Abstract
Background
Lowserum vitamin D levels are associated with susceptibility to, and severity of, multiple sclerosis. High dose vitamin D has been proposed as a potential immunomodulator in multiple sclerosis.
Objectives
We performed a single centre, investigator-led, exploratory, double-blind, randomised, placebo controlled, trial of vitamin D3 in clinically isolated syndrome and healthy control participants to assess its immunological effects. Secondary end-points included clinical and magnetic resonance imaging outcomes and safety.
Methods
Clinically isolated syndrome patients and healthy control participants were randomised to: placebo, 5000 IU or 10,000 IU vitamin D3/day (Vigantol oil). Study duration was 24 weeks.
Results
The trial did not meet its primary end point, with no difference in the frequency of pro-inflammatory CD4+ T cells (interleukin (IL)-17+/interferon (IFN)-γ+) seen. A higher level of disease freedom (67% versus 50%) was seen in those with serum 1,25 (OH) vitamin D levels>100 nmol/l but this did not reach significance. High dose vitamin D3 was well tolerated with no safety signal.
Conclusions
High dose vitamin D3 over 24 weeks was well tolerated but without immunological, magnetic resonance imaging or clinical evidence of benefit. The hypothesised therapeutic effects in clinically isolated syndrome or multiple sclerosis patients may require longer periods of administration or may only be seen in patients treated with vitamin D3 as an adjunct to established disease modifying therapies.
Keywords: Multiple sclerosis, clinically isolated syndrome, vitamin D, randomised controlled trial, immunology, cholecalciferol
Introduction
Multiple sclerosis (MS) is a relapsing inflammatory disorder affecting the central nervous system causing demyelination, axonal and neuronal injury resulting in cerebral and spinal atrophy. Its pathogenesis is not fully understood but involves a complex interplay between genetic and environmental factors.1 There is a well recognised geographical distribution of MS with increased incidence and prevalence associated with latitude from the equator.2 The association of both MS susceptibility and disease activity with low serum vitamin D levels has emerged from a number of studies showing increased prevalence with lower levels of ultraviolet (UV) radiation3,4, a higher risk of MS in offspring of women who are deficient in vitamin D5,6 which is further supported by month of birth studies;7 also, higher serum levels of vitamin D are associated with lower risk of relapse8,9 and the number of new lesions on magnetic resonance imaging (MRI) scanning.10
Vitamin D has been shown to modulate both the innate and adaptive immune systems.11 The active metabolite, 1,25 (OH) vitamin D (25(OH)D), mediates its effects through the intracellular vitamin D receptor (VDR),12 which is present in monocytes, dendritic cells, B-cells and CD4+ T cells.11,13 Activation of VDR alters transcription, proliferation and differentiation of immune cells12 and both indirectly modulates immune response by reducing the activation of pro-inflammatory T cells by antigen presenting cells14 and directly inhibiting T cell and B cell proliferation.11,15 This results in a Th2 cell driven anti-inflammatory state.13,15,16 The diverse effects seen within the immune system in response to vitamin D supplementation may explain the conflicting results from the small number of human studies carried out to date.17–23
Clinically isolated syndrome (CIS) refers to patients presenting with a first-ever symptom consistent with demyelination and without evidence of dissemination in time.24 CIS patients have a well-established natural history, with an increased risk of MS associated with the number of asymptomatic MRI T2 lesions at presentation.25 In many countries, including Ireland, a conservative approach of ‘watchful waiting’, rather than the immediate introduction of disease-modifying therapy, is taken. Vitamin D deficiency is common in Ireland,26 as in many northern European countries and may contribute to both disease susceptibility and disease severity. We hypothesised that treatment of CIS patients with vitamin D3 supplementation might reduce the risk of progression to either clinically- or MRI-confirmed MS. Therefore we carried out a randomised, double-blind, placebo-controlled trial of vitamin D3 supplementation in CIS patients to assess the immunological and therapeutic effects, if any, of vitamin D3 supplementation in these patients. Healthy control participants were also prospectively recruited to investigate if the immunological responses to vitamin D3 supplementation differed between control participants and those with CIS. We also wanted to determine whether high dose vitamin D3 is a safe and effective therapy for patients with CIS.
Participants and methods
Study participants
Inclusion criteria
CIS patients aged between 18–55 years with symptom onset within three months of screening, having two or more asymptomatic T2 lesions on brain MRI consistent with demyelination, not treated with steroids within 30 days of screening and not on any other disease-modifying therapy.
Healthy control participants (HCPs) were aged between 20–40 years with a female: male ratio of 2:1, broadly in line with the gender ratio of CIS.
Exclusion criteria
Exclusion criteria in both CIS patients and HCPs were: no history or evidence of hypercalcaemia, renal impairment, vitamin D intolerance, parathyroid dysfunction, sarcoidosis, pregnancy or refusal to use contraception, prior or current treatment with thiazide diuretics or vitamin D supplementation of greater than 1000 IU/day.
Exclusion criteria in patients with CIS were: (a) patients whose symptoms might be explained by a diagnosis other than MS; (b) patients with occurrence of an exacerbation less than six weeks prior to entry to the study; (c) previous treatment with any immunomodulating therapy in the last three months, steroids in the last four weeks or any previous treatment with mitoxantrone or other immunosuppressant.
Trial design
This was a single-centre, investigator-led, exploratory, double-blind, dose-ranging, randomised, placebo-controlled trial. A full detailed trial protocol has been previously published.27 Detailed procedures carried out at each study visit are available in the Supplementary Material, Methods section. Participants were enrolled from November 2012 and last study visit was June 2015. Both HCPs and CIS patients were recruited over the same time period and studied in parallel. Both the HCPs and CIS patients were randomised to one of three interventions: placebo oil, 5000 IU and 10,000 IU vitamin D3 per day. The vitamin D3 was in the form of Vigantol Oil, supplied by Merck KgaA (Darmstadt, Germany). Both placebo and the active product were identical in appearance.
Endpoints
The primary endpoint was the effect of oral vitamin D3 at 5000 IU and 10,000 IU per day compared to placebo on the frequency of CD4+ T cell subsets (interleukin (IL)-17+CD4+ T cells and interferon (IFN)-γ+CD4+ T cells) and cytokine (IL-17, IL-10 and IFN-γ) responses in peripheral blood mononuclear cells (PBMCs) in patients with CIS and HCPs at 16 and 24 weeks compared to baseline.
Secondary endpoints included determining if, in CIS patients, there was a difference in the response to 5000 IU and 10,000 IU vitamin D3, compared to placebo in:
Radiological activity as measured by the number of new or enlarging T2 lesions and gadolinium-enhancing lesions on brain MRI at 24 weeks compared to baseline.
Clinical activity as measured by the annualised relapse rate (ARR), percentage of patients relapse-free and time to first relapse.
Disease freedom as measured by the percentage of patients with no evidence of either radiological or clinical activity.
We also wished to determine the dose response and safety and tolerability of high dose vitamin D3 supplementation in both CIS patients and HCPs.
Study procedures
Study visits were performed at baseline and then at four-weekly intervals over a 24-week period. A follow-up visit was arranged at week 28–30. At each visit, changes in medical and neurological history or medications were recorded. All patients had a full physical examination and CIS participants has their Expanded Disability Status Scale (EDSS) score recorded and were assessed for any evidence of a relapse. Treatment adherence, as measured by the volume of study drug returned at each visit, and any adverse events were also noted.
Vitamin D and parathyroid hormone
Plasma parathyroid hormone (PTH) levels were measured at baseline, week 4, 8, 16, 24 and 28–30 using the Roche intact PTH assay on the Cobas E411 Electrochemiluminescent Immunoassay Analyser (Roche Diagnostics GmbH, Mannheim, Germany). Serum 25(OH)D levels were measured by liquid chromatography mass spectrometry (LC-MS/MS) using a Waters Acquity ultra performance liquid chromatography (UPLC) coupled to a triple quadrupole mass (TQD) (Waters Corp., Milford, USA) with a semi-automated solid phase extraction (SPE) preparation of patient serum samples (Tecan Freedom Evo 100, Switzerland). A more detailed description is available in the Supplementary Material, Methods section.
Biochemistry, including urea and electrolytes, serum creatinine, liver function and serum calcium levels adjusted for albumin level, was measured at each study visit.
Immunological outcome measures
PBMCs were isolated and cryopreserved, and immunological analyses were performed upon completion of the study. PBMCs from all time-points were thawed in batches, labelled with cell tracker dye CellTrace Violet (CTV) (Invivogen), and cultured at 2 × 106 cells/ml in a 48-well plate (0.5 × 106 cells in total) in complete Roswell Park memorial institute (RPMI) medium and were left unstimulated or stimulated with the following: 1 µg/ml anti-CD3 (eBioscience), 0.5 × 106 irradiated allogeneic PBMCs, 1 µg/ml each of purified protein derivative (PPD; Serum Staten Institute) and tetanus toxoid (TT) as recall antigens, and 0.625 µg/ml/0.375 nM each of myelin oligodendrocyte protein (MOG) and myelin basic protein (MBP) PepTivator overlapping peptides (Miltenyi Biotech). In parallel with each batch of samples the same standardised PBMC sample was included in order to monitor reproducibility between batches. After three days for anti-CD3, or seven days for antigen-specific stimuli, cell culture supernatants were removed and the concentrations of IFN-γ, IL-17 and IL-10 were analysed by enzyme-linked immunosorbent assay (ELISA) (eBioscience). In addition, the cells were restimulated with 50 ng/ml phorbol myristate acetate (PMA), 500 ng/ml ionomycin and 5 µg/ml of Brefeldin A for five hours (all Sigma), followed by staining for cell-surface CD3 and CD8 and intracellular IL-17 and IFN-γ (eBioscience). Cells were analysed by flow cytometry using a BD LSRFortessa Cell Analyser (BD Biosciences) and FlowJo software. T cell proliferation was analysed by CTV dilution.
MRI acquisition
Brain MRI was acquired at baseline and 24 weeks with contiguous axial 3 mm thick slices. Sequences included: axial and coronal T1-weighted spin echo (pre and post gadolinium), axial and sagittal PD/T2-weighted and axial fast fluid-attenuated inversion recovery (FLAIR). Baseline and 24-week scans were reviewed by two radiologists who were blinded to treatment group. MRI assessment included: (a) number of gadolinium-enhancing lesions; (b) number of new and enlarging T2 lesions; (c) total number of new lesions = number of gadolinium-enhancing lesions plus number of new and enlarging T2 lesions.
Statistical methods
Power calculations were based on a pilot study28 using α of 5% and β of 80%. A detailed description is available in the Supplementary Material, Methods section. All serum 25(OH)D levels were adjusted for season29 and these values were used in all further analysis. All data was inspected for normality using histograms, skewness and kurtosis and normality tests (Kolmogorov-Smirnov and Shapiro-Wilk). Values displayed include mean and standard deviation (SD) or median and interquartile range where appropriate. Within group differences between baseline and follow-up were assessed using the Wilcoxon Rank test as outlined in the protocol. Log10 ratio of week 16/baseline and week 24/baseline was calculated and adjusted for baseline measurements (including vitamin D level) and between group differences were compared using the t-test or Mann Whitney U test where indicated. All analysis was carried out in SPSS version 20.
Ethical approval
Ethical approval was granted by St Vincent’s University Hospital Ethics and Medical Research Committee and the study was approved by the Health Products Regulatory Authority (HPRA). The trial was registered with ClinicalTrials.gov (NCT01728922) and the European Union Clinical Trials Register (EudraCT: 2012-000635-68).
Results
Study participants
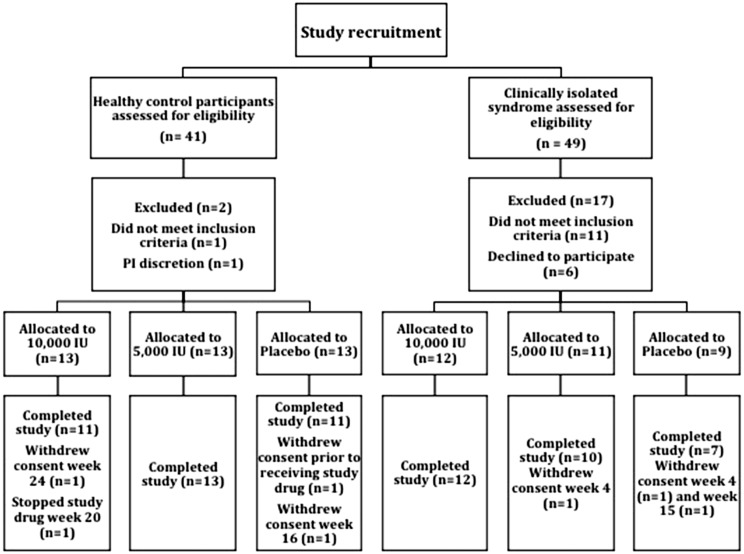
Recruitment to the study is outlined in Figure 1. Baseline characteristics of subjects in each treatment arm are outlined in Table 1. All participants were Caucasian. No significant differences were seen between each of the three treatment arms in either CIS patients or HCPs. Mean baseline EDSS was 0.9 (SD: 1), 0.9 (SD: 1.2) and 0.4 (SD: 0.5) in each of the 10,000 IU, 5000 IU and placebo groups respectively. No patients were on disease modifying treatment as per protocol.
Figure 1.
Schematic diagram outlining recruitment to the study, randomization and final analysis.
Table 1.
Baseline characteristics of both clinically isolated syndrome (CIS) patients and healthy control participants (HCPs).
| Clinically isolated syndrome (n = 29) |
Healthy control participants (n = 38) |
|||||
|---|---|---|---|---|---|---|
| 10,000 IU (n = 12) | 5000 IU (n = 10) | Placebo (n = 7) | 10,000 IU (n = 13) | 5000 IU (n = 13) | Placebo (n = 12) | |
| Female n (%) | 8 (67) | 5 (50) | 6 (86) | 6 (46) | 10 (77) | 10 (83) |
| Age (years) mean (SD) | 37.2 (8.7) | 32.7 (4.6) | 34.3 (10.6) | 30.5 (5.1) | 30.3 (3.7) | 29.1 (4.7) |
| BMI (kg/m2) mean (SD) | 30.6 (5.6) | 26.3 (3.4) | 26.7 (4.2) | 25.4 (3.2) | 23.5 (3.3) | 27.6 (4.3) |
| Ever smoked n (%) | 7 (58) | 5 (50) | 3 (43) | 2 (15) | 3 (23) | 4 (33) |
BMI: body mass index; SD: standard deviation.
Serum 25(OH)D, PTH and calcium levels
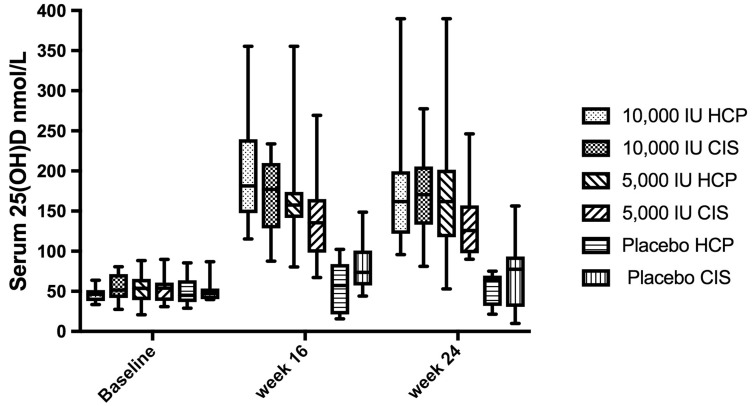
There was no difference in mean baseline serum 25(OH)D levels between CIS patients and HCP (53 vs 52 nmol/l, p = 0.8). For the CIS group, in the placebo, 5000 IU and 10,000 IU treatment arms, mean changes in serum 25(OH)D levels from baseline to week 16 were 7 nmol/l (SD: 26), 81 nmol/l (SD: 63) and 121 nmol/l (SD: 35); from baseline to week 24, mean changes were 18 nmol/l (SD: 34), 76 nmol/l (SD: 57) and 115 nmol/l (SD: 51) respectively. For the HCP group, in the placebo, 5000 IU and 10,000 IU treatment arms, mean changes in serum 25(OH)D levels from baseline to week 16 were 4 nmol/(SD: 12), 83 nmol/l (SD: 27) and 152 nmol/l (SD: 71) and from baseline to week 24 were 2 nmol/l (SD: 14), 92 nmol/l (SD: 35) and 136 nmol/l (SD: 71) respectively (Figure 2).
Figure 2.
Graph showing median (boxes: 25–75% interquartile range and error bars: minimum–maximum) seasonally adjusted serum 1,25 (OH) vitamin D (25(OH)D) levels (nmol/l) achieved in each of the treatment arms at baseline, week 16 and week 24. CIS: clinically isolated syndrome; HCP: healthy control participant.
These changes were significant in both the 10,000 IU and 5000 IU treatment arms when compared to placebo in both CIS patients and HCPs (p < 0.01). Adjusting for baseline serum 25(OH)D levels, no significant differences in serum 25(OH)D levels achieved were seen between CIS patients and HCPs in any treatment arm. No cases of hypercalcaemia (reference range: 2.2–2.6 mmol/l) were recorded in any participant. Mean plasma PTH levels were lower in both treatment arms compared to placebo at all time-points in both CIS patients and HCPs.
Immunological responses to supplementation
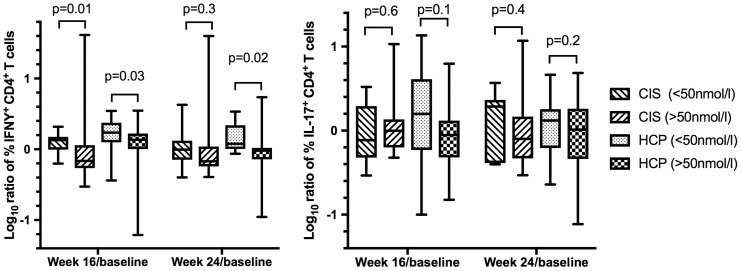
The primary outcome of the study was the change in T cell subsets (IFN-γ+CD4+/IL-17+CD4+ T cells) in response to supplementation. No significant reduction in either IFN-γ+CD4+/IL-17+CD4+ T cells was seen in any treatment arm at any time-point regardless of method of stimulation (polyclonal or antigen specific with MOG/MBP peptides, recall antigens (PPD/TT) or irradiated allogeneic PBMCs). Results of polyclonal stimulation in both CIS patients and HCPs are presented in Table 2 and antigen-specific stimulation in CIS patients in Table 3. Similarly no significant differences were observed in concentrations as measured by ELISA of IL-10, IL-17 and IFN-γ produced followed stimulation of PBMCs in any treatment arm (Supplementary Material, Table 1). Stratifying the groups based on the increase in serum 25(OH)D level (<50 nmol/L/≥50 nmol/L) seen at each time-point showed a trend towards lower IFN-γ+CD4+ proliferating T cells in both CIS patients and HCPs with significance seen at week 16 in CIS (p = 0.01) and week 16 (p = 0.03) and week 24 (p = 0.02) in HCP (Figure 3). All values were adjusted for baseline value but not for multiple comparisons.
Table 2.
Paired comparison between baseline and week 16 and baseline and week 24 of percentage interferon (IFN)-γ+CD4+ proliferating T cells and interleukin (IL)-17+CD4+ proliferating T cells amongst each treatment arm in both clinically isolated syndrome (CIS) and healthy control participants (HCPs). Values are displayed as median and interquartile range.
| CIS |
||||||
|---|---|---|---|---|---|---|
| Week 0 | Week 16 | p-Value | Week 0 | Week 24 | p-Value | |
| IFN-γ+CD4+ proliferating T cells | ||||||
| 10,000 IU | 7.3 (1.2–10.3) | 7 (2.3–8.6) | 0.646 | 7.3 (1.2–10.3) | 5.1 (2.5–7.6) | 0.169 |
| 5,000 IU | 5.8 (3.3–6.8) | 4.9 (3.6–10.2) | 0.594 | 5.8 (3.3–6.8) | 6.4 (4.7–8.6) | 0.674 |
| Placebo | 4.7 (4.3–5.6) | 7 (4.3–9) | 0.043 | 4.7 (4.3–5.6) | 6 (1.1–7.3) | 0.6 |
| IL-17+CD4+ proliferating T cells | ||||||
| 10,000 IU | 0.8 (0.1–2) | 1.3 (0.6–3.2) | 0.015a | 0.8 (0.1–2) | 0.7 (0.3–2.9) | 0.484 |
| 5000 IU | 1.1 (0.6–2.9) | 1.4 (0.8–2.9) | 0.374 | 1.1 (0.6–2.9) | 1.7 (0.5–2.4) | 0.767 |
| Placebo | 0.3 (0.2–0.9) | 0.6 (0.2–1.9) | 0.046a | 0.3 (0.2–0.9) | 0.8 (0–13) | 0.173 |
|
HCPs |
||||||
|
Week 0 |
Week 16 |
p-Value |
Week 0 |
Week 24 |
p-Value |
|
| IFN-γ+CD4+ proliferating T cells | ||||||
| 10,000 IU | 9.3 (7–12.6) | 11.3 (3.3–13.1) | 0.94 | 9.3 (7–12.6) | 8.9 (4.5–12.3) | 0.29 |
| 5000 IU | 8.5 (5–10.9) | 6.5 (3.1–11.5) | 0.59 | 8.5 (5–10.9) | 8.2 (4.8–12.3) | 0.93 |
| Placebo | 4.9 (2.5–7.5) | 5.9 (1.4–12.6) | 0.9 | 4.9 (2.5–7.5) | 6.2 (3.8–12.6) | 0.04b |
| IL-17+CD4+ proliferating T cells | ||||||
| 10,000 IU | 0.6 (0.4–0.9) | 0.5 (0.3–1.2) | 0.37 | 0.6 (0.4–0.9) | 0.7 (0.5–1.6) | 0.46 |
| 5000 IU | 1 (0.5–1.6) | 0.6 (0.3–0.9) | 0.18 | 1 (0.5–1.6) | 0.5 (0.3–1.7) | 0.86 |
| Placebo | 0.8 (0.3–1.5) | 0.8 (0.3–1.9) | 0.75 | 0.8 (0.3–1.5) | 1.3 (0.4–2.8) | 0.64 |
A significant increase in Ll-17+CD4+ T cells seen at these time-points in the 10,000 IU and placebo groups; ba significant increase in IFN-γ+CD4+ T-cells was seen at this time-points in the placebo group.
Table 3.
Paired comparison between baseline and week 16 and baseline and week 24 of percentage interferon (IFN)-γ+CD4+ proliferating T cells and interleukin (IL)-17+CD4+ proliferating T cells from antigen-specific stimulated peripheral blood mononuclear cells (PBMCs) from clinically isolated syndrome (CIS) patients. Values are displayed as median and interquartile range.
| MOG/MBP stimulation |
||||||
|---|---|---|---|---|---|---|
| Week 0 | Week 16 | p-Value | Week 0 | Week 24 | p-Value | |
| IFN-γ+CD4+ proliferating T cells | ||||||
| 10,000 IU | 0.7 (0.1–1.1) | 0.55 (0.2–1.9) | p = 0.45 | 0.7 (0.1–1.1) | 1.1 (0.5–1.9) | p = 0.04a |
| 5000 IU | 0.95 (0.3–4.4) | 1.13 (0.7–1.9) | p = 0.39 | 0.95 (0.3–4.4) | 1.3 (0.7–3.0) | p = 0.29 |
| Placebo | 0.54 (0.2–1.4) | 0.6 (0.2–1.1) | p = 0.23 | 0.54 (0.2–1.4) | 0.38 (0.2–0.9) | p = 0.17 |
| IL-17+CD4+ proliferating T cells | ||||||
| 10,000 IU | 0.42 (0.1–1.0) | 0.21 (0.1–0.7) | p = 0.24 | 0.42 (0.1–1.0) | 0.36 (0.1–0.9) | p = 0.37 |
| 5000 IU | 0.06 (0.03–0.2) | 0.07 (0.03–0.3) | p = 0.4 | 0.06 (0.03–0.2) | 0.29 (0.05–0.4) | p = 0.5 |
| Placebo | 0.1 (0.07–0.3) | 0.37 (0.05–0.4) | p = 0.23 | 0.1 (0.07–0.3) | 0.14 (0.1–0.7) | p = 0.9 |
|
PPD stimulation |
||||||
|
Week 0 |
Week 16 |
p-Value |
Week 0 |
Week 24 |
p-Value |
|
| IFN-γ+CD4+ proliferating T cells | ||||||
| 10,000 IU | 0.45 (0.2–2.1) | 0.87 (0.2–2.6) | p = 0.44 | 0.45 (0.2–2.1) | 1.2 (0.3–3.3) | p = 0.04a |
| 5000 IU | 0.61 (0.4–1.7) | 1.21 (0.6–2.1) | p = 0.29 | 0.61 (0.4–1.7) | 1.3 (0.6–2.6) | p = 0.61 |
| Placebo | 0.69 (0.05–1.6) | 1.19 (0.4–1.9) | p = 0.04a | 0.69 (0.05–1.6) | 0.37 (0.1–2.9) | p = 0.46 |
| IL-17+CD4+ proliferating T cells | ||||||
| 10,000 IU | 0.27 (0.7–3.0) | 0.27 (0.1–1.6) | p = 0.7 | 0.27 (0.7–3.0) | 0.38 (0.1–2.0) | p = 0.7 |
| 5000 IU | 0.13 (0.01–0.4) | 0.12 (0.04–0.3) | p = 0.88 | 0.13 (0.01–0.4) | 0.22 (0.04–0.4) | p = 0.8 |
| Placebo | 0.49 (0.2–1.4) | 0.56 (0.3–0.9) | p = 0.47 | 0.49 (0.2–1.4) | 0.29 (0.1–1.1) | p = 0.23 |
|
Allogeneic stimulation |
||||||
|
Week 0 |
Week 16 |
p-Value |
Week 0 |
Week 24 |
p-Value |
|
| IFN-γ+CD4+ proliferating T cells | ||||||
| 10,000 IU | 1.24 (0.7–2.7) | 1.1 (0.9–3.1) | p = 0.89 | 1.24 (0.7–2.7) | 1.74 (0.6–2.9) | p = 0.95 |
| 5000 IU | 4.5 (1.3 (1.3–7.7) | 1.5 (0.9–2.7) | p = 0.02b | 4.5 (1.3–7.7) | 1.75 (0.8–2.8) | p = 0.008b |
| Placebo | 0.89 (0.3–1.3) | 1.1 (0.6–1.7) | p = 0.69 | 0.89 (0.3–1.3) | 1.6 (0.1–1.7) | p = 0.35 |
| IL-17+CD4+ proliferating T cells | ||||||
| 10,000 IU | 1.15 (0.3–1.8) | 0.73 (0.2–1.6) | p = 0.31 | 1.15 (0.3–1.8) | 0.14 (0.1–1.4) | p = 0.07 |
| 5000 IU | 0.52 (0.2–1.2) | 0.33 (0.2–0.5) | p = 0.14 | 0.52 (0.2–1.2) | 0.43 (0.1–1.8) | p = 0.77 |
| Placebo | 0.2 (0.1–0.6) | 0.51 (0.3–0.7) | p = 0.27 | 0.2 (0.1–0.6) | 0.56 (0.3–0.8) | p = 0.35 |
A significant increase in IFN-γ+CD4+ proliferating T cells at that time point; ba significant reduction in IFN-γ+CD4+ proliferating T cells at that time point.
Figure 3.
Comparison between change in interferon (IFN)-γ+CD4+ proliferating T cells, and interleukin (IL)-17+CD4+ proliferating T cells between baseline and week 16 and baseline and week 24 between CIS and HCP. Values are expressed as a log10 ratio of week 16/baseline and week 24/baseline. The clinically isolated syndrome (CIS) and healthy control participant (HCP) groups have been stratified into two groups based on a change in serum 1,25 (OH) vitamin D (25(OH)D) of <50 nmol/l and ≥50 nmol/l seen at each time-point.
MRI results
Baseline and 24-week MRI scans were available in 26 CIS patients. An incorrect or incomplete protocol was carried out in three patients. Baseline T2 lesions and changes on follow-up MRI are outlined in Table 4. No significant differences were seen between the number of new lesions, or the proportion of participants with new activity on follow-up MRI, between groups, controlling for both baseline T2 and gadolinium-enhancing lesions.
Table 4.
Baseline T2 lesions on magnetic resonance imaging (MRI) and change in follow-up scan at 24 weeks per randomised group.
| 10,000 IU VD (n = 10) | 5000 IU VD (n = 9) | Placebo (n = 7) | |
|---|---|---|---|
| Baseline T2 lesions Mean (SD) | 18 (17) | 23 (11) | 13 (10) |
| Baseline GD-enhancing lesions Mean (SD) | 0.3 (0.7) | 0.3 (0.5) | 0.1 (0.4) |
| New T2 lesions Mean (SD) | 0.4 (0.5) | 1.4 (1.7) | 0.6 (0.8) |
| New GD-enhancing lesions Mean (SD) | 0 | 0.1 (0.3) | 0.1 (0.4) |
| Number of patients with new disease activity by MRI (%) | 5 (50%) | 5 (56%) | 3 (43%) |
GD: gadolinium; SD: standard deviation; VD: vitamin D.
Clinical outcomes
One patient had a relapse during the course of the study. Her symptoms began one day prior to her week 24 visit. She had been allocated to the 5000 IU vitamin D3 treatment group equating to mean ARR of 0.2 in this group. No patient fulfilled standard EDSS criteria for evidence of disability progression. No significant difference in EDSS between groups was seen at any time-point.
Combined clinical and radiological outcomes
Disease freedom at the end of the study as measured by percentage of participants with no evidence of MRI or clinical activity was 50% (5/10), 33% (3/9) and 57% (4/7) in the 10,000 IU, 5000 IU vitamin D3 and placebo arms respectively. Adjusting for baseline serum 25(OH)D level and, comparing CIS patients with a serum 25(OH)D level of <100 nmol/l at week 24 to those with a level ≥100 nmol/l showed a disease freedom rate of 50% (7/14) compared to 67% (8/12) (p = 0.4).
Safety and adverse events
There was one serious adverse event recorded during the study; a patient required hospitalisation for a lumbar discectomy. This event was reported immediately to the study sponsor and the patient withdrew from the study. However, the event was felt to be unrelated to the study drug. At the end of the study the patient was identified as being in the placebo group. A full adverse event listing is included as Supplementary Material, Tables 2 and 3.
Discussion
This trial showed that vitamin D3 supplementation at doses of 5000 IU and 10,000 IU per day is both safe and tolerable and results in significant increases in serum 25(OH)D levels in both healthy controls and CIS patients. It seems probable that serum 25(OH)D levels of greater than 100 nmol/l are required to produce the immunological effects of vitamin D, although no consensus level exists.29 A diminished serologic response to vitamin D3 supplementation has been described in MS patients compared to controls raising the possibility of different vitamin D pharmacokinetics in people with MS.30 In our study, a higher mean change in serum 25(OH)D levels was seen in the control group but this was not statistically significant. Given the relatively small sample size it was not possible to adjust for a number of confounders including age, sex and body mass index (s) across each of the treatment arms.
Analysis of the primary outcome showed no difference in the frequency of pro-inflammatory CD4+ T cells (IL-17+CD4+/IFN-γ+CD4+) between baseline and either time-point in any of the treatment arms. No difference was seen between randomisation groups or in response to varying methods of PBMC stimulation (polyclonal or antigen-specific). A significant reduction in proliferating IFN-γ+CD4+ T-cells was seen in both control participants (week 16 and 24) and CIS patients (week 16) when the groups were stratified according to change in serum 25(OH)D level but these results were not adjusted for multiple comparisons and need to be interpreted with caution. Measurement of anti-inflammatory (IL-10) and pro-inflammatory (IL-17/IFN-γ) cytokines from stimulated PBMCs by ELISA also failed to show any significant changes in response to supplementation or between randomisation groups.
The role of IL-17+CD4+ T cells in MS pathogenesis has been shown in the experimental autoimmune encephalitis (EAE) model, and is also supported by high levels of IL-17 in MS plaques and cerebrospinal fluid.31 In vitro studies have suggested that vitamin D significantly inhibits IL-17 production and increases IL-10 in relapsing remitting MS (RRMS) patients and controls.11 However, the results of a small number of published clinical trials exploring various immunological outcomes in response to vitamin D supplementation in MS have been conflicting and somewhat disappointing in identifying the underlying molecular immunological mechanism of vitamin D supplementation.17–23 A recent study by Sotirchos et al. showed a reduction in IL-17+CD4+ T cells at week 24 in RRMS patients receiving high dose (10,400 IU/day) vitamin D3.17 These effects were not observed in the low dose group (800 IU/day) group and no significant change was seen in IFN-γ+CD4+ T cells in either group. A Dutch sub-study of a larger clinical trial exploring high dose (up to 14,000 IU/day) vitamin D3 supplementation, as an add-on therapy to interferonβ-1 a in patients with RRMS, showed no difference in either IL-17+CD4+ or IFN-γ+CD4+ T cells at 48 weeks follow-up.22 The investigators found a reduction in the proportion of anti-inflammatory IL-4+CD4+ T cells in the placebo group and suggested that supplementation may contribute to immune homeostasis early in the disease course.
There were methodological differences in the processing of PBMCs between these studies and our own which may have contributed towards the different percentages of IL-17+CD4+ and IFN-γ+CD4+ T cells seen. The experimental design of our study was in part similar to that of Sotirchos et al. in that intracellular cytokines were measured by restimulation after an initial in vitro stimulation period using cryopreserved PBMC. However, we cultured cells for three rather than five days with anti-CD3, and included additional antigen-specific stimuli. The rationale for including antigen-specific stimuli was that vitamin D3 is likely to exert at least some of its anti-inflammatory effects on T cells via antigen-presenting cells, and such effects may not be apparent after polyclonal stimulation. The allogeneic stimulation would be expected to stimulate naïve allo-specific T cells and would therefore reveal possible effects of vitamin D3 on T cell differentiation, whereas recall antigens PPD/TT or putative MS auto-antigens MOG/MBP would restimulate antigen-specific memory T cells. In contrast, Muris et al. measured intracellular cytokine production directly ex vivo without cryopreservation or an in vitro stimulation period.22 Although our study was designed to examine the effects of vitamin D3 supplementation on T cell responses either directly or indirectly via the antigen presenting cell, it is possible that vitamin D3 may also exert other immunologic effects that were not examined here. It has been suggested that vitamin D may have immunomodulatory effects on B cells32, regulatory T cells33 and dendritic cells.34
The discordance between published results to date may suggest alternative hypotheses as to the observed association between vitamin D deficiency and MS. It has been argued that low serum 25(OH)D levels seen early in the disease course are a result of the disease itself and supplementation is unlikely to affect disease activity.35 Alternative environmental factors need to be considered. Ultraviolet exposure has been shown to have immunomodulating effects on skin lymphocytes36 and thus, observed vitamin D deficiency may only be a marker of lack of sunlight exposure.
Exploration of secondary endpoints showed no difference between treatment arms and placebo with regard to either radiological or clinical activity. A higher level of disease freedom (67% versus 50%) was seen in patients with serum 25(OH)D levels >100 nmol/l but this was not significant. The trial was not powered to meet this endpoint and the trial duration was probably too short to detect a treatment effect of vitamin D3 supplementation.
It is possible that high dose vitamin D3 therapy on its own may not be sufficiently effective as an anti-inflammatory or immunomodulatory therapy in MS or CIS. There is convincing evidence, from a post-hoc analysis, that higher serum 25(OH)D levels in patients recruited into the BENEFIT and BEYOND studies had additional therapeutic effects on disease activity in patients on betaferon-1 b.37,38 Recent results from the SOLAR study of high dose vitamin D3 supplementation as an add-on therapy to interferonβ-1 a, showed a non-significant reduction in the annualised relapse rate of 30% in the vitamin D3 group at 48 weeks.39 A significant reduction in both unique new lesions and percentage change in T2 lesion volume on MRI was also seen suggesting a therapeutic benefit of supplementation. That study was hampered by difficulty with recruitment leading to a reduced trial duration. Longer follow-up and larger numbers are needed to confirm these findings. A number of trials are ongoing to address this issue.40,41
The strengths of this study are that it was a double-blind randomised controlled trial and the first clinical trial of vitamin D3 therapy in treatment-naïve CIS patients. The HCP group allowed us to establish if immunological effects observed were unique to a CIS population. There were limitations to our study. Power calculations were based on a small pilot study28 and may have resulted in the study being underpowered. Difficulty with recruitment in the CIS group also led to smaller numbers than originally anticipated. The CIS group had a higher mean age than controls, which is a potential confounder when comparing results of some of the secondary immunologic outcomes and vitamin D pharmacokinetics. Our results were adjusted for baseline values but not for multiple comparisons, which may limit their interpretability.
Conclusion
In conclusion, this study demonstrates that vitamin D3 supplementation at doses up to 10,000 IU daily is safe and tolerable in both HPCs and CIS patients. High dose vitamin D3 supplementation did not reduce pro-inflammatory IL-17+CD4+ T cells; there were possible minor effects on IFN-γ+ CD4+ T cells, but no changes were observed in concentrations of IL-10, IL-17 or IFN-γ produced by stimulated PBMCs. The potential role of vitamin D3 as an immunomodulator needs to be further clarified in larger randomised controlled trials.
Supplementary Material
Acknowledgments
The authors would like to thank all participants for their willingness for taking part in this trial.
Conflicts of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Merck KGaA (Darmstadt, Germany) reviewed the manuscript for medical accuracy only before journal submission. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: The authors acknowledge the support of MS Ireland for part funding the study, Merck KgaA (Darmstadt, Germany) who provided the placebo oil and study drug (Vigantol Oil) and Biogen Idec in providing funding for the lead clinical researcher (KO’C) through the Newman Clinical Fellowship programme.
Supplemental Data
Supplementary material is available for this article online.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Simpson S, Jr, Blizzard L, Otahal P, et al. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J Neurol Neurosurg Psychiatry 2011; 82: 1132–1141. [DOI] [PubMed] [Google Scholar]
- 3.Ramagopalan SV, Handel AE, Giovannoni G, et al. Relationship of UV exposure to prevalence of multiple sclerosis in England. Neurology 2011; 76: 1410–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orton SM, Wald L, Confavreux C, et al. Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology 2011; 76: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salzer J, Hallmans G, Nystrom M, et al. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012; 79: 2140–2145. [DOI] [PubMed] [Google Scholar]
- 6.Munger KL, Aivo J, Hongell K, et al. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the finnish maternity cohort. JAMA Neurol 2016; 73: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson R, Giovannoni G, Ramagopalan S. The month of birth effect in multiple sclerosis: Systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry 2013; 84: 427–432. [DOI] [PubMed] [Google Scholar]
- 8.Simpson S, Jr., Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 2010; 68: 193–203. [DOI] [PubMed] [Google Scholar]
- 9.Mowry EM, Krupp LB, Milazzo M, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol 2010; 67: 618–624. [DOI] [PubMed] [Google Scholar]
- 10.Mowry EM, Waubant E, McCulloch CE, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol 2012; 72: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009; 132: 1146–1160. [DOI] [PubMed] [Google Scholar]
- 12.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 2008; 4: 404–412. [DOI] [PubMed] [Google Scholar]
- 13.Smolders J, Menheere P, Kessels A, et al. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 2008; 14: 1220–1224. [DOI] [PubMed] [Google Scholar]
- 14.Bhalla AK, Amento EP, Serog B, et al. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 1984; 133: 1748–1754. [PubMed] [Google Scholar]
- 15.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007; 179: 1634–1647. [DOI] [PubMed] [Google Scholar]
- 16.Boonstra A, Barrat FJ, Crain C, et al. 1Alpha,25-dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 2001; 167: 4974–4980. [DOI] [PubMed] [Google Scholar]
- 17.Sotirchos ES, Bhargava P, Eckstein C, et al. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology 2016; 86: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimball S, Vieth R, Dosch HM, et al. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Metab 2011; 96: 2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolders J, Peelen E, Thewissen M, et al. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PloS One 2010; 5: e15235–e15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, et al. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation 2015; 22: 400–404. [DOI] [PubMed] [Google Scholar]
- 21.Toghianifar N, Ashtari F, Zarkesh-Esfahani SH, et al. Effect of high dose vitamin D intake on interleukin-17 levels in multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. J Neuroimmunol 2015; 285: 125–128. [DOI] [PubMed] [Google Scholar]
- 22.Muris AH, Smolders J, Rolf L, et al. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNbeta; the SOLARIUM study. J Neuroimmunol 2016; 300: 47–56. [DOI] [PubMed] [Google Scholar]
- 23.Aivo J, Hanninen A, Ilonen J, et al. Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-beta. J Neuroimmunol 2015; 280: 12–15. [DOI] [PubMed] [Google Scholar]
- 24.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol 2012; 11: 157–169. [DOI] [PubMed] [Google Scholar]
- 26.Lonergan R, Kinsella K, Fitzpatrick P, et al. Multiple sclerosis prevalence in Ireland: Relationship to vitamin D status and HLA genotype. J Neurol Neurosurg Psychiatry 2011; 82: 317–322. [DOI] [PubMed] [Google Scholar]
- 27.O'Connell K, Kelly S, Kinsella K, et al. Dose-related effects of vitamin D on immune responses in patients with clinically isolated syndrome and healthy control participants: Study protocol for an exploratory randomized double-blind placebo-controlled trial. Trials 2013; 14: 272–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen AC, Kelly S, Basdeo SA, et al. A pilot study of the immunological effects of high-dose vitamin D in healthy volunteers. Mult Scler 2012; 18: 1797–1800. [DOI] [PubMed] [Google Scholar]
- 29.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006; 296: 2832–2838. [DOI] [PubMed] [Google Scholar]
- 30.Bhargava P, Steele SU, Waubant E, et al. Multiple sclerosis patients have a diminished serologic response to vitamin D supplementation compared to healthy controls. Mult Scler 2016; 22: 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher JM, Lalor SJ, Sweeney CM, et al. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 2010; 162: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolf L, Muris AH, Hupperts R, et al. Illuminating vitamin D effects on B-cells – the multiple sclerosis perspective. Immunology 2016; 147: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prietl B, Treiber G, Mader JK, et al. High-dose cholecalciferol supplementation significantly increases peripheral CD4(+) Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur J Nutr 2014; 53: 751–759. [DOI] [PubMed] [Google Scholar]
- 34.Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology 2016; 148: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghashut RA, Talwar D, Kinsella J, et al. The effect of the systemic inflammatory response on plasma vitamin 25 (OH) D concentrations adjusted for albumin. PloS One 2014; 9: e92614–e92614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maverakis E, Miyamura Y, Bowen MP, et al. Light, including ultraviolet. J Autoimmun 2010; 34: J247–J257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald KC, Munger KL, Kochert K, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol 2015; 72: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 38.Ascherio A, Munger KL, White R, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 2014; 71: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smolders J, Vieth R, Holmøy T, et al. High dose cholecalciferol (vitamin D3) oil as add-on therapy in subjects with relapsing–remitting multiple sclerosis receiving subcutaneous interferonB-1a. Mult Scler 2016; 22. [Google Scholar]
- 40.Bhargava P, Cassard S, Steele SU, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: Study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials 2014; 39: 288–293. [DOI] [PubMed] [Google Scholar]
- 41.Dorr J, Ohlraun S, Skarabis H, et al. Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS Trial): Study protocol for a randomized controlled trial. Trials 2012; 13: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.