Abstract
A rate-limiting aspect of transgenic mouse models of mammary adenocarcinoma is that primary tumor burden in mammary tissue typically defines study end-points. Thus, studies focused on elucidating mechanisms of late-stage de novo metastasis are compromised, as are studies examining efficacy of anti-cancer therapies targeting mediators of metastasis in the adjuvant setting. Numerous murine mammary cancer models have been developed via targeted expression of dominant oncoproteins to mammary epithelial cells yielding models variably mimicking histopathologic and transcriptome-defined breast cancer subtypes common in women1. While much has been learned regarding the biology of mammary carcinogenesis with these models, their utility in identifying molecules regulating growth of late-stage metastasis are compromised as mice are typically euthanized at earlier time points due to significant primary tumor burden. Moreover, since a significant percentage of women diagnosed with breast cancer receive adjuvant therapy after surgical resection of primary tumors and prior to presence of detectable metastatic disease, preclinical models of de novo metastasis are urgently needed as platforms to evaluate new therapies aimed at targeting metastatic foci. To address these deficiencies, we developed a murine model of de novo mammary cancer metastasis, wherein primary mammary tumors are surgically resected, and metastatic foci subsequently develop over a 115 day post-surgical period. This long latency provides a tractable model to identify functionally significant regulators of metastatic progression in mice lacking primary tumor, as well as a model to evaluate preclinical therapeutic efficacy of agents aimed at blocking functionally significant molecules aiding metastatic tumor survival and growth.
Keywords: Cancer Research, Issue 125, mammary cancer, murine model, metastasis, surgical resection, adjuvant therapy, lung, MMTV-PyMT
Introduction
Women in North America have a ~12% lifetime risk of developing breast cancer2; a majority of these individuals will have primary tumors removed via surgery, and depending on cancer subtype, will then receive targeted, endocrine, chemo- and/or radiation therapy in the adjuvant setting3. Examples include, women diagnosed with hormone receptor-positive cancers receiving anti-estrogen therapies and women with HER2-positive tumors receiving HER2-targeted therapies with radiation/chemotherapy, whereas no targeted therapies are yet available for triple negative tumors3. Despite advances in radiation, chemotherapy, personalized and hormone-based therapies that supplement surgical resection, disease recurs in 30-70% of women diagnosed with stage II or III disease4, as therapies are largely ineffective in eradicating metastatic disease in distant organs, including lung, bone, brain and/or liver5. This is especially significant given that when metastatic disease occurs in the absence of primary tumor regrowth, this implies that disseminated malignant cells were likely already present in secondary organs at the time of definitive surgery. Thus therapies able to eradicate or slow growth of metastatic tumors are urgently needed.
While de novo mouse models of mammary carcinogenesis have been remarkably informative in revealing mechanisms regulating neoplastic progression1, existing models also have several limitations. One of these is the fact that de novo transgenic models typically develop primary tumors in multiple mammary glands, wherein primary tumor burden limits duration of studies. While primary tumor cell escape and metastatic seeding likely occur early in neoplastic progression in these models, frank development of metastatic tumors occurs late, and depending on the mouse model and strain background, is often partially penetrant1. This further limits the utility of de novo models for discovery of molecules regulating metastasis in secondary organs, and for evaluating preclinical efficacy of therapeutics in the adjuvant setting.
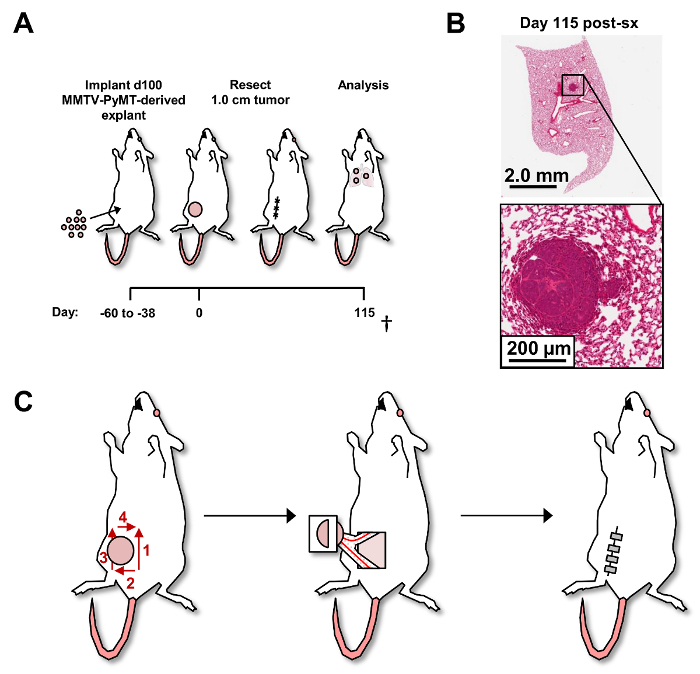
To circumvent these limitations, we developed a de novo autochthonous model of mammary carcinoma metastasis to lungs. Parental transgenic females (i.e., MMTV-PyMT on the FVB/n strain background for studies described herein) bearing late-stage de novo mammary tumors are aged to ~100 days6, at which point their primary tumors are surgically resected and enzymatically dissociated into single cell suspensions. Suspensions (1 x 106 cells) are in turn orthotopically explanted into 6-7-week-old recipient syngeneic female mice, where single primary mammary tumors develop over a 38 to 60 day period (Figure 1A). At a defined tumor size (172 to 450 mm3), recipient mice are anesthetized and primary tumors are surgically resected such that tumor regrowth at the surgical site is minimized, consistent with surgery in women (Supplementary Figure 1). On the FVB/n strain background, mice develop histologically-detectable metastatic foci in lungs with 45% penetrance by ~115 days post-surgery (Figure 1B). With this extended latency of metastatic tumor growth, the model is uniquely positioned for adjuvant therapy delivery, and for elucidating and evaluating underlying biology influencing metastatic progression following surgical removal of primary tumors.
Protocol
Animals used in the following protocol are covered by Oregon Health & Science University's Institutional Animal Care and Use Committee (IACUC), which is designed to be compliant with the Animal Welfare Act regulations and Public Health Service (PHS) Policy.
Maintenance of sterile conditions: Sterilized instruments should be used and between mice, should be wiped clean with sterile gauze, rinsed in PBS followed by sterilization with disinfectant 70% ethanol for at least 15 minutes. A surgical cap, facemask, gown, and gloves should be worn for survival surgeries. Pre-operative preparation of the animal for survival surgeries is included in the following protocol. Refer to Table 1 for a list of reagents and equipment.
1. Isolation and Preparation of Single Cell Suspensions from Primary Mammary Tumors
Anesthetize donor female 100 day old transgenic MMTV-PyMT (FVB/n) mice and maintain under continuous sedation by administering 2% isoflurane via an anesthesia mask. Confirm that the mouse is properly anesthetized with an absent foot pinch reflex.
In a sterile setting, resect primary mammary tumors from 100-day old transgenic female MMTV-PyMT (FVB/n) mice by separating mammary tumor from overlying skin and surrounding adipose tissue and/or lymph nodes using sterile scissors. Euthanize the anesthetized mice by cervical dislocation.
- With sterile scissors or a scalpel, mince primary tumors manually into small pieces (~1.0 mm3). Place tumor pieces in 3.0 mg/mL collagenase A and 4.0 U/mL DNase I dissolved in DMEM. Use ~10 mL of the above digestion medium per 1.0 cm diameter tumor.
- Perform digestion in a sterile 25 mL bottle with a sterile stir bar at ~125 rpm and 37 °C for 40 min.
Stop the digestion by adding fetal bovine serum (FBS) to a final dilution of 10% and place the entire mixture on wet ice where it is maintained.
Filter the digested tumor suspension through a 0.7 µm nylon strainer into a 50 mL conical tube and discard any tumor remaining in the strainer. Centrifuge the supernatant at 300 x g at 4 °C.
Resuspend the pellet in 10 mL DMEM per 1.0 cm tumor and re-filter through a 0.7 µm nylon strainer. Count cell concentration, then centrifuge at 300 x g at 4 °C.
Resuspend pellet in 10% dimethyl sulfoxide with 90% FBS at a concentration of 2 x 107 live cells/mL. Store single-cell suspensions of whole primary tumor at -80 °C.
2. Orthotopic Injection of Mammary Tumor
Partially thaw frozen primary tumor suspensions at 37 °C until the frozen pellet can be released from the cryogenic tube into 20 mL DMEM and count the cells. Centrifuge at 300 x g at 4 °C and resuspend cells in a 1:1 DMEM: growth factor-reduced solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma at a concentration of 1 x 107 cells/mL (see the Table of Materials).
Place the anesthetized recipient female syngeneic mouse ventral side up and maintain under continuous sedation by administering 2% isoflurane via an anesthesia mask.
Sterilize the right 4th mammary gland injection site using aerosolized 70% ethanol and applying Poly(vinylpyrrolidone)-Iodine with a sterile cotton swab.
Inject 100 µL (1 x 106 live cells from frozen primary tumor suspensions) bevel-side up into uncleared right 4th mammary gland of the 6 to 10 week-old female FVB/n mouse using a 29 G 0.3 mL insulin syringe.
3. Surgical Resection of Orthotopic Mammary Tumor
38-60 days following tumor cell injection, euthanize mice not exhibiting orthotopic tumor volumes ranging between 172 to 450 mm3 in volume [length x (width2)/2] (about 75% of injected mice).
Place anesthetized mice displaying proper tumor size ventral side up under continuous sedation by administering 2% isoflurane via an anesthesia mask and confirm the mouse is properly anesthetized with an absent foot pinch reflex. Apply vet ointment to the eyes for prevention of dryness while under sedation.
Spray 70% ethanol to sterilize the surgical area surrounding the primary tumor, then apply Poly(vinylpyrrolidone)-Iodine with a sterile cotton swab. NOTE: Hair removal at the surgical site resulted in skin excoriations and/or infections for ~5% mice and was therefore excluded from this step (data not shown).
As shown in Figure 1C, make an initial skin incision using blunt scissors medial-caudal to the tumor.
Next, make a superior excision of the skin (Figure 1C) medial to the tumor. Pay attention to the need to cauterize any vasculature feeding the tumor located on the skin before extending the incision.
Continue the skin incision laterally (posterior to the tumor), then make a superior skin excision (lateral to tumor), and medial excision (superior to tumor) (Figure 1C).
After the skin has been excised circumferentially around the tumor (Figure 1C), lift overlying skin attached to the tumor using forceps while blunt dissecting the tumor away from the abdominal wall musculature and keeping the mammary glands intact.
Identify (by blunt dissection) and cauterize large vessels running through the 4th and 5th mammary glands.
Excise approximately half of the 4th and 5th mammary glands at the cauterization site to free the tumor, overlying skin, and segments of the mammary glands (Figure 1C).
In the event of bleeding, identify actively bleeding vessels and immediately cauterize them. If more than 250 µL of blood is lost, exclude the mouse from study and euthanize it.
Close excision sites with wound clips using a wound clip applier (Figure 1C). NOTE: Remove the wound clips 10 days post-surgery with a wound clip remover.
- Administer warm sterile saline subcutaneously (4% of the animal's body weight) and keep the animal warm with a heat lamp. Check the animals every 5-10 min during recovery from anesthesia. NOTE: Administration of either bupivacaine or lidocaine increased post-operative mortality (data not shown). Mice that showed signs of pain (hunching, unwilling to move, failure to groom) 1 h after surgery were euthanized.
- Once the mouse has fully recovered, return it to the company of other mice.
4. Isolation and Processing of Blood and Lung for Flow Cytometry and Histology
At the study endpoints, prepare mice for various histopathologic assessments if desired. 90 min before euthanasia, give each mouse an intraperitoneal injection of bromodeoxyuridine (BrdU, 50 µg/g mouse weight) at a concentration of 6.25 µg/µL in 1x PBS. NOTE: Frozen stocks of dissolved bromodeoxyuridine are used within 1 month after preparation.
10 min before euthanasia, with the mouse anesthetized, collect retroorbital blood (>500 µL) using heparinized capillary tubes, and subsequently transfer to tubes coated with dipotassium EDTA held on ice.
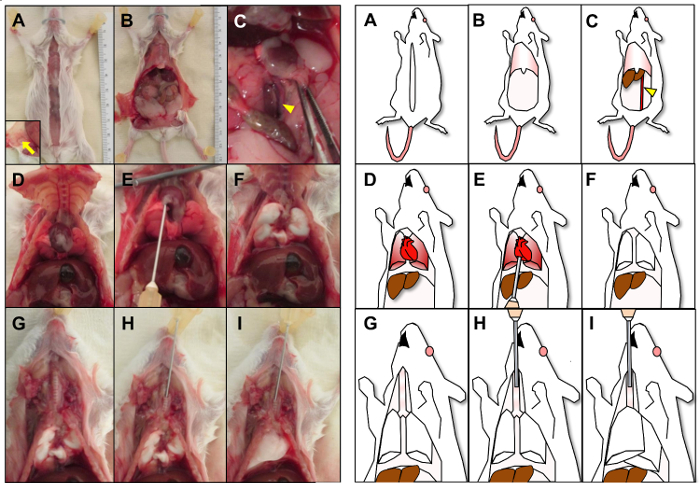
To remove lungs and remaining mammary tissue, make a midline incision with scissors from the lower abdomen to the mouth to expose the thoracic and peritoneal cavities (Figure 2A), and peel the skin laterally to also expose the remaining right 4th and 5th mammary glands.
Excise the remaining mammary gland tissue and examine it to rule out primary tumor regrowth by assessing serial sectioned formalin-fixed paraffin-embedded (FFPE) tissue by hematoxylin and eosin (H&E) staining (Supplementary Figure 1).
To remove lungs, open the abdominal wall to the diaphragm with scissors, then euthanize the animal by cutting the abdominal aorta to drain blood prior to perfusion of lungs (Figure 2B-2C).
Cut the diaphragm along the rib cage from an abdominal approach making sure to avoid the lung and heart. Then, expose the thorax by cutting through the lateral sides of the rib cage (Figure 2D).
Using a 23-gauge needle on a 20 mL syringe, perfuse lungs with ~5.0 mL DPBS (~10 mL/min) through the right ventricle of the heart until the lungs turn entirely white (Figure 2E-2F). Immediately cut off the heart from the main vessels so blood does not re-perfuse into the lung.
For lung tissue to be fixed and processed for histopathologic assessments, inject ~1.0 mL 10% formalin at 4 °C into the exposed trachea bevel side up toward the lungs using a 23-gauge needle (Figure 2G-2H). Cease injection once lungs are completely expanded and filled with fixative (Figure 2I).
Excise lung lobes from trachea, and emersion fix lung tissue in neutral-buffered formalin for subsequent paraffin embedding or OCT-freezing medium, per standard histopathologic procedures.
Quantitatively evaluate metastatic burden in lungs by serial sectioning of FFPE lung tissue and evaluate microtome sections (100 µm, thirteen sections), by H&E staining (Figure 1B).
Representative Results
Greater than 75% of recipient mice receiving 1 x 106 cells from primary mammary tumors derived from MMTV-PyMT mice, develop single mammary adenocarcinomas ranging in size from 172 to 450 mm3 within 38-60 days (data not shown). Mice eligible for randomization are then enrolled into study groups following surgical resection of primary tumors as shown (Figure 1C). Primary tumor regrowth was identified in less than 2% of mice that underwent surgical resection of primary tumor (Supplementary Figure 1). 45% of recipient mice evaluated by this protocol developed histologically detectible metastatic foci by day 115 post-tumor resection (Figure 1B). To affirm histology of metastases in areas identified containing metastatic cells by H&E staining, adjacent tissue sections were evaluated by PyMT PCR (data not shown).
Figure 1: Post-surgical resection of primary tumors and development ofde novo pulmonary metastasis. (A) Experimental schema of murine mammary adenocarcinoma metastasis model. † denotes that all mice were cardiac perfused and injected with BrdU on the day of euthanasia. (B) Representative H&E where detection of metastatic foci was assessed by serial sectioning of FFPE lung tissue with lobes separated. Metastatic foci (>5 cells) were determined by H&E staining every 100 µm reflecting 1,300 µm of tissue. Lungs from 11 mice were analyzed. (C) Schema of surgical resection of primary mammary tumor. Red numbers and arrows denote the order and direction of skin incisions bordering the primary tumor (left). The right 4th and 5th mammary glands with major vessels are shown attached to the primary tumor (middle) followed by wound closure with wound clips (right). Please click here to view a larger version of this figure.
Figure 2: Isolation, perfusion and fixation of lung. Picture (left) and corresponding cartoon (right) are shown of lung isolation, perfusion and fixation. (A) A midline incision is shown with inset image displaying retracted skin exposing the right 4th and 5th mammary glands (arrow). (B) The abdominal wall is shown opened to the diaphragm. (C) After retraction of intestine, the abdominal aorta (arrowhead) is identified and cut open. (D) The diaphragm and lateral sides of the rib cage are cut to expose the thoracic cavity. (E) The lung is perfused through the right ventricle of the heart until the lungs turn entirely white (F). (G) The exposed trachea is identified followed by injecting formalin (H) into the trachea until the lungs have expanded (I). Please click here to view a larger version of this figure.
Supplementary Figure 1: Primary tumor regrowth at surgical site. Representative H&E (top) and gross (bottom) images of the remaining right 4th and 5th mammary gland post-surgery, showing absence of tumor regrowth with inguinal lymph node (A-B) and mammary gland with primary tumor regrowth (C-E). Please click here to download this figure.
Discussion
Modifications and troubleshooting:
When blunt dissecting tumor away from the abdominal wall, the tumor may remain adherent to the abdominal wall. This was observed in <5% of mice injected with tumor (data not shown). For mice with tumors adherent to the abdominal wall, the mouse should be euthanized as resection is difficult without primary tumor regrowth.
Limitations of model/technique:
Whereas other investigators have reported presence of fluorescently-labeled single metastatic cells disseminated to liver, kidney, spleen and brain, in addition to lung, following reimplantation of mammary terminal end buds derived from MMTV-PyMT mice7, aside from lung, we observed several mice with metastatic foci in liver, the penetrance of which has yet to be determined. Other sites of potential metastatic burden, such as lymph nodes, spleen, bone, and brain, were not evaluated. A limitation of this technique also included post-operative excoriations and infection of the skin when shaving the surgical site. Because of this, sterility of the surgical site was limited to application of 70% ethanol followed by Poly(vinylpyrrolidone)-Iodine.
Critical steps within the protocol:
Blunt dissection of the tumor away from the abdominal wall is a critical step (step 3.7) where avoidance of the vessels within the mammary gland should be performed. Steps 3.8 and 3.9 are also critical steps where there is greatest risk for uncontrolled bleeding. The uncauterized proximal and distal portions of vessel should be identified post-cauterization to easily visualize sources of bleeding.
Significance with respect to existing methods:
Mouse models of human cancer mimicking stages of disease progression, kinetics and histopathology provide invaluable tools within which to identify and evaluate new targets for therapy, as well as potential efficacy of new therapeutic agents targeting those molecules/pathways. While tail-vein and/or cardiac injection of established cancer cell lines are often used as experimental models of metastasis, these fail to recapitulate critical steps in the metastatic process, and instead reflect ectopic organ colonization assays where aspects of tumor cell survival can be evaluated8. Moreover, whereas some existing transgenic mouse models of de novo mammary carcinogenesis development do provide model systems enabling study of steps involved in metastasis, significant primary tumor burden typically limits duration of study. Thus groups have injected cultured neoplastic cells derived from MMTV-PyMT primary tumors to allow for surgical resection9. Our method expands from these techniques as it does not select for neoplastic cell lines grown from tumors in vitro and directly introduces the complete, heterogeneous primary tumor to recipient mice. Additionally, the long latency of lung metastasis formation in our model allows for a better therapeutic window for various treatment studies.
Future applications:
Regarding evaluating efficacy of therapeutics in these models, because primary tumors typically develop in all mammary glands, surgical resection of all primary tumors and adjuvant evaluation of therapies aimed at minimizing growth of metastatic colonies is not possible. Because of these issues, we developed an autochthonous model of metastatic dissemination wherein metastatic dissemination of tumor cells occurs de novo, and following surgical resection of primary tumor, an extended latency period is established that allows for identification of metastasis in the lung. Thus, this model mirrors human breast cancer metastasis and affords a unique system to evaluate efficacy of adjuvant delivered therapies for impact on regulating disease-free survival and/or overall survival with defined endpoints per IACUC guidelines.
Since a large proportion of women with breast cancer are treated by surgical resection of primary tumors10, and those that progress subsequently develop distal metastasis, this implies that dissemination and seeding had occurred prior to surgical resection. Distal organ microenvironments provide unique niches for surviving and/or proliferating metastatic cells11. Thus, it is imperative that model systems mimic these facets such that molecules and pathways operative in secondary sites, that are likely distinct from primary tumors, can be identified, studied, and therapies targeting them accurately evaluated for efficacy. The model developed herein provides these aspects for study.
Disclosures
The authors have no disclosures of conflict with data presented herein.
Acknowledgments
The authors thank Jo Hill for histopathology assistance, Dr. John Gleysteen for instruction in surgical technique, Tessa Diebel for videography assistance, all members of the Wong and Coussens laboratories for critical insight and discussions, and the OHSU Knight Cancer Institute for financial support. The authors acknowledge support from T32GM071388-10 and T32CA106195-11 to CEG, the NCI/NIH, the Department of Defense Breast Cancer Research Program, the Susan G Komen Foundation, the Breast Cancer Research Foundation, and a Stand Up To Cancer - Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant (SU2C-AACR-DT14-14) to LMC, a Women's Health Circle of Giving Foundation Award to MHW, and the Brenden-Colson Center for Pancreatic Health to MHW and LMC.
References
- Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006;8:212. doi: 10.1186/bcr1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader NNA, Krapcho M, editors. SEER Cancer Statistics Review 1975-2008. Bethesda, MD: National Cancer Institute; 2011. based on November 2010 SEER data submission, posted to the SEER web site. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- National Comprehensive Cancer Network. Breast Cancer (Version 3.2014) 2016. Accessed November 25. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [DOI] [PubMed]
- Kataja V, Castiglione M, Group EGW. Locally recurrent or metastatic breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19 Suppl 2:11–13. doi: 10.1093/annonc/mdn072. [DOI] [PubMed] [Google Scholar]
- Margolese RG, Hortobagyi GN, Buchholz TA, et al. In: Management of Metastatic Breast Cancer. Holland-Frei Cancer Medicine. 6th. Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Hamilton (ON): BC Decker; 2003. [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, et al. Genes that mediate breast cancer metastasis to the lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, et al. FLT1 signalling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212(9):1433–1448. doi: 10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkooijen HM, et al. Patients' refusal of surgery strongly impairs cancer survival. Ann Surg. 2005;242(2):276–280. doi: 10.1097/01.sla.0000171305.31703.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastasis. Nat Rev Cancer. 2017;17(5):302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]


