Abstract
Insects rely on their sense of smell to guide a wide range of behaviors that are critical for their survival, such as food-seeking, predator avoidance, oviposition, and mating. Myriad chemicals of varying volatilities have been identified as natural odorants that activate insect Olfactory Receptor Neurons (ORNs). However, studying the olfactory responses to low-volatility odorants has been hampered by an inability to effectively present such stimuli using conventional odor-delivery methods. Here, we describe a procedure that permits the effective presentation of low-volatility odorants for in vivo Single-Sensillum Recording (SSR). By minimizing the distance between the odor source and the target tissue, this method allows for the application of biologically salient but hitherto inaccessible odorants, including palmitoleic acid, a stimulatory pheromone with a demonstrated effect on ORNs involved in courtship and mating behavior1. Our procedure thus affords a new avenue to assay a host of low-volatility odorants for the study of insect olfaction and pheromone communication.
Keywords: Neuroscience, Issue 125, Single-sensillum Recording, Drosophila, trichoid sensillum, long-chain fatty acid, palmitoleic acid, Or47b ORNs
Introduction
Drosophila ORNs respond to a vast number of odorants, with widely ranging carbon chain lengths and a variety of functional groups, including esters, alcohols, ketones, lactones, aldehydes, terpenes, organic acids, amines, sulfur compounds, heterocyclics, and aromatics2,3. Odorants varied in their physicochemical features can have markedly different volatilities, indicated by the vapor pressure of the compound. Notably, biologically relevant odorants for Drosophila melanogaster differ tremendously in their volatility. For example, Ir92a ORNs respond to ammonia4, which is highly volatile, with a vapor pressure of 6,432 mmHg at 20 °C. In contrast, Or67d ORNs respond to a male pheromone, cis-vaccenyl acetate (cVA)5,6, the vapor pressure of which is 43 mmHg at 20 °C.
Studying the olfactory response to odorants of low volatility is particularly challenging with conventional odor-delivery methods, in which odorants are delivered via a carrier air stream over a relatively long distance (i.e. several centimeters). As such, the reported olfactory responses to a given low-volatility odorant can vary greatly, depending on the design of the odor-delivery system. For example, the reported response of Or67d ORNs to a high dose of cVA ranges from ~407 - >200 spikes/s6. Moreover, the ineffective delivery of cVA with conventional delivery methods is likely attributed to false-negative results, leading to the interpretation that cVA by itself is not sufficient to activate Or67d ORNs8. This interpretation was later challenged by another study using a close-range odor-delivery method9. It is therefore imperative to develop a robust odor-delivery system for the effective presentation of odorants of low volatility.
Recently, we identified several long-chain cuticular fatty acids as ligands for Or47b ORNs. They are housed in the type 4 Antennal Trichoid Sensillum (at4). Among the long-chain fatty acid odorants, we found that palmitoleic acid functions as an aphrodisiac pheromone that promotes male courtship by activating Or47b ORNs1. However, in another study using a conventional odor-delivery method, methyl laurate was shown to elicit responses from Or47b ORNs, while palmitoleic acid evoked no response when presented from the same distance10. Compared to cVA, long-chain fatty acids are even less volatile, with vapor pressures less than 0.001 mmHg at 25 °C11. The inherently low volatility of long-chain fatty acid odorants, which precludes efficient presentation to the antenna via conventional odor-delivery systems, likely accounted for the false-negative results10. This inconsistency highlights the inadequacy of conventional odor-delivery systems in presenting low-volatility odorants. It was previously shown that the effective delivery of fly cuticular odors requires close proximity between the odor source and the target tissue6. Thus, to fully characterize the effects of biologically active pheromones while mimicking the distance from which they are likely encountered by fruit flies in nature12,13, we agreed that minimal distance must be accorded high priority in our procedure.
Our method holds further advantages, including compatibility with standard electrophysiology rigs and techniques. Pre-existing rig setups require minimal modification to accommodate this protocol, and most SSR steps require only minor adjustments. This renders our technique readily accessible to researchers experienced in SSR. Furthermore, our technique allows for the presentation of low-volatility odorants with sharp onset and offset, correlating stimulus delivery with neuronal response. Finally, the hardware layout facilitates rapid exchanges between odorant cartridges, expediting data collection over a desired dosage range.
We begin by reviewing the preparation of reference and recording electrodes, Adult Hemolymph-Like (AHL) solution, odorant delivery cartridges, and the corresponding olfactometer. We then discuss the preparation of the palmitoleic acid odorant solutions, followed by the preparation of the fly for recording. We proceed to consider the criteria for selecting a trichoid sensillum to record and more closely examine the positioning of the odorant cartridge before presenting representative data acquired using this method. Finally, we conclude by exploring useful applications of this technique, some encountered issues, and their solutions.
Protocol
1. Preparation of the Hardware for at4 Recording
Use a pipette puller instrument to prepare electrodes with aluminosilicate glass capillaries (O.D 1.0 mm, I.D. 0.64 mm). Blunt the tip of the reference electrode slightly with a pair of fine forceps to facilitate insertion into the clypeus of the fly (i.e. a rounded plate at the front of the fly head, above the mouthparts). NOTE: 7-day-old WT males (Berlin) were used in this study. Use AHL saline solution14 as the electrolyte for both electrodes.
Prepare 1 L of AHL by mixing 900 mL of distilled water with 6.312 g of NaCl, 0.373 g of KCl, 0.337 g of NaHCO3, 0.1120 g of NaH2PO4, 1.892 g of Trehalose ּ2H2O, 3.423 g of sucrose, 1.192 g of HEPES, and 8.2 mL of 1M MgCl2. Using distilled water, bring the total volume up to 1 L. Bring the pH to 7.4 using 1 N NaOH and sterilize the solution with a vacuum-driven filter system. For long-term storage, keep the AHL aliquots at 4 °C. NOTE: Consistent deliveries of palmitoleic acid are contingent upon uniformity between cartridges. It is critical that each cartridge is assembled in a reproducible manner.
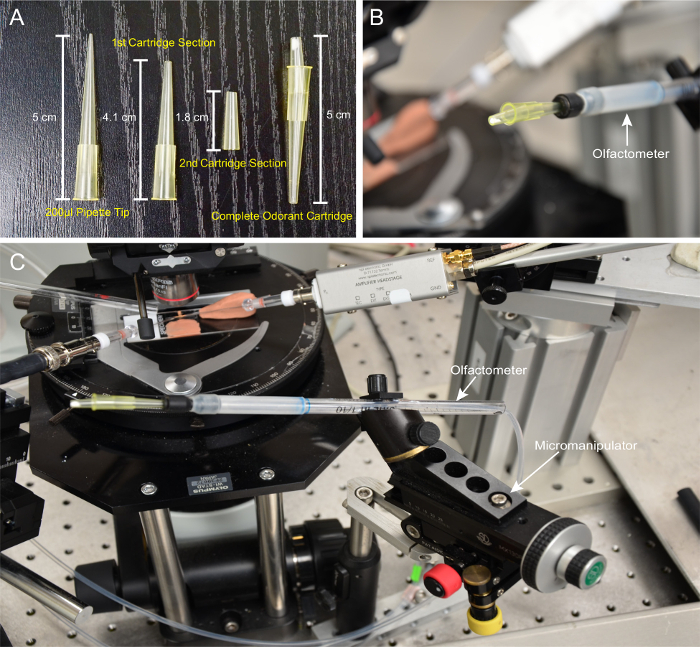
Using a razor blade, remove 0.9 cm from the tip of a 200 µL pipette tip to create the first cartridge section, measuring 4.1 cm; refer to the dimensions detailed in Figure 1A. Use another 200 µL pipette tip and remove 1.7 cm and 1.5 cm from the tip and the base, respectively, to create the second cartridge section, measuring 1.8 cm (Figure 1A). Use a ruler to ensure reproducibility.
Use a ⅛" hole puncher to cut discs from filter paper.
Use forceps to place a filter paper disc at the tip of the second cartridge section. Visually confirm that there is an opening in the cartridge tip through which air can pass.
Attach the first and second cartridge sections together, as shown in Figure 1A. Angle the second cartridge section downward to facilitate square aiming at the prep (Figure 1B).
Connect the cartridge with the odor-delivery tube, which is mounted on a micromanipulator. NOTE: This design allows the cartridge to be swiveled outward to facilitate exchange (Figure 1C).
Set the constant humidified airflow to 2 L/min in one mass controller and the odorant flow to 500 mL/min in another mass controller.
Using the software (see the Table of Materials), program the procedure to administer a 500 ms odor puff.
2. Preparation of Palmitoleic Acid Odorant Solutions for Delivery
NOTE: Or47b ORNs respond to both cis- and trans-palmitoleic acid. As palmitoleic acid is unstable at RT, stocks are stored at -20 °C and used within a month upon opening. Ethanol is the solvent of choice for palmitoleic acid.
Use a vortex mixer to thoroughly mix 10 µL of cis- or trans-palmitoleic acid stocks or dilutions with 90 µL of 100% ethanol for ten-fold serial dilutions in 1.7 mL microtubes. Prepare fresh palmitoleic acid dilutions daily prior to experiments and use within a day. NOTE: For odorants that are not soluble in ethanol, a glass vial is recommended for preparing odor dilutions with other types of organic solvents.
Using a P10 micropipette, apply 5 µL of cis-palmitoleic acid solutions of the desired dilutions to the filter paper in each corresponding cartridge. NOTE: The highest dosage (10-1) contains 450 µg of the compound. For trans-palmitoleic acid solutions, apply 4.5 µL instead so that the highest dosage (10-1) also contains 450 µg of the compound.
To completely evaporate the solvent, place the palmitoleic acid cartridges in a vacuum desiccator for 1 h at RT and 7.59 mmHg of pressure. NOTE: The cartridges can be used for up to 4 h at RT.
3. Preparation of Drosophila for Ready Access to the at4 Sensilla for In Vivo Electrophysiological Recordings
NOTE: WT flies (Berlin) are reared in standard cornmeal medium at 25°C in a 12:12 light-dark cycle. Upon eclosion, flies are separated by sex into groups of ten, whereby they are group-housed until 7 d of age. Or47b ORNs in both male and female flies respond to palmitoleic acid. For simplicity, only male flies are examined in the current study.
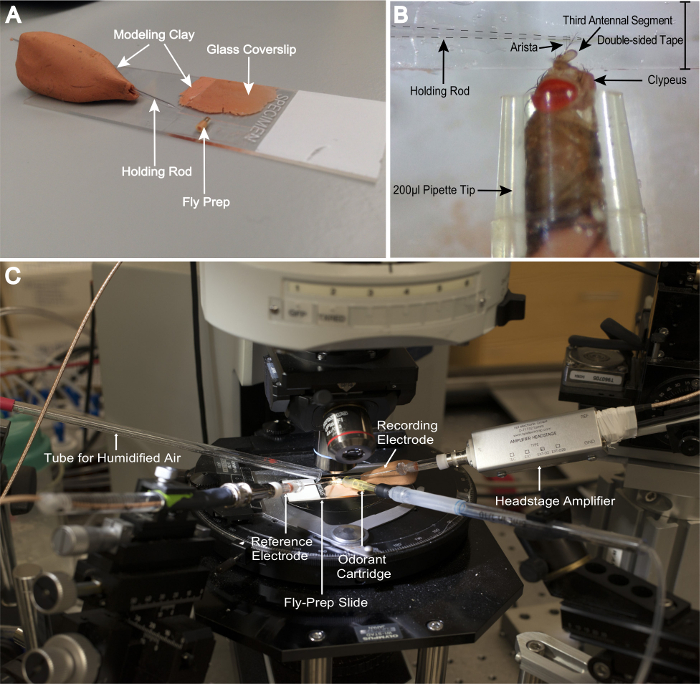
Assemble a fly-prep slide: on a glass slide, place a glass coverslip (18 x 18 mm2) on a small amount of modeling clay, forming an ~3° angle with the glass slide. Place double-sided tape on the inner edge of the coverslip and on the area of the slide immediately below. Replace with fresh tape for every day of recording (Figure 2A).
Use a fly aspirator15 to collect the fly of interest in the tubing and then fit a 200-µL pipette tip over the end of the tubing. Simultaneously flick the tube forward while blowing air into the tube to push the fly to the end of the pipette tip. Use a razor blade to cut just below the body of the fly and 2 heads' lengths above the fly.
Tamp the bottom of the pipette tip with modeling clay, pushing the fly upward until both the antennae and the clypeus are exposed (Figure 2B). To avoid killing the fly, add only enough clay to expose the antennae and aristae, as this prevents the fly's abdomen from being crushed. Furthermore, add clay slowly and gently to prevent any sudden constriction. Confirm that the fly is alive by checking for antennal or proboscis movement.
Use forceps to maneuver the pipette tip that houses the fly. Orient the head so that the clypeus is facing to the right of the observer. Adjust the prep along the coverslip using fine forceps until the lateral side of the antenna lies against the taped coverslip surface (Figure 2B).
Place a holding rod on the arista to secure the antenna to the double-sided tape to prevent movement (Figure 2B). NOTE: The holding rod is pulled from a borosilicate glass capillary with a pipette puller and held in position with modeling clay (Figure 2A).
Place the prep on the stage of the rig (Figure 2C). Using the microscope, confirm that the trichoids are visible along the distal-lateral edge of the third segment of the antenna. NOTE: Ideally, the sensilla should be clearly silhouetted against the background, which simplifies their identification and facilitates recording (Figure 3). In this preparation, the majority of the accessible trichoid sensilla are of the at4 type.
Keep the prep under constant humidified airflow (2 L/min) delivered via a separate air delivery tube from a distance of about 2 cm from the prep (Figure 4), as described previously2,15.
4. Recording of at4 Sensillum Activity from Or47b ORNs in the at4 Trichoids in Response to Palmitoleic Acid
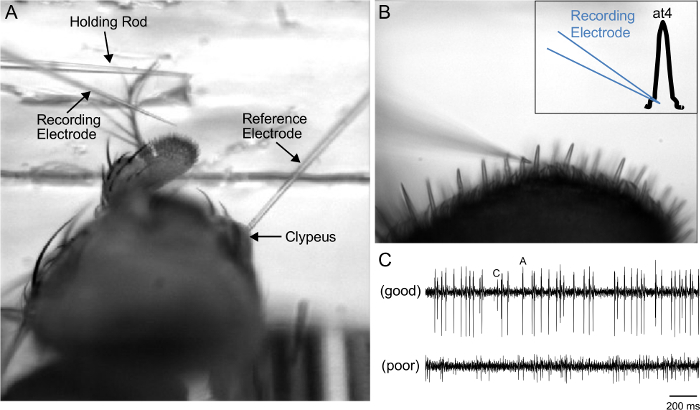
Insert the reference electrode into the clypeus (Figure 3A). To avoid tissue damage, ensure that the electrode is inserted just below the surface, where it can contact the hemolymph under the cuticle, with a swift and smooth motion.
Lower the recording electrode slowlyuntil it enters the same plane of view as the target sensillum (Figure 3B). Record under a 50X objective lens. NOTE: The tough trichoidal cuticle necessitates inserting the recording electrode into the sensillar base, whose wider area provides a larger target that reduces the likelihood that the electrode is deflected away (Figure 3B, inset).
- Before applying odor stimuli to a sensillum, observe the following selection criteria; any trichoid failing to meet these standards should be rejected and another sensillum chosen instead.
- Observe a high signal-to-noise ratio (see Figure 3C for an example).
- Observe that the basal firing rate of the at4A neurons is around or under 20 Hz. NOTE: This criterion is specific for at4A because the basal firing rate for the neuron is higher than that of the basiconic ORNs2. A much higher basal firing indicates that the neurons may have been damaged during electrode insertion.
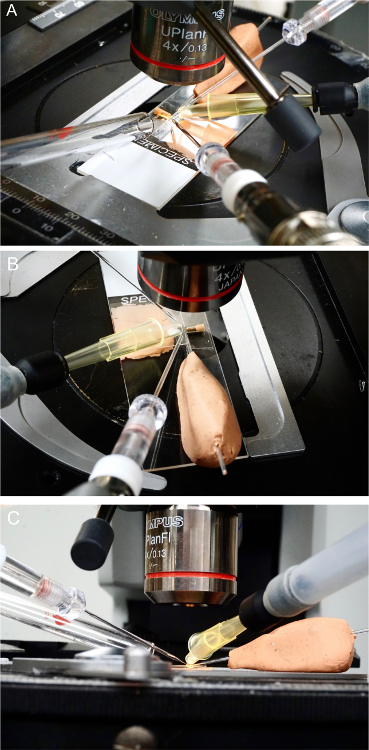
Connect the cartridge to the odorant-delivery tube. Start with the solvent control and then the odorants, from low to high concentrations. Use the micromanipulator to maneuver the cartridge towards the prep while aiming the cartridge squarely at the head of the prep. Visually confirm that the cartridge is pointed directly at the antenna (Figure 4) from a few millimeters away. NOTE: The goal is to orient the opening of the cartridge directly at the antenna and position it in close proximity to the target tissue.
Ensure that the odorant cartridge is separated from the recording electrode on its right by 1 - 2 mm and from the fly prep slide below by approximately 1 mm. NOTE: In the setup described here, the odorant cartridge is closely bordered by the recording electrode, the reference electrode, and the fly-prep slide (Figure 4). NOTE: Pay attention to the distance between the cartridge and the recording/reference electrodes. A distance of around 4 mm is recommended1. Unintentional contact may terminate the signal and break the tip of the recording electrode, damaging the current neuron and complicating further recordings. NOTE: Consider the distance separating the cartridge and the fly-prep slide. Touching the coverslip may also dislodge the recording electrode to disrupt the recording.
Press "Record" in the data acquisition software to begin the recording. NOTE: For each 10 s recording, a single 500 ms odor pulse is delivered directly to the antenna, as described in step 1.9.
After odorant application, carefully retract the cartridge before replacing it with a cartridge of the next-highest concentration. Continue until the entire dosage range is obtained. NOTE: It is recommended that only one Or47b ORN is recorded from each fly to avoid any possible effects of adaptation.
Thoroughly rinse the recording electrode with distilled water after finishing recording for the day.
Analyze and plot the data using commercially available offline analysis software.
Representative Results
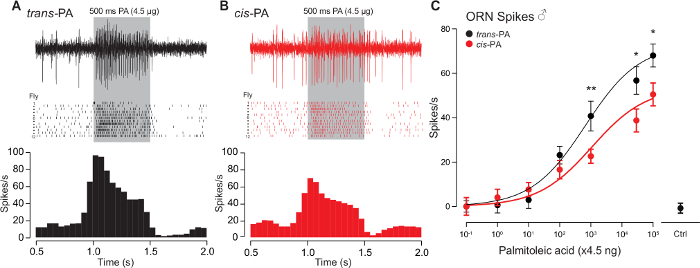
Our technique was successfully applied to determine the relative efficacy of the trans (Figure 5A) versus cis (Figure 5B) isomers of palmitoleic acid. Our representative data demonstrates that trans-palmitoleic acid is a more effective ligand for Or47b ORNs when compared to the cis isoform (Figure 5C). A single neuron was recorded from each fly, with twelve flies recorded per dosage curve, for a total of 24 flies. The collective data were obtained from three independent repeats of the experiments, with 8 flies recorded in each. The error bars represent the s.e.m.
Of note, the distance between the opening of the odor cartridge and the head of the fly has a significant influence on the outcome of the recording. To elicit a significant response to palmitoleic acid in Or47b ORNs, we presented the odorant at close range, around 4 mm away from the antenna1 (Figure 6A). When palmitoleic acid is presented further away from the antenna (~11 mm), we could hardly observe any significant response from the same Or47b ORNs (Figure 6B). These results highlight the importance of the close-range presentation of palmitoleic acid (Figure 6C-D). The data were collected from parallel experiments from 6 male flies (Berlin, 7 d old). A single Or47b ORN was recorded/fly. The error bars represent the s.e.m.
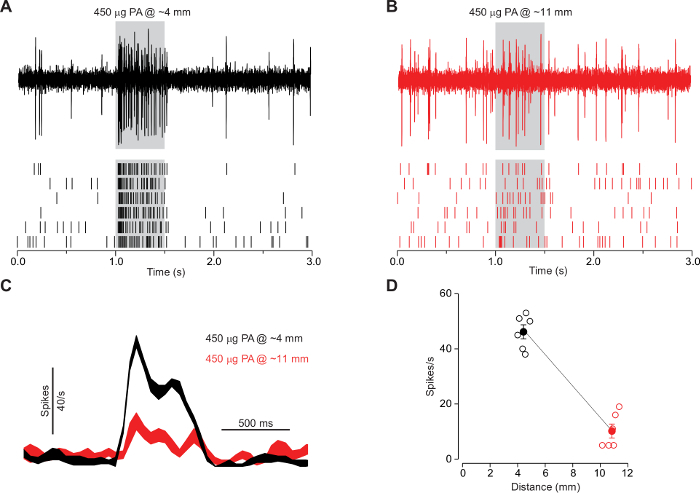
Figure 1: Cartridge and Olfactometer Setup. (A) Preparation of odor cartridges. From left to right: a standard 200-µL pipette tip, the first and second cartridge sections, and a completed odorant cartridge. (B) The cartridge connected to the olfactometer, showing the downward angling of the second section. (C) Olfactometer setup depicting the odor delivery tube mounted on the micromanipulator, with an attached odorant cartridge. Please click here to view a larger version of this figure.
Figure 2: Drosophila Preparation. (A) A complete preparation, showing the relative positions of the fly, coverslip, and holding rod. (B) Close-up view of the prep, showing the positioning of the fly, its antennal orientation, and its clypeus. The holding rod is placed over the arista, securing the third antennal segment to the double-sided tape. (C) Rig setup. All major components are annotated. Please click here to view a larger version of this figure.
Figure 3: Identification of the at4 Sensillum for SSR. (A) 4X view of the prep, showing the reference electrode inserted in the clypeus, the holding rod atop the arista, and the recording electrode positioned near the third antennal segment. (B) 50X view of the electrode, poised for insertion into the at4 trichoid. Inset: Illustration of the position of the recording electrode. (C) Representative SSR traces of baseline spike activity, demonstrating good (top) or poor (bottom) signal-to-noise ratio. Good signal-to-noise ratio permits the reliable identification of at4A and at4C spikes. Please click here to view a larger version of this figure.
Figure 4: Cartridge Placement. (A) The odorant cartridge is aimed squarely at the head of the fly from a distance of a few mm. (B) Another view of the prep and olfactometer from a different angle. (C) A close-up view of the prep and olfactometer, showing the position of the odorant cartridge above the fly-prep slide. Please click here to view a larger version of this figure.
Figure 5: Representative Traces and Dosage Curves of Or47b ORNs in Response to cis- or trans-palmitoleic Acid. (A-B) SSR from the at4A ORNs that express the Or47b receptor with trans- (A) or cis-palmitoleic acid (B). Recordings were performed with 7-day-old WT Berlin males. Corresponding spike rasters (middle) and a peri-stimulus time histogram (bottom, binned at 50 ms) are shown below the sample traces (n = 12). (C) Dose-response curves comparing the Or47b ORN spike responses to cis- or trans-palmitoleic acid. Mean ±s.e.m. (*p <0.05; **p <0.01; t-test). Ctrl: Negative control without palmitoleic acid. Please click here to view a larger version of this figure.
Figure 6: The activation of at4A by palmitoleic acid requires close-range stimulation. (A-B) SSR from the at4A ORNs in 7-day-old wildtype Berlin males. cis-palmitoleic acid was delivered at a close range (~4 mm) or further away (~11 mm) (n = 6). (C) Comparison of the corresponding spike responses (binned at 50 ms, smoothed peri-stimulus time histograms). (D) Comparison of the corresponding average spike responses. The responses of at4A to palmitoleic acid drop markedly as the stimulus distance increases. Reprinted with permission from Figure S4 in reference1. Please click here to view a larger version of this figure.
Discussion
Here, we described a procedure by which the responses of Or47b ORNs to palmitoleic acid can be robustly induced and recorded. We modified a conventional long-distance odor delivery method2,7,10 to troubleshoot the problem of insufficient pheromone odorant delivery. We addressed the issue of low odorant volatility by delivering the compound via odorant cartridges, the opening of which are positioned within millimeters of the prep. When consideration is given to the consistent construction and placement of each odorant cartridge, this protocol manifests itself as an effective method of presenting otherwise inaccessible odorants in a reproducible manner.
The close-range odor presentation procedure described here is significant with respect to existing odor delivery methods. It permits a variety of future applications, including screening other low-volatility odorants for responses in not only ORNs housed in trichoid sensilla1, but those found in any sensillum type. The procedure allows for the efficient delivery of pheromone odorants via a pulse of air instead of by physically moving a glass capillary carrying the odorants towards the antennae6. Our modification minimizes the possibility of touching the tissue directly with the odorant-containing glass capillary, as supported by the experimental results in which we observed palmitoleic acid-elicited responses only after we delivered the odor pulse. In addition, our method provides excellent temporal control of rapid odor onset and offset.
It should be noted that, despite the demonstrated potential of the procedure, it is not without limitations. In our procedure, the positioning of the cartridge relies entirely upon manual adjustment, which renders it technically difficult to place the cartridge precisely at the same location from trial to trial. In addition, special attention to critical steps of the protocol is required to ensure it is successfully executed. Occasionally, highly variable responses to a given odor concentration are encountered. In most cases, the cause is traced to inconsistent cartridge placement. In addition, stringent selection criteria for at4 sensilla must be observed before recording. Uniform at4A spike sizes of high signal-to-noise ratios (Figure 3C) are a key benchmark, while a modest basal firing rate indicates the absence of neuronal damage. The degree of technical difficulty of this procedure is more than offset by its ability to deliver pheromone odorants from ranges that closely simulate the observed proximity between a courting male and the target female.
In summary, our method of odorant presentation offers access to palmitoleic acid for use in SSR from Or47b ORNs. However, the application of this technique is not limited to a single pheromone, but is readily adaptable to any other low-volatility odorant of choice, making it a versatile analytical technique when assaying previously inaccessible odorants.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Ye Zhang for the help with the sample traces and Tin Ki Tsang for the help with the pictures. This work was supported by a Ray Thomas Edwards Foundation Early Career Award and an NIH grant (R01DC015519) to C.-Y.S. and NIH grants (R01DC009597 and R01DK092640) to J.W.W.
References
- Lin H-H, et al. Hormonal modulation of pheromone detection enhances male courtship success. Neuron. 2016;90(6):1272–1285. doi: 10.1016/j.neuron.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125(1):143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Silbering AF, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31(38):13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S, Ai M, Shin SA, Suh GSB. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc Nat Acad Sci USA. 2013;110:1321–1329. doi: 10.1073/pnas.1215680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446(7135):542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10(5):623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin JD, Ha TS, Jones DNM, Smith DP. Activation of Pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133(7):1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Diaz C, Reina JH, Cambillau C, Benton R. Ligands for pheromone-sensing neurons are not conformationally activated odorant binding proteins. PLoS Biol. 2013;11(4):e1001546. doi: 10.1371/journal.pbio.1001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck HKM, et al. Pheromones mediating copulation and attraction in Drosophila. Proc Nat Acad USA. 2015;112:2829–2835. doi: 10.1073/pnas.1504527112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappa CD, Lovejoy ER, Ravishankara AR. Evaporation rates and vapor pressures of the even-numbered C8-C18monocarboxylic acids. J Phys Chem A. 2008;112(17):3959–3964. doi: 10.1021/jp710586m. [DOI] [PubMed] [Google Scholar]
- Kimura K-I, Sato C, Yamamoto K, Yamamoto D. From the back or front: the courtship position is a matter of smell and sight in Drosophila melanogaster males. J Neurogenet. 2015;29(1):18–22. doi: 10.3109/01677063.2014.968278. [DOI] [PubMed] [Google Scholar]
- Grosjean Y, et al. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478(7368):236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112(2):271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Pellegrino M, Nakagawa T, Vosshall LB. Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. J Vis Exp. 2010. pp. e1–e5. [DOI] [PMC free article] [PubMed]


