Abstract
The epithelial-mesenchymal transition (EMT) is a fundamental characteristic of carcinoma cells. EMT is generally associated with a change in cellular morphology from cobblestone to spindle shape, reduced expression of epithelial markers such as E-cadherin, and enhanced expression of mesenchymal markers such as N-cadherin. This EMT-associated reciprocal expression of E-cadherin and N-cadherin has been called the “cadherin switch”. Downregulation of E-cadherin enables cells to dissociate from colonies while upregulation of N-cadherin is associated with increased invasiveness. The transcription factor Snail1 induces these changes in various epithelial cell lines, including canine MDCK cells and human A431 cells. In the present study, we introduced a Snail1 expression vector into human DLD-1 cells and isolated stable transfectants. These cells showed changes in morphology, reduced expression of epithelial marker E-cadherin and occludin, and elevated invasion and migration. However, neither expression of N-cadherin protein nor its corresponding mRNA was detected. Therefore, elevated N-cadherin expression is not required for invasiveness of the cells.
Abbreviations: APC, adenomatous polyposis coli; EMT, epithelial–mesenchymal transition
Keywords: DLD-1, EMT, Invasion, N-cadherin, Snail
Highlights
-
•
The transcription factor Snail induces colorectal cancer DLD-1 cells to undergo EMT.
-
•
E-cadherin expression was downregulated but N-cadherin expression was not induced.
-
•
Transfected cells showed elevated invasion and migration.
-
•
N-cadherin expression is not required for invasiveness of the cells.
1. Introduction
The transition of epithelial cells to a mesenchymal phenotype (EMT) is a fundamental characteristic of carcinoma cells [1]. A lineage tracing study using genetically engineered mouse models for pancreatic adenocarcinoma demonstrated that EMT of pancreatic epithelial cells leads to their migration into the surrounding stroma and entry into the bloodstream. Importantly, these events were observed before the formation of solid tumors in the mice [2].
During EMT, cells lose their epithelial characteristics, including polarity and specialized cell–cell contacts, while downregulating E-cadherin expression. They instead acquire mesenchymal characteristics by upregulating N-cadherin and fibronectin expression, resulting in weaker adhesion ability that allows them to move away from their epithelial cell community and integrate into surrounding tissue, even at remote locations [3], [4].
Several transcription factors, including the Snail (Snail1)/Slug family, Twist, δEF1/ZEB1, SIP1/ZEB2, and E12/E47 reportedly respond to different microenvironmental stimuli and function as molecular switches in the EMT program [5], [6], [7]. Snail1 is a prominent inducer of EMT and strongly represses E-cadherin expression [5], [6], [7]. Hence, Snail1 expression in cell lines such as MDCK, A431, HT29, and HCT116 induces EMT and its accompanying changes in morphology, E-cadherin downregulation, and N-cadherin upregulation [8], [9], [10], [11].
Cadherins are a class of type I transmembrane proteins that play important roles in cell adhesion by forming adherens junctions. E-cadherin is expressed in epithelial cells and is responsible for the formation and maintenance of epithelial sheets. Downregulating or inhibiting E-cadherin expression thus results in cell dissociation [12]. N-cadherin is expressed mainly in mesenchymal cell types, including nerve tissues, myocytes, and fibroblasts [13]. N-cadherin expression has been reported to enhance invasiveness, cell migration, metastasis, and angiogenesis of a variety of cancers, including those of the bladder, breast, esophagus, and thyroid [14], [15], [16]. Knockdown of N-cadherin expression in the BxPC-3 pancreatic cancer cell line led to decreased tumor size and metastases in an orthotopic animal model [17]. Moreover, monoclonal antibodies against the ectodomain of N-cadherin slowed the growth, invasion, and metastasis of prostate cancer cells in a xenograft model [18]. These studies suggest that N-cadherin plays an important role in cancer metastasis.
In the present study, we show that Snail1 expression in human colon adenocarcinoma DLD-1 cells induces a change in their morphology from cobblestone to spindle shape, and downregulates epithelial markers including E-cadherin and the tight junction component occludin. Furthermore, DLD-1 cells expressing Snail1 showed increased motility and invasiveness. However, we did not detect either N-cadherin protein or its corresponding mRNA in these cells. Thus, contrary to previous observations that increased N-cadherin expression confers invasiveness in cells induced to undergo EMT [14], [15], [16], we have observed that N-cadherin expression is not necessary for DLD-1 cells to acquire invasive properties.
2. Materials and methods
2.1. Cell lines and transfection
DLD-1, a human colorectal adenocarcinoma cell line (provided by Dr. Shintaro T. Suzuki, Kwansei Gakuin University), and A431, a human squamous cell carcinoma cell line (provided by Dr. Kiyotoshi Sekiguchi, Osaka University), were grown as previously described [9]. Cells were transfected using the calcium phosphate method with 10 μg of either plasmid DNA containing an HA-tagged human Snail1 construct (pC-Snail-HA) or with control vector containing a Discosoma sp. red fluorescent protein (DsRed) as previously described [9], [12]. At least three independent clones were selected for each construct to ensure that any observed effects were not due to phenotypic variability introduced by clonal selection. A human fibrosarcoma cell line HT1080 (provided by Dr. Kiyotoshi Sekiguch, Osaka University) was used as a positive control cell expressing mesenchymal markers. SW480 and HCT116, colon cancer cell lines, were provided by Dr. Akira Kikuchi, Osaka University The mammalian expression vectors containing HA-tagged N-cadherin (pC-NcadHA) were previously described [12].
2.2. Antibodies
Mouse monoclonal antibodies (mAbs) against E-cadherin, N-cadherin, p120, Laminin5, and fibronectin were purchased from BD Biosciences (Lexington, KY, USA). Mouse mAb against vimentin and rabbit polyclonal antibody against occludin were obtained from Zymed Laboratories (South San Francisco, CA, USA). Mouse mAb against Snail was purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse mAb against vinculin was purchased from Sigma-Aldrich (St Louis, MO, USA). Rat mAb against HA was purchased from Roche Applied Science (Mannheim, Germany). All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
2.3. Immunofluorescence staining
For immunofluorescence staining, cells were grown on coverslips, fixed with 3% paraformaldehyde in PBS for 20 min at room temperature, and subsequently permeabilized with 0.1% Triton X-100. Coverslips with fixed cells were immunostained with primary and secondary antibodies as previously described [9]. Immunostained cells were analyzed using an Olympus fluorescence microscope (Tokyo, Japan) or a confocal laser scanning microscope (LSM700; Zeiss).
2.4. Immunoblotting
For immunoblot determination of protein levels, proteins were separated on electrophoresis on 8% polyacrylamide gels, and transferred to nitrocellulose membranes. After blocking, membranes were incubated with specific primary antibodies before probing with peroxidase-conjugated secondary antibodies. After washing with PBS containing 0.1% Tween-20, blots were visualized by enhanced chemiluminescence (ECL; Amersham International, Little Chalfont, UK) as previously described [9]. Each experiments were performed at least three times.
2.5. Semi-quantitative RT-PCR
Total RNA was extracted and reverse transcribed as described previously [10]. The resulting cDNA was used as a template for PCR; PCR conditions were optimized for each primer pair as previously described [19]. The following primer combinations were used: N-cadherin (CDH2), sense (5′-CTCTTCTGAGCATGCCAAGT-3′) and antisense (5′-GAGGGATGACCCAGTCTCTC-3′); vimentin (VIM), sense (5′-AATGGCTCGTCACCTTCGTGAA-3′) and antisense (5′-CAGATTAGTTTCCCTCAGGTTCA-3′); fibronectin (FN1), sense (5′-GTGCCTGGGCAACGGA -3′) and antisense (5′- CCCGACCCTGACCGAAG -3′); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), sense (5′- GCATCCTGGGCTACACTG -3′) and antisense (5′- GTGAGGAGGGGAGATTCAG -3′). Each experiments were performed at least three times.
2.6. Dissociation assay
Cells were washed with PBS before incubation for 2 h in DMEM supplemented with 10% FCS containing 2.4 U/ml of dispase (Gibco) as previously described [12]. Detached cells were observed with a microscope without adding mechanical stress.
2.7. Migration assay
Migration was measured using Transwell® with 8.0 µm pore polycarbonate membrane insert (Corning, NY, USA). The lower chambers were filled with DME medium containing 5% FBS as a chemoattractant and serum-free DME medium as a control. The upper insert chambers were seeded with 4×104 cells in serum-free DME medium. After incubation for 4 h at 37 °C, the cells were fixed, stained, and counted.
2.8. Invasion assay
Cell invasion was measured using BioCoat MatriGel Invasion Chambers (BD Biosciences) according to the manufacturer's instructions. The lower chambers were filled with either DME medium containing 5% FBS as a chemoattractant or serum-free DME medium as a control. The upper insert chambers were seeded with 2.5×104 cells in serum-free DME medium. After incubation for 22 h at 37 °C, the cells were fixed, stained, and counted.
2.9. Gene expression microarray and data analysis
Total RNA was prepared from DsRed+, and Snail+ DLD-1 cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and purified as previously described [12]. cRNA was amplified and labeled using a Quick Amp Labeling Kit (Agilent Technologies, Santa Clara, CA, USA) and hybridized to a 44 K Agilent 60-mer oligomicroarray (Human Oligo Microarray Kit). Hybridized microarray slides were scanned using an Agilent scanner. The relative hybridization intensities and background hybridization values were calculated using Agilent Feature Extraction Software (version 9.5.1.1). Microarray data analysis was supported by Cell Innovator (Fukuoka, Japan).
2.10. Statistical analysis
Statistical analysis was performed by Student's t test. Differences were considered to be significant at p<0.05.
3. Results
3.1. Ectopic Snail1 expression in DLD-1 cells induces morphological changes and cell dissociation
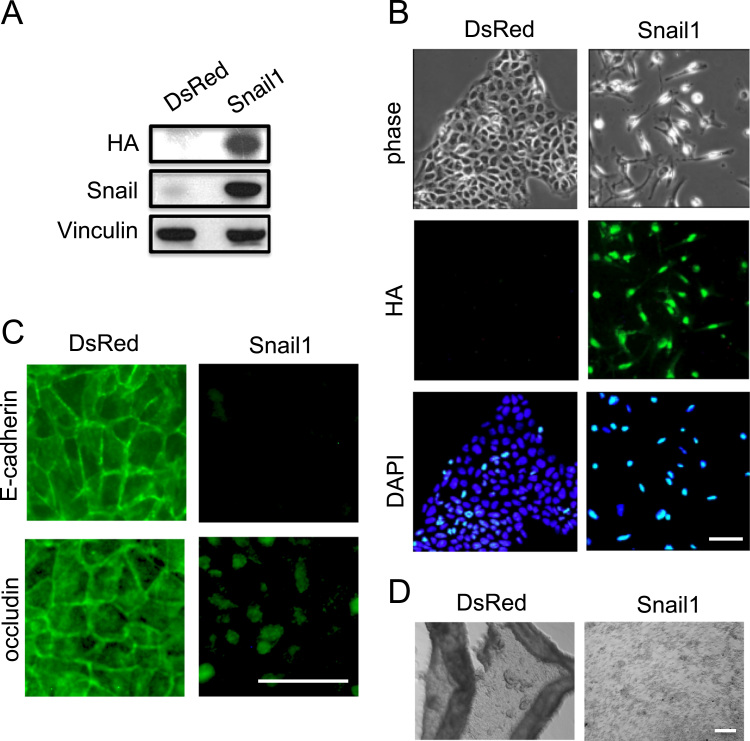
DLD-1 cells, a cell line derived from human colorectal cancer, display epithelial properties such as a cobblestone cell shape. We introduced a control vector encoding DsRed or an expression vector encoding HA-tagged Snail1 protein into DLD-1 cells and isolated stable transfectants, designated DsRed+ or Snail+ cells, respectively. Immunoblot analysis with anti-HA and Snail antibodies revealed that Snail+ cells were positive for the exogenous Snail1 protein but DsRed+ cells were not (Fig. 1A). Like the parental DLD-1 cells, DsRed+ cells show epithelial morphology (Fig. 1B). Snail1 expression was accompanied by a change in cell morphology from epithelial to mesenchymal; cells expressing Snail1 became spindle-shaped whereas control DsRed+ cells remained cobblestone-shaped (Fig. 1B). Snail tagged with HA epitope at its C-terminus was detected in the nucleus by anti-HA immunofluorescence staining (Fig. 1B). Thus, consistent with previous experiments using MDCK or A431 cells [7], [8], ectopic expression of Snail1 induces morphological changes characteristic of EMT. Immunofluorescence staining with anti-E-cadherin or anti-occludin antibodies revealed that membrane staining of E-cadherin or occludin was lost in Snail+ cells (Fig. 1C).
Fig. 1.
Ectopic expression of Snail1 in DLD-1 cells induces morphological changes, downregulation of E-cadherin expression, and cell dissociation. (A) Immunoblot determination of Snail1 protein levels in Snail+ and control DsRed+ cells using either anti-HA or anti-Snail. Anti-vinculin was used as a loading control. (B) Phase contrast micrographs showing morphological (epithelial to fibroblastic) changes in response to Snail1 expression. Immunofluorescence staining with anti-HA and DAPI was carried out to determine the nuclear localization of Snail-HA. Bar, 25 µm. (C) Immunostaining with either anti-E-cadherin or anti-occludin of Snail+ and DsRed+ control cells. Bar, 50 µm. (D) DsRed+ cells detach from the dish as cell sheets while Snail+ cells become either single cells or form small aggregates. Bar, 100 µm.
Cells undergoing EMT show a loss of cell–cell adhesion. Cell dissociation assays demonstrated significant differences in the cell–cell adhesion of DsRed+ cells and Snail+ cells (Fig. 1D). Since the primary cell–cell adhesion molecule of DLD-1 cells is E-cadherin, decreased cell–cell adhesion of Snail+ cells is consistent with the absence of staining of these cells by E-cadherin-specific antibodies (Fig. 1C).
3.2. Ectopic Snail1 expression in DLD-1 cells changes expression levels of epithelial and mesenchymal markers but does not induce N-cadherin expression
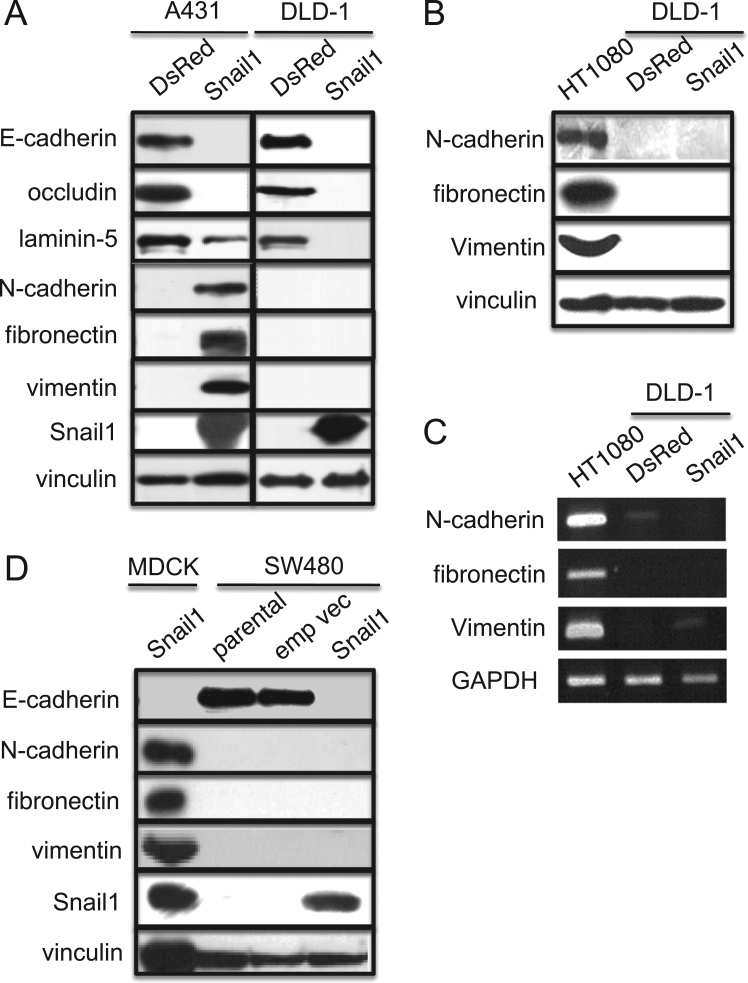
Immunoblot analysis revealed that Snail1 expression in A431 cells induces the downregulation of E-cadherin and occludin expression, and the upregulation of N-cadherin and fibronectin expression (Fig. 2A). These changes are characteristic of cells undergoing EMT [9], [10]. The same analysis of DLD-1 cells revealed the downregulation of E-cadherin and occludin, but the upregulation of N-cadherin and fibronectin was not observed (Fig. 2A).
Fig. 2.
Snail1 expression in DLD-1 cells induces downregulation of epithelial markers but does not induce upregulation of mesenchymal markers, which is distinct from A431 cells. (A) Immunoblot determination of levels of epithelial marker proteins E-cadherin, occludin, and laminin5, and mesenchymal marker proteins N-cadherin, fibronectin, and vimentin, in A431 cells. Anti-vinculin was used as a loading control. Immunoblotting for the same markers was carried out to determine their levels in DLD-1 cells. (B) Immunoblot detection of N-cadherin, fibronectin, and vimentin in HT1080 human fibrosarcoma cells but not in control (DsRed) cells and Snail+ cells. Anti-vinculin was used as a loading control. (C) Semi-quantitative RT-PCR determination of mRNAs encoding N-cadherin, fibronectin, and vimentin in control (DsRed) cells and Snail+ cells. GAPDH mRNA was used as an internal control. (D) Immunoblot determination of levels of E-cadherin, N-cadherin, fibronectin, vimentin, and Snail in SW480 cells. Parental SW480 cells (parental), stable transfectant of SW480 cells transfected with an empty vector control (emp vec) or Snail expression vector (Snail) were analyzed. Anti-vinculin was used as a loading control. Immunoblotting for the same markers was carried out to determine their levels in MDCK cells expressing Snail.
Using an Agilent Whole Human Genome microarray, we compared the gene expression profiles of DLD-1 cells expressing either Snail1 or DsRed. The levels of mRNAs encoding E-cadherin (CDH1), occludin (OCLN), and the three subunits of laminin 5 (LAMA3, LAMB3, and LAMC2) were decreased in Snail+ cells (Table 1). The level of mRNA encoding an α subunit of type IV collagen (COL4A2), a component of the epithelial cell basement membrane, was also decreased. In contrast to the decreased expression of epithelial cell markers, the expression levels of mRNAs encoding mesenchymal markers integrin α1 subunit (ITGA1), type I collagen (COL1A1), ZEB1 (ZEB1), vimentin (VIM), LEF1 (LEF1), and β-catenin (CTNNB1) [20] were increased (Table 1). Surprisingly, the mRNA expression levels for N-cadherin (CDH2), and fibronectin (FN1), however, did not change greatly in Snail+ cells (Table 1), which agrees with immunoblot analysis of the respective protein levels. We used semi-quantitative RT-PCR to verify our microarray findings. The mRNA expression levels of N-cadherin and fibronectin did not increased in Snail+(Fig. 2B). Although our microarray results indicated a significant increase in VIM mRNA, our RT-PCR results did not. The latter results are consistent with our inability to detect vimentin by immunoblotting in DLD-1 cells (Fig., 2A). It is unclear why our RT-PCR and microarray results do not agree but differences in the two methodologies could be reason for the discrepancy.
Table 1.
Changes in relative expression levels of selected marker genes following Snail1 expression in DLD-1 cells.
| Gene symbol | GenBank Accession | –Snail1→+Snail1 |
|---|---|---|
| CDH1 | NM_004360 | 0.001 |
| OCLN | NM_002538 | 0.078 |
| LAMA3 | NM_198129 | 0.067 |
| LAMB3 | NM_001017402 | 0.026 |
| LAMC2 | NM_005562 | 0.139 |
| COL4A2 | NM_001846 | 0.226 |
| ITGA1 | NM_181501 | 534 |
| COL1A2 | NM_000089 | 463 |
| ZEB1 | NM_001128128 | 69.1 |
| VIM | NM_003380 | 27.5 |
| LEF1 | NM_016269 | 3.83 |
| CTNNB1 | NM_001098210 | 4.20 |
| CDH2 | NM_001792 | 0.24 |
| CDH11 | NM_001797 | 0.99 |
| FN1 | NM_054034 | 1.18 |
| MMP-2 | NM_004530 | 3.39 |
| MMP-7 | NM_002423 | 0.98 |
| MMP-9 | NM_004994 | 1.07 |
Gene expression profiles of Snail1+ cells were compared using Agilent Whole Human Genome microarrays. Data are presented as the intensities of signals in Snail1+ cells relative to the corresponding signals from control DsRed+ cells.
CDH1, E-cadherin; OCLN, occludin; LAMA3, laminin alpha 3; LAMB3, laminin beta3; LAMC2, laminin gamma2; COL4A2, collagen type IV alpha 2; ITGA1, integrin, alpha 1; COL1A2, collagen, type I, alpha 2; VIM, vimentin; LEF1, lymphoid enhancer-binding factor 1; CTNNB1, catenin (cadherin-associated protein), beta 1; CDH2, N-cadherin; CDH11; cadherin-11; FN1, fibronectin; MMP-2, matrix metalloproteinase 2; MMP-7, matrix metalloproteinase 7; MMP-9, matrix metalloproteinase 9.
To determine the absent upregulation of N-cadherin and fibronectin in Snail+ cells is specific for DLD-1 cells, we established Snail+ cells using SW480 cells and HCT116 cells, another colon cancer cells. Immunoblot analysis of revealed that Snail1 expression SW480 cell (Fig. 2D) and HCT116 cells (not shown) indeed downregulated E-cadherin expression but did not upregulate N-cadherin and fibronectin expression.
3.3. Ectopic Snai1l expression in DLD-1 cells enhances motility and invasiveness
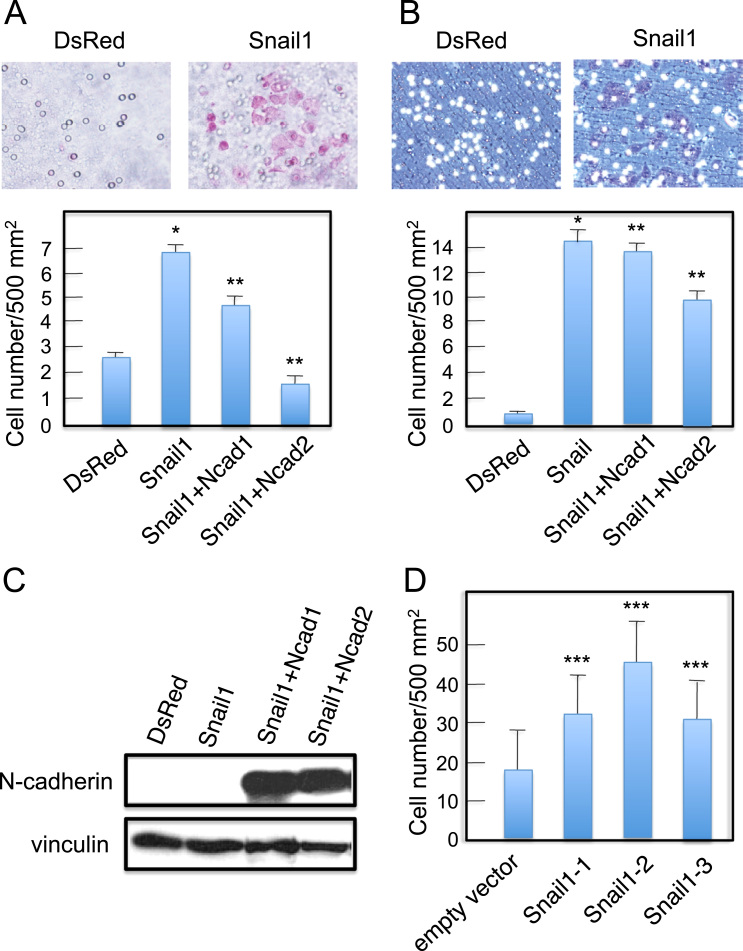
Cells undergoing EMT exhibit increased motility and invasiveness. We used cell migration assays to determine differences in the directional migration and motility of Snail+ and control DsRed+ cells. We observed that Snail+ cells migrated faster than control DsRed+ cells (Fig. 3A). Furthermore, we used a Matrigel invasion assay to determine that Snail+ cells had an increased invasive capacity compared with the control (Fig. 3B). Together, these results indicate that Snail1 expression in DLD-1 cells enhances their motility and invasive properties without N-cadherin expression. Although parental SW480 cells showed 40 times higher invasion compared with parental DLD-1 cells, we could detect increase in invasion of Snail+ SW480 cells (Fig. 3D).
Fig. 3.
Snail1 expression in colon cancer cells increases their motility and invasiveness but additional N-cadherin expression does not increase these activities. (A) Migration assay to determine motility of Snail+ DLD-1 cells and Snail+ DLD-1 cells expressing N-cadherin (Snail+Ncad cells) compared with control DsRed+ DLD-1 cells. Values represent the mean±S.E.; n=at least 3 times. (B) Invasion assay using Matrigel to determine invasiveness of Snail+ DLD-1 cells and Snail+DLD-1 cells expressing N-cadherin (Snail+Ncad cells) compared with DsRed+ DLD-1 cells. Values represent the mean±S.E.; n=at least 3 times. (C) Immunoblot detection of N-cadherin in stable N-cadherin transfectants of Snail+ DLD-1 cells. Vinculin was used as a loading control. (D) Invasion assay using Matrigel to determine invasiveness of Snail+ SW480 cells compared with control (transfected with an empty vector) SW480 cells. Values represent the mean±S.E.; n=at least 3 times. *p<0.01 compared with control (DsRed). **p<0.05 compared with Snail1. ***p<0.01 compared with control (empty vector).
To determine the impact of N-cadherin expression on Snail-induced EMT in DLD-1 cells, we established Snail+ DLD-1 cells expressing N-cadherin (Snail+Ncad cells) (Fig. 3C). Snail+Ncad cells, however, did not show significant increase in motility and invasiveness (Fig. 3A and B).
4. Discussion
The cadherin switch, which involves switching from E-cadherin expression to N-cadherin expression, is associated with the epithelial-mesenchymal transition (EMT). The cadherin switch has been implicated in the transition from benign tumors to invasive, malignant cancer, and in the subsequent metastatic dissemination of tumor cells [21]. Ectopic expression of N-cadherin increases tumor cell motility, implicating cadherin switching in the regulation of cell behavior [16], [22]. We previously showed that upregulation of N-cadherin accompanies the downregulation of E-cadherin during EMT induced by either Snail1 expression [9], [10] or LEF1 expression [23]. Contrary to these observations, we found in the present study that Snail1 expression in DLD-1 cells does not induce N-cadherin expression. Nevertheless, Snail1 expression in DLD-1 cells does induce other changes that are characteristic of cells that have undergone EMT, including morphological changes, downregulation of epithelial markers, upregulation of mesenchymal markers, and increased invasiveness. Therefore, we believe that Snail1 expression in DLD-1 cells induces them to undergo EMT.
It has been shown that N-cadherin promotes invasiveness by complex formation with fibroblast growth factor receptor (FGFR) [24] and by enhancing FGF-stimulated ERK phosphorylation leading to MMP-9 gene expression [24], [25]. In this study, we found that Snail1 expression in DLD-1 cells promotes invasiveness without N-cadherin expression. Consistent with these observations, our microarray analysis did not detect an increase in the expression of matrix metalloproteinases, including MMP-9, in Snail+ cells (Table 1). Cadherin-11 is another member of the cadherin family. Cadherin-11 is expressed in some invasive cancer cells [26] and its expression is upregulated upon EMT induction [27]. Like N-cadherin, cadherin-11 expression has been shown to increase the invasiveness of cells [28]. Microarray analysis, however, revealed no increased expression of cadherin-11 in Snail+ cells (Table 1) or other cadherins (data not shown). Therefore, some cadherin-independent mechanism, or mechanisms, may be operating in Snail+ DLD-1 cells.
DLD-1 is a human colon cancer cell line that expresses a truncated adenomatous polyposis coli (APC) protein [29]. APC is a tumor suppressor gene that plays a critical role in cell growth by regulating expression of the Wnt pathway protein, β-catenin. Truncated forms of APC fail to downregulate β-catenin expression, hence β-catenin levels in DLD-1 cells are elevated. When in the nucleus, β-catenin binds Tcf/Lef transcription factors and recruits the transcriptional coactivator CREB-binding protein (CBP)/p300 to the site of activation of target genes [30]. Recently, CBP was reported to be the critical factor in Snail-mediated target gene transactivation [31]. Therefore, it is plausible that the excess β-catenin present in DLD-1 cells depletes CBP from Snail1, rendering Snail1 unable to transactivate transcription of N-cadherin and fibronectin. Our preliminary transfection experiments with a CBP expression vector did not reveal any increase in the levels of mRNAs encoding these proteins in the presence of excess CBP (data not shown).
In the present study we showed that DLD-1 cells stably expressing Snail did not upregulate N-cadherin and fibronectin expression. Despite absent upregulation of these molecules, the cells showed increased invasiveness. The clinical relevance of the findings is not known as yet. Contrary to our observation that Snail1 expression does not up-regulate fibronectin in DLD-1 and SW-480 cells, it has been reported that the expression of fibronectin in these cells was increased after transfection with Snail1 expression constructs [32], [33]. We do not know the reason for the discrepancy of the results. The different methodology could be one of the reasons. In our experiments we isolated stable Snail1 transfectants and used these cells. Wang et al. [32] used cells maintained in complete medium for 24 h after transfection. The level of Snail1 protein in stable transfectants is low compared with transient transfectants and may not be sufficient for induction of fibronectin. In the present study, we used SW-480 cells expressing high levels of E-cadherin, whereas Solanas et al. [33] used a cell population expressing low levels of E-cadherin. This also could be another reason for the discrepancy. Overexpression of PRL-3, a metastasis-associated phosphatase, in DLD-1 cells induces up-regulation of fibronectin and Snail1, and down-regulation of E-cadherin [34]. It is unclear whether upregulation of fibronectin expression is mediated by Snail1. Therefore, as discussed by the authors, up-regulation of Snail1, together with other transcriptional events, leads to E-cadherin down-regulation and fibronectin upregulation.
Although microarray data (Table 1) and RT-PCR assays (Fig. 2C) revealed downregulation of N-cadherin expression in Snail-overexpressing DLD-1 cells, immunoblot analysis did not detect N-cadherin expression (Fig. 2A). The former two experiments detect N-cadherin mRNA but the latter detects N-cadherin protein. This could be the reason for the conflicting results.
N-cadherin overexpression in Snail1-positive cells inhibits migration and invasion (Figs. 3A and 3B). In addition, a difference between clones was observed. Additional analyses are necessary to answer these raised questions.
Acknowledgements
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M.O. We thank Kumiko Sato for secretarial assistance. We also thank the Joint Research Laboratory at the Kagoshima University Graduate School of Medical and Dental Sciences for use of their facilities.
Footnotes
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2016.08.017.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Rhim A.D., Mirek E.T., Aiello N.M., Maitra A. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisberg M., Neilson E.G. Biomarkers for epithelial- mesenchymal transitions. J. Clin. Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Lamouille S., Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cano A., Pérez-Moreno M.A., Rodrigo I., Locascio A., Blanco M.J. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell. Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 6.Batlle E., Sancho E., Francí C., Domínguez D., Monfar M. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell. Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 7.Barrallo-Gimeno A., Nieto M.A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 8.Qian X., Anzovino A., Kim S., Suyama K. N-cadherin/FGFR promotes metastasis through epithelial-to- mesenchymal transition and stem/progenitor cell-like properties. Oncogene. 2014;33:3411–3421. doi: 10.1038/onc.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkubo T., Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J. Cell Sci. 2003;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi M., Okubo T., Miyashita Y. Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. J. Biol. Chem. 2008;283:23514–23523. doi: 10.1074/jbc.M801125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan F., Samuel S., Evans K.W., Lu J. Overexpression of Snail induces epithelial – mesenchymal transition and a cancer stem cell– like phenotype in human colorectal cancer cells. Cancer Med. 2012;1:5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozawa M., Kobayashi W. Cadherin cytoplasmic domains inhibit the cell surface localization of endogenous E-cadherin, blocking desmosome and tight junction formation and inducing cell dissociation. PLoS One. 2014;9:e105313. doi: 10.1371/journal.pone.0105313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatta K., Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- 14.Giroldi L.A., Bringuier P.P., Shimazui T., Jansen K. Changes in cadherin-catenin complexes in the progression of human bladder carcinoma. Int. J. Cancer. 1999;82:70–76. doi: 10.1002/(sici)1097-0215(19990702)82:1<70::aid-ijc13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Rieger-Christ K.M., Pezza J.A., Dugan J.M., Braasch J.W. Disparate E-cadherin mutations in LCIS and associated invasive breast carcinomas. Mol. Pathol. 2001;54:91–97. doi: 10.1136/mp.54.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazan R.B., Phillips G.R., Qiao R.F., Norton L. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell. Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shintani Y., Hollingsworth M.A., Wheelock M.J., Johnson K.R. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745–11753. doi: 10.1158/0008-5472.CAN-06-2322. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka H., Kono E., Tran C.P., Miyazaki H. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat. Med. 2010;16:1414–1420. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kume K., Haraguchi M., Hijioka H., Ishida T. The transcription factor Snail enhanced the degradation of E-cadherin and desmoglein 2 in oral squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 2013;430:889–894. doi: 10.1016/j.bbrc.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 20.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheelock M.J., Shintani Y., Maeda M., Fukumoto Y. Cadherin switching. J. Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 22.Nieman M.T., Prudoff R.S., Johnson K.R., Wheelock M.J. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi W., Ozawa M. The transcription factor LEF-1 induces an epithelial-mesenchymal transition in MDCK cells independent of β-catenin. Biochem. Biophys. Res. Commun. 2013;442:133–138. doi: 10.1016/j.bbrc.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Suyama K., Shapiro I., Guttman M., Hazan R.B. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–314. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 25.Hulit J., Suyama K., Chung S., Keren R. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res. 2007;67:3106–3116. doi: 10.1158/0008-5472.CAN-06-3401. [DOI] [PubMed] [Google Scholar]
- 26.Pishvaian M.J., Feltes C.M., Thompson P., Bussemakers M.J. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59 947-52. [PubMed] [Google Scholar]
- 27.Sarrió D., Rodriguez-Pinilla S.M., Hardisson D., Cano A. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 28.Huang C.F., Lira C., Chu K., Bilen M.A. Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res. 2010;70:4580–4589. doi: 10.1158/0008-5472.CAN-09-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishida S., Yamamoto H., Ikeda S., Kishida M. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J. Biol. Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 30.Hecht A., Vleminckx K., Stemmler M.P., van Roy F., Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu D.S., Wang H.J., Tai S.K., Chou C.H. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell. 2014;26:534–548. doi: 10.1016/j.ccell.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Quah S.Y., Dong J.M., Manser E. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:2922–2926. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- 33.Solanas G., Porta-de-la-Riva M., Agustí C., Casagolda D., Sánchez-Aguilera F. E-cadherin controls beta-catenin and NF-kappaB transcriptional activity in mesenchymal gene expression. J. Cell Sci. 2008;121:2224–2234. doi: 10.1242/jcs.021667. [DOI] [PubMed] [Google Scholar]
- 34.Kwon C.H., Park H.J., Choi J.H., Lee J.R. Snail and serpinA1 promote tumor progression and predict prognosis in colorectal cancer. Oncotarget. 2015;6:20312–20326. doi: 10.18632/oncotarget.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material