212 (CLIN-013-07F)
Keywords: endoscopy, UGI bleeding, stigmata of hemorrhage, clinical trial
INTRODUCTION
For more than 40 years stigmata of recent hemorrhage (SRH) have been utilized to guide decisions about endoscopic treatment for peptic ulcer and other types of non-variceal upper gastrointestinal (UGI) bleeding.1–5 Current guidelines rely on endoscopic SRH to estimate risks of rebleeding, describe visual guides to endoscopic hemostasis and provide recommendations based upon systematic review of published study results about non-variceal UGI bleeding.6–9 Although residual arterial blood flow has been reported to be an independent predictor of rebleeding for non-variceal UGI lesions, arterial flow at endoscopy has been infrequently studied or used to guide treatment.10–12
Our hypothesis was that arterial blood flow monitoring with Doppler endoscopic probe during endoscopy and endoscopic treatment of severe non-variceal hemorrhage would significantly improve patient care outcomes compared to standard treatment based upon SRH alone without blood flow monitoring. Our primary outcome was clinically defined severe rebleeding from the index lesion within 30 days and secondary outcomes were rates of surgery, major complications, deaths, and blood product transfusions within 30 days of the index bleed. The association between residual arterial blood flow after endoscopic treatment and rebleeding was also assessed.
METHODS
This RCT was designed to compare the clinical outcomes of standard visually guided hemostasis of severe hemorrhage from non-variceal UGI lesions (ulcers, Dieulafoy’s lesions or Mallory Weiss tears without portal hypertension) to Doppler probe assisted treatment with blood flow monitoring. The treatment allocation was 1 to 1 in a parallel treatment design.
This study was approved by the Institutional Review Boards (IRBs) of the West Los Angeles Veterans Administration and the Ronald Reagan University of California Los Angeles Medical Centers. Prior to starting this study, it was registered with ClinicalTrial.gov as NCT00732212 (CLIN-013-07F). The clinical trials registration of the VA research proposal included two separate RCT’s, one on non-variceal UGI bleeding and the other on variceal-portal hypertensive lesions. The non-variceal lesion study is reported in this manuscript and was conducted between February 2009 and January 2015 at both medical centers. The study was suspended for 9 months because of slow enrollment. The study was resumed after the following changes were made: IRB approval of surrogate consenting and inclusion of sicker patients- American Society for Anesthesia [ASA] grades III and IV.
Prior to study initiation, all treating physicians were trained on the use of the Doppler endoscopic probe, as previously reported by us.12 Also, the same training methods about SRH and endoscopic treatments were used in this RCT as described in the recent DEP cohort study. 12 The same endoscopists treated all patients in each arm of the study. They were all skilled endoscopists who had previously been trained by the PI (D.M.J.) in endoscopic hemostasis. The number of endoscopists who assessed, screened and randomized patients on this RCT was 8. These are all general gastroenterologists who are experienced in managing patients with UGI hemorrhage similar to other large referral centers.
Severe GI bleeding was clinically defined as presence of hematemesis, melena, or hematochezia; signs or symptoms of hypovolemia (hypotension, tachycardia, orthostatic change in pulse and blood pressure, dizziness or syncope); along with a hemoglobin concentration decrease from baseline of 2 grams per deciliter or more (from previous outpatient hemoglobin or after IV resuscitation before red blood cell-RBC-transfusion); and transfusion of 1 or more units of packed RBC’s for hypovolemia, resuscitation and acute blood loss anemia. Patients with less severe bleeding or those not hospitalized were excluded. Patients were screened for inclusion either if bleeding started before presentation to the hospital or while they were hospitalized for other causes (e.g. inpatient bleeding). Endoscopic inclusion criteria were: 1) benign appearing peptic ulcers that were at least 5 mm in size and had some SRH. SRH were divided into two categories: major SRH defined as spurting or pulsatile bleeding, non-bleeding visible vessel, or adherent clot; or lesser SRH defined as a flat spot or oozing bleeding without clot or visible vessel, 2) a Dieulafoy’s lesion with major SRH, 3) a Mallory Weiss tear with pulsatile arterial bleeding. Other inclusion criteria were written informed consent (from the patient or a surrogate), ASA grade of I–IV before urgent endoscopy, and life expectancy of 30 days or more. Exclusion criteria were severe coagulopathy not correctable by blood product transfusions (e.g. platelet count < 20,000, international normalized ratio – INR- > 2.5 or partial thromboplastin time of twice normal), uncooperative or non-compliant patients including those unwilling to continue hospitalization as directed by the managing physicians or to return for follow-up, active UGI malignancy, ASA grade of V, hypotension necessitating IV drugs to maintain blood pressure and a malignant appearing ulcer. Consent for study inclusion was obtained prior to urgent endoscopy for those who met clinical and laboratory inclusion criteria. Patients were then randomized at the bedside during urgent endoscopy if they met endoscopic criteria. Therapeutic panendoscopes (Olympus or Pentax with 3.8 mm suction channel and target jet irrigation) were utilized.
A card inside the sealed envelope designated which treatment to use - either standard endoscopic treatment or Doppler assisted hemostasis. Cards and notebooks had been prepared before the study started by the statistician using permuted blocks of four for randomization.
For patients randomized to the standard visually guided endoscopic treatment group, either endoscopic hemoclipping (11 mm size opened, Boston Scientific Corporation) or multipolar electrocoagulation (MPEC 10 French size, 8 – 10 second pulses/tamponade station, firm pressure on and next to SRH) was used with or without dilute epinephrine pre-injection (1:20,000 concentration mixed with normal saline), as previously described.12–15 End points of endoscopic treatment were control of active bleeding and flattening the visible vessel, either through hemoclip use or coaptive coagulation with firm tamponade on the SRH.12–16 For either treatment group, lesions with adherent clots were first injected with dilute epinephrine, shaved down with cold guillotining, and the residual pedicle or visible vessel was treated with application of hemoclips or MPEC probe, as previously described.13,15 For patients randomized to the Doppler probe group, the probe was used to detect arterial blood flow prior to epinephrine injection or visually guided endoscopic hemostasis and after this treatment, on the stigmata and out from it as previously described.12 The probe is FDA approved and is composed of a control unit and one-time use, disposable Doppler probe (Vascular Technology Incorporated - VTI, Nashua, NH).
The following was the treatment algorithm used by the investigators for chronic ulcers with active arterial bleeding, NBVV, or adherent clot (after baseline DEP assessment before epinephrine injection and guilloting off the clot or shaving it down): epinephrine injection followed by MPEC until hemostasis or concerns about complications. If there was more bleeding or persistence of arterial signal in the DEP group, application of hemoclips (resulting in triple therapy) was used. If there were acute, small (< 10mm), or less fibrotic ulcers with NBVV or adherent clots or Dieulafoys lesions and Mallory Weiss Tears, injection of epinephrine and hemoclips were used.
In the Doppler group, patients with flat spots in ulcers were treated at endoscopy only if arterial flow was detected. Neither those patients with a negative Doppler signal nor patients with flat spots in the standard treatment group received endoscopic therapy, in accordance with current treatment guidelines.7–9
If residual arterial blood flow was detected after initial treatment in the Doppler group, more hemoclips were placed over the site of the positive Doppler signal. In cases of firm or fibrotic ulcer bases where hemoclips would not adhere, more MPEC was applied if that was deemed safe by the investigator.
Patients, their families, and the managing medical-surgical teams were blinded as to whether Doppler endoscopic probe was utilized or not. Decisions about transfusion of red blood cells and other blood products and the medical-surgical (or angiographic) management after randomization were made by the blinded medical-surgical physicians caring for the study patients during the hospitalization and after hospital discharge.
Medical treatment after endoscopy was as follows: patients with ulcers and Dieulafoy’s lesions received high dose proton pump (Pantoprazole) infusion – PPI - (80 mg bolus and 8 mg/hour) for 72 hours, followed by twice daily oral PPI for 30 days (omeprazole 20 mg, Pantoprazole 40 mg, or Lansoprazole 30 mg). Patients with a Mallory Weiss tear were treated with anti-emetics initially and PPI twice daily for 7 days.
H. pylori infection was considered present if any of the following was positive for the ulcer patients: IgG serology, stool antigen, or gastric biopsy. For those ulcer patients with H.P. infection, treatment with three or four drug therapies was started within 5–7 days of the index bleed. In patients requiring secondary prophylaxis to prevent heart or cardiovascular events, aspirin, anti-coagulants, or dual anti-platelet agents were resumed within 4–5 days of the randomization.
The clinical criteria for rebleeding after randomization were clinical signs of rebleeding (recurrent hematemesis, melena, and/or hematochezia), acute signs of hypovolemia, a 2 gram/deciliter or more decrease (from baseline after initial endoscopy and resuscitation) in hemoglobin concentration, and transfusion of 1 or more units of RBC’s. A diagnosis of rebleeding required all three of these. These criteria were chosen to be more stringent than most of the RCT’s of severe ulcer or NVUGI bleeding including those studies forming the basis for recent guidelines of NVUGI bleeding, 6–9 Doppler studies, 17–20 and for a recent, large international RCT of ulcer hemorrhage. 21 At the discretion of the managing physicians (who were blinded to the endoscopic treatment) either repeat endoscopy, angiography, or surgery were performed for severe rebleeding unless a severe complication or death precluded these. Angiography with embolization or surgery was performed when hemorrhage could not be controlled initially, was within 12 hours of randomization, or for rebleeding from the same lesion in spite of repeat upper endoscopy and hemostasis.
All patients who lived were followed up to 30 days after randomization. Some patients died before 30 days but were followed until time of death. Each patient was followed prospectively each day and had hemoglobins checked daily until hospital discharge. After discharge (if it was before 30 days), they were contacted by telephone and/or had clinic follow-up visits at 30 days. Patients were also instructed to return to the same hospital for any signs of GI bleeding.
The primary clinical outcome was index lesion rebleeding within 30 days after randomization. This was prospectively ascertained using the clinical algorithm for rebleeding as detailed above. Secondary outcomes were rates of surgery, angiography, major complications, death and blood product transfusions within 30 days after randomization as well as length of hospital stay.
All authors had access to the study data and reviewed and approved the final manuscript.
Sample Size Calculation
Based upon prior prospective studies by our group,12–15 we estimated that the 30 - day rebleeding rate in the standard treatment group would be 20% and for the endoscopic Doppler group would be 5%. To achieve an 80% power with two tailed alpha of 0.05, the sample size was 75 patients per group. With an estimated 5% drop out rate, we planned to randomize 79 patients per group. However, as the trial proceeded, there were no dropouts, so the goal for randomization was reduced to 150 patients.
Statistical Methods
Data were prospectively collected, de-identified, and entered onto electronic data files. SAS 9.4 (SAS Inc, Cary NC) was used for data management and statistical analyses. Data analysis compared the background characteristics, endoscopic findings, and 30 day outcomes according to the two treatments. The cut off p value for statistical significant was 0.05 in two sided testing. Proportions were compared using Fisher exact tests and means were compared using the Wilcoxon rank sum test (Mann-Whitney U test), since most continuous data such as hospital days did not follow the normal distribution. Computations were carried out using StatXact 8.0 (Cytel Inc, Cambridge Mass) and SAS 9.4. All data analyses were performed according to an intension to treat basis and included all 148 patients who were randomized. Time to lesion rebleeding was also determined and compared by log rank test.
One interim analysis was performed when 60% of the patients were randomized and followed up for 30 days. This was used by the VA Data and Safety Monitoring Committee (DMC) to monitor accrual, dropouts, complications, and safety, but not to assess efficacy. This was not used as a means to stop this study early which was the responsibility of the VA Data and Monitoring Committee. This gave us the opportunity to reassess drop-out rates (which were 0%) and therefore to reduce the sample size estimate for enrollment by 5%.
The interactions between treatment group (standard vs. Doppler) and enrollment date were also analyzed in two ways: early vs. late period and year of enrollment. In the first analysis, the early period was on or before June 23, 2012, which was the median date of entry. Late was after that date. For the second analysis, year of enrollment was used. All the patient characteristics and risk factors in Table 1 and the primary outcome (rebleed with 30 days) were analyzed. Each specified variable and 30 day lesion rebleeding were compared between the two treatment groups separately by time period using the Chi-Square or Fisher exact tests (for categorical variables) or the Wilcoxon rank sum test (for continuous variables). In addition, we assessed whether the relationship between treatment group and the specific variable or rebleeding varied significantly according to time period, using the logistic or linear regression module, as appropriate.
Table 1.
Patient Characteristics
| Standard | Doppler | P value | |
|---|---|---|---|
|
| |||
| Patients | 76 | 72 | |
|
| |||
| Age* | 66.34 ± 16.1 | 65.18 ± 15.6 | 0.505 |
|
| |||
| Female/Male | 14/62 | 15/57 | 0.836 |
|
| |||
| Inpatient Bleed | 14 (18.4%) | 15 (20.8%) | 0.836 |
|
| |||
| Ulcers ≥ 20 mm | 12 (15.8%) | 12 (16.7%) | 0.999 |
|
| |||
| CURE Prognosis Score (1–6)* | 2.95 ± 0.99 | 2.94 ± 1.17 | 0.622 |
|
| |||
| American Society of Anesthesia Grade | 0.899 | ||
| I | 5 (6.6%) | 5(6.9%) | |
| II | 21 (27.6%) | 24 (33.3%) | |
| III | 43 (56.6%) | 37 (51.4%) | |
| IV | 7 (9.2%) | 6 (8.3%) | |
|
| |||
| Cirrhosis | 12 (15.8%) | 10 (14.0%) | 0.820 |
| Child Pugh Score* | 9.5 ± 2.9 | 8.0 ± 2.6 | 0.258 |
|
| |||
| Hypotension | 35 (46.1%) | 34 (47.2%) | 0.755 |
|
| |||
| H. Pylori positive Ulcers | 23 (35.9%) | 23 (37.8%) | 0.986 |
|
| |||
| UCLA/VA | 44/32 | 39/33 | 0.648 |
|
| |||
| Smoking – Yes/No | 3/73 | 2/70 | 0.694 |
|
| |||
| Drinking – Yes/No | 14/62 | 15/57 | 0.836 |
|
| |||
| Aspirin | 28 (36.8%) | 39 (54.2%) | 0.034 |
|
| |||
| Other anti-platelet drugs | 9 (11.8%) | 8 (11.1%) | 0.889 |
|
| |||
| Non-steroidal anti-inflammatory drugs | 19 (25%) | 20 (27.8%) | 0.701 |
|
| |||
| Warfarin | 13 (17.1%) | 8 (11.1%) | 0.296 |
|
| |||
| Other Anti-coagulant | 4 (5.3%) | 7 (9.7%) | 0.359 |
|
| |||
| Both anti-platelet and anti-coagulant drug | 6 (7.9%) | 5 (6.9%) | 0.826 |
|
| |||
| Endoscopic Diagnosis | 0.258 | ||
| DU’s | 32 (42%) | 30 (41.7%) | |
| (posterior DU’s) | 12 (37.5%) | 8 (26.7%) | |
| GU’s | 24 (31.6%) | 20 (27.8%) | |
| (lesser curve GU’s) | 12 (50%) | 9 (45%) | |
| ** EU’s/ HH ulcers | 2 (2.6%) | 5 (6.9%) | |
| Anastomotic ulcers | 4 (5.3%) | 7 (9.7%) | |
| Dieulafoy’s lesion | 13 (17.1%) | 6 (8.3%) | |
| Mallory Weiss Tears | 1 (1.3%) | 3 (4.2%) | |
|
| |||
| Baseline hemoglobin* | 7.6 ± 1.5 | 7.9 ± 1.7 | 0.348 |
|
| |||
| Baseline red cell transfusions* | 3.1 ± 2.3 | 3.4 ± 2.9 | 0.657 |
|
| |||
| Baseline fresh frozen plasma transfusions * | 0.95 ± 2.3 | 0.6 ± 1.5 | 0.406 |
|
| |||
| Baseline platelet transfusions * | 0.92 ± 6.7 | 0.14 ± 0.5 | 0.661 |
Legend
Mean ± Standard deviation.
EU’s are Esophageal Ulcers. HH are ulcers in a hiatal hernia.
RESULTS
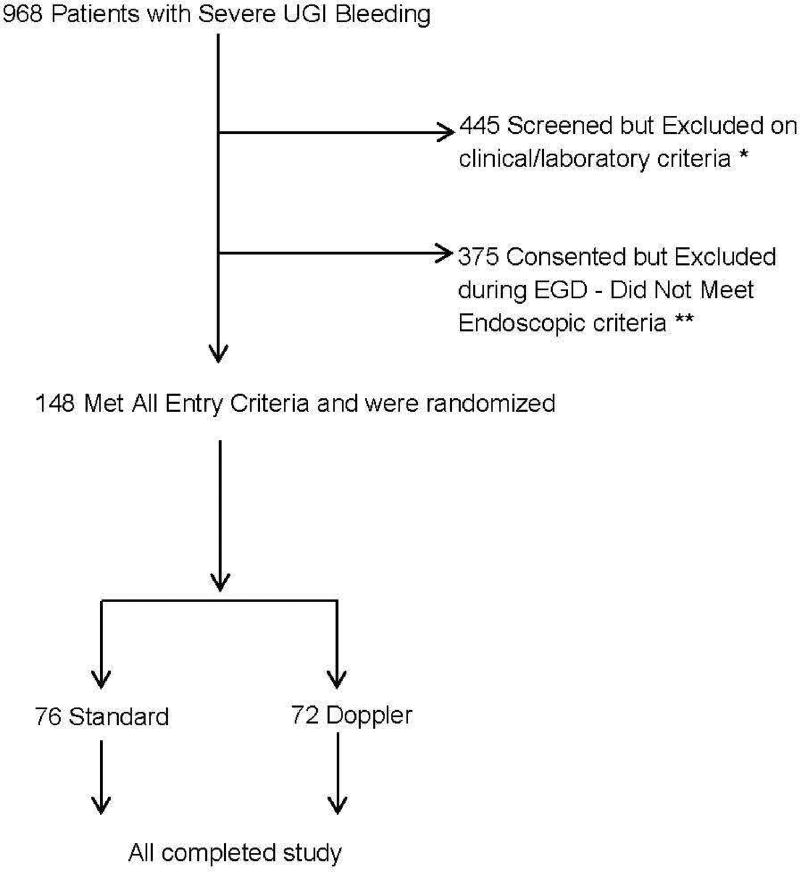
By the completion of this study of non-variceal UGI bleeding, 968 patients with severe UGI hemorrhage were assessed for potential enrollment. Because of clinical and/or laboratory exclusion criteria, 445 patients were excluded on screening prior to endoscopy. See Figure 1 for details. Another 375 patients who met clinical inclusion criteria were excluded after an upper endoscopy showed they failed to meet endoscopic criteria. Also see Figure 1 for further details. A total of 148 patients meeting clinical and endoscopic criteria were randomized. Each endoscopist randomized 4 – 20 patients (of the total 148 non-variceal patients) and helped manage another 40 – 150 patients with UGI hemorrhage who were screened but excluded on clinical or laboratory criteria or excluded on EGD after meeting clinical and laboratory entry criteria (of a total of 820 patients from this non-variceal UGI RCT).
Figure 1.
CONSORT diagram of Doppler Endoscopic Probe Randomized Controlled Trial.
* The specific reasons were ASA 5 or very poor prognosis – 30 day survival not expected including those without organ transplantation (176 patients), no consent for study including surrogate (110 patients), uncooperative, non-compliant, or unable to return for study follow-up (45 patients), refused to consent (32 patients), UGI malignancy (26 patients), hypotensive on pressors (17 patients), not severe enough hemorrhage (13 patients), did not meet entry criteria.
There was no statistically significant difference between the intervention (Doppler) and the standard (control) group as far as demographic characteristics, laboratory values, distribution of bleeding lesions (Table 1), or distribution of the stigmata of recent hemorrhage (Table 2). The only difference was a higher proportion of patients using aspirin in the intervention (Doppler) group (54.2% vs. 36.8%, p = 0.034) – Table 1. In this study, 84.5% of the patients had peptic ulcers (63 duodenal ulcers, 44 gastric ulcers, 7 esophageal or hiatal hernia ulcers, and 11 anastomotic ulcers), 12.8 % had Dieulafoy’s lesions, and 2.7% had bleeding Mallory Weiss tears (Table 1).
Table 2.
Differences in Rebleeding by Stigmata of Recent Hemorrhage and Use of Doppler Probe.
| Stigmata | Standard | Doppler | P value | Difference (Standard- Doppler) |
95% Confidence Invervals |
|---|---|---|---|---|---|
| Active Arterial bleed | 5/10 (50.0%) | 4/14 (28.6%) | 0.403 | 21.4% | (− 17.6%, 60.4%) |
| Non-bleeding visible vessel | 7/27 (25.9%) | 4/26 (15.4%) | 0.501 | 10.5% | (− 11.0%, 32.1%) |
| Adherent Clot | 4/16 (25%) | 0/12 (0%) | 0.113 | 25.0% | (− 1.1%, 49.9%) |
| Flat Spots | 3/16 (18.8%) | 0/16 (0%) | 0.226 | 18.8% | (0.4%, 37.9%) |
| Oozing bleeding | 1/7 (14.3%) | 0/4 (0%) | 0.428 | 14.3% | (− 11.6%, 40.2%) |
| TOTALS | 20/76 (26.3%) | 8/72 (11.1%)* | 0.0214 | 15.2% | (2.9%, 27.5%) |
Legend. Shown are patients (and percentage) with lesion rebleeding for individual stigmata and for all patients (TOTALS) according to endoscopic treatment.
p values by Fisher Exact tests.
The overall rebleeding rate was significantly lower for the Doppler group than the standard group 20/76 (26.3%) vs. 8/72 (11.1%) –p = 0.0214. The 30 day lesion rebleeding rates according to each stigmata of recent hemorrhage (on index endoscopy) are reported in Table 2. For all stigmata combined by treatment, there was a significant difference in the primary outcome - 30 day rebleed rates. For the Doppler treatment group compared to standard treatment – with an odds ratio of 0.35 (with 95% confidence intervals of 0.143 to 0.8565). However, for each individual SRH, there were no significant differences in rebleed rates.
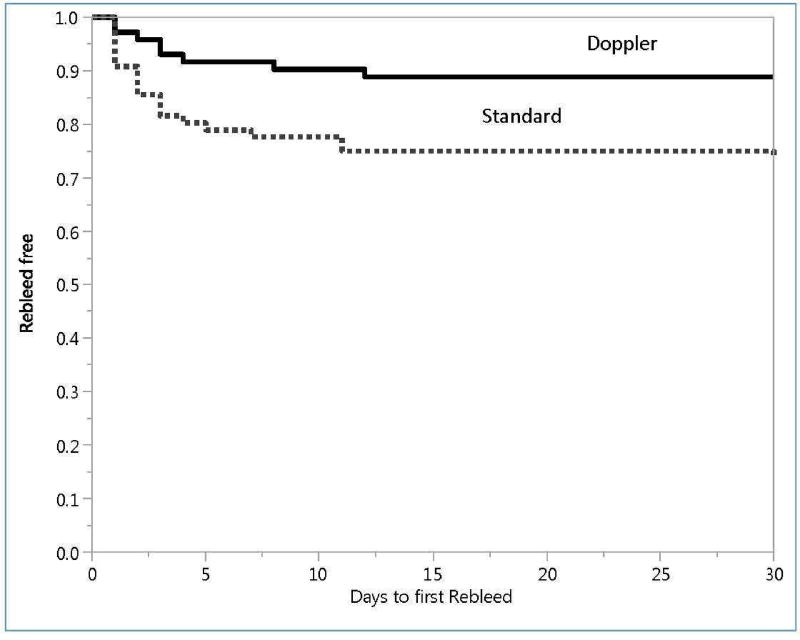
The time to lesion rebleeding for Doppler and standard treatments is shown in Figure 2. The difference was also significant (p = 0.0174). The median times (and ranges) to rebleeding were similar for the standard treatment – 2 days (1 – 30 day range) - and Doppler groups – 3 days (1–12 days).
Figure 2.
Proportion of patients without rebleeding (Rebleed free) during 30 days after randomization. Top curve is Doppler patients and lower curve is standard treated patients. Product limit plots, compared by Log-rank test. p = 0.0174.
** These included esophageal varices or portal hypertensive lesions (87 patients) and no SRH or UGI lesions that did not meet endoscopic inclusion criteria (288 patients).
Secondary outcomes are presented in Table 3. Patients randomized to the Doppler group also had significantly lower rates of surgery and major complications. The two treatment groups did not differ for any other outcome (angiography for rebleeding; length of hospital or intensive care unit stay; transfusion of red cells, fresh frozen plasma or platelets; other GI bleeds, or mortality). There was 1 perforation in the standard group and none in the Doppler group. Other major complications in the standard group were two cerebral vascular accidents related to rebleeding and one pneumoperitoneum after endoscopic retreatment for rebleeding, managed medically.
Table 3.
Primary and Secondary Outcomes Within 30 days
| Standard | Doppler | difference | 95% Confidence Intervals lower |
95% Confidence Intervals upper |
P value |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Patients | 76 | 72 | ||||
|
| ||||||
| Routine 30 day Outcomes Index Lesion Rebleed | 20 (26.3%) | 8 (11.1%) | 15.2% | 2.7% | 27.7% | 0.021 |
|
| ||||||
| Surgery for Rebleeds | 4 (5.3%) | 0 (0%) | 5.3% | 0.0% | 12.8% | 0.048 |
|
| ||||||
| Angiography for Rebleeds | 1 (1.3%) | 4 (5.6%) | −4.2% | −12.3% | 2.5% | 0.200 |
|
| ||||||
| Death | 3 (4.0%) | 1 (1.4%) | 2.6% | −3.9% | 9.8% | 0.337 |
|
| ||||||
| Major | 4 (5.3%) | 0 (0%) | 5.3% | 0.0% | 12.8% | 0.048 |
| Complications CVA | 2 (2.6%) | 0 (0%) | ||||
| Perforation | 1 (1.3%) | 0 (0%) | ||||
| Pneumopertoneum | 1 (1.3%) | 0 (0%) | ||||
|
| ||||||
| Other Gastrointestinal Bleeds | 3 (4.0%) | 4 (5.6%) | −1.6% | −10.1% | 6.2% | 0.714 |
|
| ||||||
| Transfusions and Hospital Days * | ||||||
|
| ||||||
| More red cell units transfused | 1.09 ± 2.94 | 0.56 ± 2.41 | 0.53 | −0.34 | 1.40 | 0.230 |
|
| ||||||
| More units of fresh frozen plasma transfused | 0.12 ± 0.49 | 0.06 ± 0.29 | 0.06 | −0.07 | 0.19 | 0.502 |
|
| ||||||
| More unit of platelets transfused | 0.03 ± 0.23 | 0.06 ± 0.33 | −0.03 | −0.12 | 0.06 | 0.890 |
|
| ||||||
| Hospital Days | ||||||
|
| ||||||
| Length of Stay in the Intensive care unit (in days) | 4.21 ± 8.40 | 3.04 ± 3.04 | 1.17 | −0.86 | 3.20 | 0.220 |
|
| ||||||
| Length of hospital stay (in days) | 7.00 ± 8.79 | 6.65 ± 8.48 | 0.35 | −2.46 | 3.16 | 0.997 |
Legend.
Numerical variables: Mean ± Standard deviation.
As a summary, for the standard group with rebleeds (20/76): 9 had repeat EGD, 4 had EGD’s scheduled but 3 died beforehand and 1 was cancelled due to a severe CVA, 4 had surgery, and 1 angiography. For the Doppler group with rebleeds (8/72): 7 had repeat EGD (and 2 of these later had angiography also) and 1 had neither EGD, angiography, or surgery.
The other 3 GI bleeds in the standard group were 1 esophageal varices, 1 Crohn’s disease (terminal ileal ulcers), and 1 intra-peritoneal bleed. For the Doppler group, the 4 other bleeds were 1 esophageal varices, 1 gastric angiomas, 1 antral erosions (after anti-coagulation), and 1 post-bulbar ulcer (whose index lesion was a bulbar ulcer with a visible vessel).
These was a strong association between residual blood flow after endoscopic hemostasis and rebleeding rates. During the index endoscopy, 23.6% (17/72) of patients randomized to the Doppler group had residual blood flow detected after the initial visually guided endoscopic treatment and 76.5% (13/17) of those patients received further endoscopic hemostatic treatment until less (only a faint Doppler signal – 5/13 – and 4 of the 5 later rebled) or no more residual blood flow was detected (8/13 – and none rebled). The other 4 patients did not receive further treatment because of the concern for complications and all 4 rebled. Therefore, 8 of 9 (88.9%) of patients in the Doppler group with residual blood flow which was not obliterated later rebled, compared with 0 of 8 (0%) in those whose residual blood flow was obliterated with additional hemostasis – p = 0.0004 (Fisher exact test).
For analysis of interactions between treatment group (standard vs. Doppler) and enrollment period (early vs. late), there were no significant interactions with respect to any of the baseline variables. Nor was there a significant interaction between treatment group vs. time period for the primary outcome of lesion rebleeding. The probability of same rebleeding tended to be higher for the standard treatment group than the Doppler group, regardless of the time period in either the early vs. late or the enrollment year analysis. For the early vs. late analysis, the difference in rebleeding rates between the two treatment groups was about 14% for both periods with an interaction p value of 0.7638. See Table 4 for details of the second analysis (by year of enrollment and treatment). The interaction p-value of that logistic regression was p = 0.6531, is similar to the analysis of early vs. late enrollment.
Table 4.
Enrollment Year × Treatment Interaction for 30 day Rebleeding
| Year Enrolled |
n | Standard- # rebleeds |
pct rebleed |
n | Doppler- # rebleeds |
pct rebleed |
|---|---|---|---|---|---|---|
|
| ||||||
| 2008 – 09 | 9 | 0 | 0.0% | 10 | 0 | 0.0% |
| 2010 | 6 | 1 | 16.7% | 4 | 0 | 0.0% |
| 2011 | 12 | 3 | 25.0% | 14 | 2 | 14.3% |
| 2012 | 14 | 6 | 42.9% | 17 | 3 | 17.6% |
| 2013 | 20 | 9 | 45.0% | 18 | 1 | 5.6% |
| 2014–15 | 15 | 1 | 6.7% | 9 | 2 | 22.2% |
|
| ||||||
| Total | 76 | 20 | 26.3% | 72 | 8 | 11.1% |
DISCUSSION
The important new findings of this RCT are that monitoring of arterial blood flow underneath the SRH in patients with severe non-variceal UGI hemorrhage and using it as a guide to endoscopic hemostasis improved clinical outcomes. Specifically, those were significantly lower rates of rebleeding, surgery, and complications for the Doppler group compared to the standard hemostasis group where endoscopic hemostasis was guided by stigmata of recent hemorrhage without Doppler. In the Doppler group, residual arterial blood flow after endoscopic hemostasis of SRH was highly associated with lesion rebleeding. Use of Doppler endoscopic probe as a guide to risk stratification and endoscopic hemostasis during emergency endoscopy was safe.
This is the first large RCT that utilized Doppler endoscopic probe as both a guide to risk stratification and for directing definitive endoscopic hemostasis of non-variceal UGI hemorrhage. These results are highly clinically significant and relevant. This also is the first RCT to include both major stigmata of hemorrhage and lesser stigmata for non-variceal UGI lesions with severe hemorrhage. We also combined currently recommended medical therapy with standard of care endoscopic hemostasis for different SRH. Two previously reported RCT’s using Doppler probe lacked one or more of these important features, or were negative, and utilized a much more complicated DEP unit.17,18 Several other cohort studies using Doppler probe for risk stratification reported encouraging early results for peptic ulcer hemorrhage but did not include other non-variceal UGI lesions.10,11,19,20
Patients with severe NVUGIB requiring hospitalization are the most likely to benefit from DEP for risk assessment and as a guide to endoscopic hemostasis. DEP may have been associated with improved outcomes in different ways in patients with NVUGIB according to different SRH. First, patients with major stigmata (spurting, non-bleeding visible vessel, and adherent clot) benefited the most because about 24% had residual arterial blood flow after standard visually guided endoscopic hemostasis. Residual blood flow increased the risk of rebleeding and further endoscopic treatment with DEP guidance in the current RCT reduced the rebleeding rate and improved other outcomes compared to visually guided (standard) hemostasis (Tables 2 and 3). Secondly, those patients with flat spots (FIIC) could be risk stratified to endoscopic hemostasis if DEP positive at baseline or no endoscopic hemostasis and medical treatment if DEP is negative. Third, oozing (FIB) bleeding (without other SRH such as a clot or vessel) could be stratified into low risk (DEP negative) versus higher risk (DEP positive before endoscopic treatment). After endoscopic hemostasis in the oozing group, the rebleeding rate was low, so oozing patients benefited less from DEP; similar to our recent cohort study.12
The number needed to treat (NNT) to prevent one episode of rebleeding was 7 patients with the Doppler probe. The NNT varied according to the type of SRH. It was 4 for adherent clots, 5 for flat spots, 5 for spurting bleeding, 7 for oozing, and 10 with non-bleeding visible vessels. Although the confidence intervals are wide for rebleeding and the subgroups were too small to show statistically significant differences, these are clinically relevant (Table 3).
The high rebleeding rates of patients with major stigmata of hemorrhage were associated with incomplete initial hemostasis and high rates of residual arterial blood flow underneath the SRH, as we reported both in this RCT and in a recent prospective cohort study of severe ulcer hemorrhage.12 The rates of residual blood flow after visually guided endoscopic hemostasis in the Doppler treated patients varied by stigmata from 0% for oozing bleeding to 28% for major stigmata, consistent with our prior cohort study where these rates were 0% for oozing and 27.4% for major stigmata.12 Even after further endoscopic treatment for residual blood flow in the majority of the patients in the Doppler group, there still was a high rebleeding rate for the spurting bleeding and non-bleeding visible vessel subgroups (28.6% and 15.4%). This related to untreated patent arteries in some patients who did not receive further endoscopic hemostasis or others where the signal was faint but not completely obliterated. Other possible reasons for the high rebleeding rates may be large artery size (perhaps too large to effectively treat with current through the endoscope hemoclips or with thermal coaptive coagulation), only transient interruption of arterial blood flow by treatment, incomplete coaptive coagulation or mechanical closure of the artery underlying the stigmata, fibrinolysis, coagulopathies, large ulcer size, and medications.
For clinically high risk patients such as those enrolled in this study, there is the opportunity for significant improvements in both coaptive coagulation and mechanical closure of the underlying arteries of non-variceal lesions. One potential candidate for further study is a large, over-the-endoscope hemoclip that may be able to close larger or deeper underlying arteries and obliterate blood flow more effectively than current trans-endoscopic hemoclips and potentially be safer than additional thermal coagulation for treatment of residual arterial blood flow.22 Besides surgery, another option is selective angiographic embolization targeted to the artery under the endoscopically placed hemoclips, if blood flow persists or rebleeding occurs after what is judged by the endoscopist to be safe as maximum endoscopic treatment. Five of our patients with rebleeding had angiographic embolization, as shown in Table 3.
A discussion of potential limitations and weaknesses of this study will give the reader further perspective. These include: use of a new technology that may be hard to learn; that this RCT is small and no other similar studies have been reported to confirm the results; concerns about the small numbers of patients with some major SRH such as spurting bleeding or adherent clot which could make results of statistical comparisons inconclusive for individual SRH; higher rebleed rates than are reported in other international RCT’s of bleeding ulcers; and there was no inclusion of baseline Rockall or Glasgow-Blatchford scores so that an equal distribution of baseline risk could not be confirmed by those who utilize these scores. Other potential limitations and weaknesses may be the quality of the RCT and lack of comparability of the patients because of the long duration of the study and an early suspension which could limit generalizability of results. Finally, there are concerns about increasing the cost of care by adding a new technology.
We address each of these for the readers to give them our perspective. First, this is relatively new technology and a new type of Doppler endoscopic probe. However, unlike the more technically complex endoscopic ultrasound endoscopies (EUS), the DEP unit does not produce a visual image but rather an auditory output which is gated by depth and much easier to apply and interpret than EUS endoscopes or probes which require much more training and experience.12 Also, the DEP system utilized in this study with single use endoscopic probes is FDA approved is newer and much simpler than those more complex and cumbersome systems previously used in Europe and the United Kingdom one or two decades ago, which often had multiple depth settings, multichannel recorders, or oscilloscope outputs requiring technician support for recording and interpretation. 10,17–20 Secondly, in regard to confirmatory studies, there are no other recent RCT’sreported. Third, we agree that there were small numbers of patients with some major SRH and conclusions about differences in rebleeding rates in Table 2 of individual SRH are inconclusive because of large confidence intervals. However, this study was not designed nor powered to differentiate rebleeding rates of different individual SRH for the two treatments. Instead, our primary goal was to compare overall rebleeding rates according to treatment and those were significantly different (Table 2 and Figure 2). Fourth, concerning higher rebleeding rates in this RCT compared to other recent international bleeding ulcer studies, this is accounted for by the higher risk of our patients including high ASA scores (about 60% in categories III or IV); high rates of inpatient start of bleeding (18–21%); high prevalence of large ulcers (16–17%); inclusion of cirrhotics (14–16%); and frequent use of anti-platelet drugs or aspirin (48–65%); or anti-coagulants (21–22%). We also included Dieulafoy’s lesions and Mallory Weiss Tears in high risk patients, whereas international trials focus on ulcers. Nevertheless, the gender, heterogeneous ethnicity, low prevalence of H. pylori, and these other risk factors are representative of patients managed in referral centers in the United States such as ours. 12–15,23 Our patients differ from those commonly included in RCT’s of ulcer hemorrhage from Asia or other countries that were previously reported and therefore included as evidence in current guidelines. 6–9 The latter patients are typically younger, have fewer co-morbidities and lower ASA scores, but have higher prevalences of H. pylori infection which all improve their prognosis, their response to PPI’s, and reduce their risks of rebleeding compared to the current RCT. It may be a limitation of this RCT that Rockall or Glasgow – Blatchford scores were not included. However, based upon other scores that we have utilized in our prior interventional studies (ASA, CURE prognosis score, and Child-Pugh) and detailing the known risk factors for rebleeding23 that are compared in Table 1, the treatment groups were very similar at baseline and these did not explain the outcomes reported. Fifth are potential limitations of quality control and comparability of patients because our RCT took a long time to complete and was suspended once. It was carefully monitored by an independent VA Data and Safety Monitoring Committee (DMC) which stopped the study at one point because of slow enrollment (as detailed above) and approved all protocol changes such as surrogate consenting and inclusion of sicker patients (ASA III and IV) for enrollment. Most similar interventional studies are not performed in the US and exclude such sick patients. Our group has a track record of completing well-designed, clinically relevant, credible, and new RCT’s in GI bleeding. 12–15 Some readers may think that a small number of patients were recruited, that the two centers were in the same city, and because patients were recruited over more than four years, time might have confounded the results. However, the study met recruitment goals (which was a relatively large size), included 148 patients, and reported clinically relevant outcomes (rebleeding, surgery, and major complications) which were all significantly improved with the Doppler group. Furthermore, there was no evidence that enrollment date affected the distributions of baseline variables nor the primary outcome (See Table 4).
Last, about the potential limitation related to cost. An updated cost-effectiveness study utilizing current techniques will be required to formally evaluate cost effectiveness However, our current RCT results corroborate those of a prior cost minimization analysis about potential savings with Doppler endoscopic probe utilization for treatment of severe peptic ulcer hemorrhage based upon anticipated costs in health care management.2
Our conclusions are: 1) The use of Doppler endoscopic probe as a guide to endoscopic risk stratification and hemostasis for patients with severe UGI hemorrhage from peptic ulcers, Dieulafoy’s lesions, and Mallory Weiss tears reduced 30-day rebleeding rate, surgery, and major complications compared to standard visually guided endoscopic hemostasis. 2) Doppler guided treatment during emergency endoscopy was safe. 3) Residual arterial blood flow after endoscopic hemostasis was associated with a significantly higher rebleeding rate than successful obliteration of blood flow. We recommend that current guidelines for management of NVUGI bleeding incorporate these new findings.
Acknowledgments
This study was primarily funded by a VA Clinical Merit Review Grant- CLIN-013-07F (to Dr. Jensen, PI) and also partially supported by NIH-NIDDK P30 CURE DDRC Center Grant 41301 (Human Studies Core).
Abbreviations in the Manuscript
- ASA
American Society for Anesthesia (score)
- CI
Confidence intervals
- CURE: DDRC
Center for Ulcer Research and Education: Digestive Diseases Research Center
- CVA
Cerebral vascular accident
- DEP
Doppler endoscopic probe
- EGD
Esophagogastroduodenoscopy (Panendoscopy)
- FDA
Federal Drug Administration
- GI
Gastrointestinal
- H (pylori = p)
Helicobacter pylori
- INR
International normalized ratio
- IRB
Institutional review boards
- MPEC
Multipolar electrocoagulation
- N
Number
- NH
New Hampshire
- NIH
National Institutes of Health
- NNT
Number needed to treat
- NSAID’s
Non-Steroidal anti-inflammatory drugs
- PPI
Proton pump inhibitor
- RBC’s
Red blood cell (transfusions)
- RCT
Randomized Controlled Trial
- SRH
Stigmata of recent hemorrhage
- SAS
Statistical Analysis Software
- UCLA
(Ronald Reagan) University of California Los Angeles (Medical Center)
- UGI
Upper gastrointestinal
- US
United States
- VA DMC
Veterans Administration Data Monitoring Committee
- VTI
Vascular Technology Incorporated
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Jensen is a consultant for VTI. No other co-authors have disclosures.
Author Contributions
All authors contributed to enrollment of patients and data acquisition, critical revision of the manuscript for important intellectual content, technical support, and supervision of the research coordinator for their patients.
Dr. Jeffrey Gornbein was the biostatistician for the study and contributed to data management and statistical analysis as well as to the study concept and study design. Drs. Jensen, Kovacs, and Ohning also contributed to the study concept and design and administrative support for the study. Dr. Jensen obtained the funding as a Clinical VA Merit Review Grant, supervised study personnel, drafted the manuscript, and was the principal investigator of the study.
References
- 1.Storey DW, Bown SG, Swain CP, Salmon PR, Kirkham JS, Northfield TC. Endoscopic prediction of recurrent bleeding in peptic ulcers. N Engl J Med. 1981;305:915–6. doi: 10.1056/NEJM198110153051603. [DOI] [PubMed] [Google Scholar]
- 2.Swain CP, Storey DW, Bown SG, et al. Nature of bleeding vessel in recurrently bleeding gastric ulcers. Gastroenterology. 1986;90:595–608. doi: 10.1016/0016-5085(86)91113-3. [DOI] [PubMed] [Google Scholar]
- 3.Johnston JH. The sentinel clot and invisible vessel: pathologic anatomy of bleeding peptic ulcer. Gastrointest Endosc. 1984;30:313–15. doi: 10.1016/s0016-5107(84)72431-x. [DOI] [PubMed] [Google Scholar]
- 4.Swain P. Perception and interpretation: The problem of the visible vessel. Endoscopy. 1998;30:570–74. doi: 10.1055/s-2007-1001346. [DOI] [PubMed] [Google Scholar]
- 5.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394–7. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 6.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928–37. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 7.Barkun A, Bardou M, Kuipers EJ, Sung J, Hunt R, Martel M, Sinclair P for the International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with non-variceal upper gastrointestinal bleeding (ICON-UGIB) Ann Intern Med. 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 8.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–360. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 9.Gralnek IM, Dumonoceau J-M, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1–a46. doi: 10.1055/s-0034-1393172. [DOI] [PubMed] [Google Scholar]
- 10.Fullarton GM, Murray WR. Prediction of rebleeding in peptic ulcers by visual stigmata and endoscopic Doppler ultrasound criteria. Endoscopy. 1990;22:68–71. doi: 10.1055/s-2007-1012795. [DOI] [PubMed] [Google Scholar]
- 11.Wong RC. Endoscopic Doppler US probe for acute peptic ulcer hemorrhage. Gastrointest Endosc. 2004;60:804–12. doi: 10.1016/s0016-5107(04)02046-2. [DOI] [PubMed] [Google Scholar]
- 12.Jensen DM, Ohning GV, Kovacs TOG, Ghassemi K, Jutabha R, Dulai GS, Machicado GA. Doppler Endoscopic probe as a guide to risk stratification and definitive hemostasis of peptic ulcer bleeding. Gastrointest Endosc. 2016;83:129–36. doi: 10.1016/j.gie.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen DM, Kovacs TOG, Jutabha R, Machicado GA, Gralnek IM, Savides TJ, Smith J, Jensen ME, Alofaituli G, Gornbein J. Randomized, Controlled trial of Medical Therapy Compared to Endoscopic Therapy for Prevention of Recurrent Ulcer Hemorrhage in Patients with Non-bleeding Adherent Clots. Gastroenterol. 2002;123:407–413. doi: 10.1053/gast.2002.34782. [DOI] [PubMed] [Google Scholar]
- 14.Jensen DM. Heat probe for hemostasis of bleeding peptic ulcers: Techniques and results of randomized controlled trials. Gastrointest Endosc. 1990;Supplement to 36:S42–S49. [PubMed] [Google Scholar]
- 15.Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, or combination methods. Techniques in Gastrointestinal Endoscopy. 2005;7:124–31. [Google Scholar]
- 16.Johnston JH, Jensen DM, Auth D. Experimental comparison of endoscopic Yttrium-aluminum-Garnet laser, electrosurgery, and heater probe for canine gut arterial coagulation: The importance of vessel compression and avoidance of tissue erosion. Gastroenterology. 1987;92:1101–1108. doi: 10.1016/s0016-5085(87)91065-1. [DOI] [PubMed] [Google Scholar]
- 17.Kohler B, Maier M, Benz C, Riemann JR. Acute ulcer bleeding: A prospective randomized trial to compare Doppler and Forrest classifications in endoscopic diagnosis and therapy. Dig Dis Sci. 1997;42:1370–4. doi: 10.1023/a:1018877602113. [DOI] [PubMed] [Google Scholar]
- 18.Van Leerdam ME, Rauws EAJ, Geraedts AA, et al. The role of endoscopic Doppler US in patients with peptic ulcer bleeding. Gastrointest Endosc. 2003;58:677–84. doi: 10.1016/s0016-5107(03)02033-9. [DOI] [PubMed] [Google Scholar]
- 19.Kohler B, Riemann JF. Endoscopic Doppler Ultrasound. Gastrointest Endosc. 2004;60:493–5. doi: 10.1016/s0016-5107(04)01717-1. [DOI] [PubMed] [Google Scholar]
- 20.Jakobs R, Zoepf T, Schilling D, Siegel EG, Riemann JF. Endoscopic Doppler ultrasound after injection therapy for peptic ulcer hemorrhage. Hepatogastroenterology. 2004;51:1206–9. [PubMed] [Google Scholar]
- 21.Sung JJ, Barkun A, Kuipers EJ, et al. and the Peptic Ulcer Bleed Study Group. Intravenious esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized controlled trial. Ann Int Med. 2009;50:455–64. doi: 10.7326/0003-4819-150-7-200904070-00105. [DOI] [PubMed] [Google Scholar]
- 22.Kirschniak A, Subotova N, Zeiker D, et al. A new over-the-scope clip (OTSC) for treatment of gastrointestinal bleeding, perforations, and fustulas. Surg Endosc. 2011;25:2901–2905. doi: 10.1007/s00464-011-1640-2. [DOI] [PubMed] [Google Scholar]
- 23.Camus M, Jensen DM, Kovacs TOG, et al. Independent Risk Factors of 30 day Outcomes in 1264 Patients with Peptic Ulcer Bleeding in USA - Large Ulcers do Worse. Alim Pharm Ther. 2016;43:1080–1089. doi: 10.1111/apt.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen VK, Wong RC. Endoscopic Doppler ultrasound versus endoscopic stigmata-directed management of acute peptic ulcer hemorrhage: a multi-model cost analysis. Dig Dis Sci. 2007;52:149–160. doi: 10.1007/s10620-006-9506-5. [DOI] [PubMed] [Google Scholar]